Published online Feb 27, 2015. doi: 10.4254/wjh.v7.i2.261
Peer-review started: August 28, 2014
First decision: September 16, 2014
Revised: November 3, 2014
Accepted: November 17, 2014
Article in press: November 19, 2014
Published online: February 27, 2015
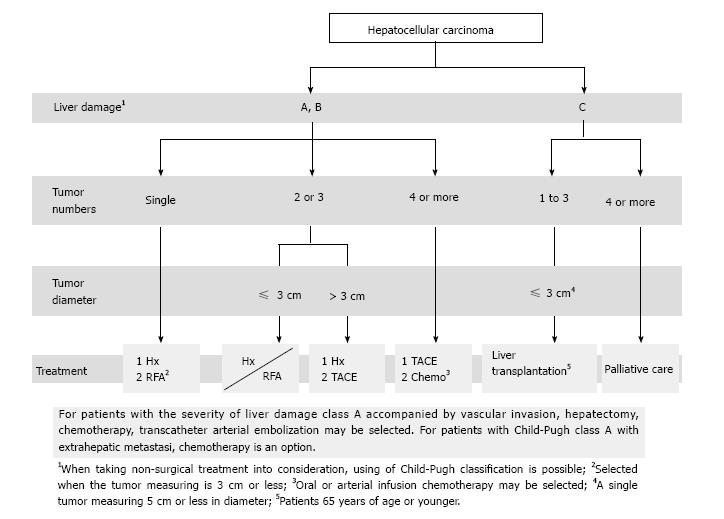
In the Algorithm for Diagnosis and Treatment in the Japanese Evidence-Based Clinical Practice Guidelines for Hepatocellular Carcinoma, the treatment strategy is determined by three major factors: liver function and the number and size of tumors. The algorithm is quite simple, consisting of fewer components than the Barcelona-Clinic Liver Cancer staging system. In this article, we describe the roles of the treatment algorithm in hepatectomy and perioperative management of hepatocellular carcinoma.
Core tip: In the Algorithm for Diagnosis and Treatment in the Japanese Evidence-Based Clinical Practice Guidelines for Hepatocellular Carcinoma, the treatment strategy is determined by three major factors: liver function and the number and size of tumors. The algorithm is quite simple, consisting of fewer components than the Barcelona-Clinic Liver Cancer staging system. In this article, we describe the roles of the treatment algorithm in hepatectomy and perioperative management of hepatocellular carcinoma.
- Citation: Nakayama H, Takayama T. Role of surgical resection for hepatocellular carcinoma based on Japanese clinical guidelines for hepatocellular carcinoma. World J Hepatol 2015; 7(2): 261-269
- URL: https://www.wjgnet.com/1948-5182/full/v7/i2/261.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i2.261
Hepatocellular carcinoma (HCC) is the sixth most common neoplasm worldwide and the third most frequent cause of cancer-related death. More than 0.7 million people were diagnosed with HCC in 2008, indicating an incidence of 16 per 0.1 million people[1]. The distribution of HCC is regional, with approximately 80% of HCC cases found in Eastern Asia and central Africa. The risk factors in these areas are hepatitis B and aflatoxin, but those in North America, Europe, and Japan are hepatitis C and alcohol.
The spread of the concept of evidence-based medicine (EBM) has provided an opportunity for development of treatment guidelines. In Western countries, the Barcelona Clinic Liver Cancer (BCLC) staging system was published as practice guidelines in 2005 and updated in 2011, and is recommended for use by the American Association for the Study of Liver Diseases (AASLD)[2] and the European Association for the Study of the Liver (EASL). In Japan, the Clinical Practice Guidelines for Hepatocellular Carcinoma were published in 2005[3,4] and then revised in 2009 and 2013 to add new information[5]. The “treatment algorithm” listed in the guidelines has become well disseminated as a standard method for selection of optimal treatment based on liver function and tumor conditions[6]. Here, we describe the roles of the treatment algorithm in hepatectomy for HCC and we discuss current knowledge on hepatectomy in Japan.
Staging systems for liver cancer have three elements: (1) tumor stage (TNM system); (2) hepatic functional reserve; and (3) integrated stage, a combination of (1) and (2). The International Union Against Cancer (UICC) published the UICC TNM classification of malignant tumors in 1968 and added liver cancer to the TNM classification in 1987. Now, the seventh edition is used from 2009[7]. The UICC-TNM classification is based on the staging system of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual, with a database from multicenter research by the International Cooperative Study Group on Hepatocellular Carcinoma[8]. The UICC-TNM classification is a simplified version of the AJCC Manual, and the 7th edition set the cutoff for tumor size as 5 cm. In 1983, the Liver Cancer Study Group of Japan published the “General Rules for Clinical and Pathological Studies of Primary Liver Cancer” (henceforth referred to as the “General Rules”), which included the Japanese TNM classification[9] and was prepared based on a database developed by the Liver Cancer Study Group. In the latest edition, the stages are classified using a cutoff tumor size of 2 cm, single/multiple lesions, and vascular invasion. In a comparison of these two staging systems in Japanese patients, Minagawa et al[10] found that both systems allowed clear stratification of patients into prognostic groups, but that the General Rules were more appropriate for stratifying patients with early-stage HCC[10].
The Child-Pugh classification is most commonly used for evaluation of hepatic functional reserve[11,12]. This classification has five parameters: serum bilirubin, serum albumin, ascites, hepatic encephalopathy, and prothrombin activity, which are used to assess liver function in three classes: A, B and C. The indocyanine green retention rate at 15 min (ICGR15) is also used in Japan, eastern Asia, and some European countries as a more detailed index for assessment of hepatic functional reserve. ICGR15 is useful for prediction of postoperative mortality[13] and as a marker of liver function for determining the extent of hepatectomy[14]. The General Rules also have a liver damage classification system that uses ICGR15, as well as serum bilirubin, serum albumin, ascites, and prothrombin activity[9,15]. The degree of liver damage has replaced the Child-Pugh classification to evaluate liver function in Japan. For serious liver failure patients, model for end stage liver disease (MELD) is used to indicate liver transplantation[16].
Integrated Stage score for liver function and tumor stage, including OKUDA[17], Cancer of the Liver Italian Program (CLIP)[18], Chinese University Prognostic Index (CUPI)[19], Japan Integrated Staging (JIS)[20], modified-JIS[15], and Tokyo[21], is effective for prognostic assessment in HCC (Table 1). Kudo et al[20] proposed the JIS score, which unified TNM staging in the General Rules and the Child-Pugh classification[20]. The JIS is superior to the CLIP system [a combination of the Child-Pugh classification, tumor morphology, α-fetoprotein (AFP), and portal vein tumor thrombosis] in terms of (1) clear stratification of scores; (2) prognostic predictive power in HCC with a score of 0; and (3) differentiation of scores in patients with a poor prognosis. Thus, the JIS score is useful for prediction of prognosis of patients, but is not appropriate for comparison of treatment modalities or selection of optimal treatment.
| Classification | Year | Background | Variables | ||
| Tumor status | Liver function | Health status | |||
| Okuda staging | 1985 | 850 Japanese patients | 50% liver involvement | Ascites, bilirubin, albumin | - |
| BCLC staging | 1999 | Selected papers | Size, number, vascular invasion, Okuda stage | Child-Pugh, bilirubin, porta hypertension | Performance status |
| CLIP score | 2000 | 435 Italian patients | 50% liver involvement, vascular invasion, AFP | Child-Pugh | - |
| CUPI | 2002 | 926 Chinese patients | TNM, AFP | Bilirubin, albumin, alkaline phosphatase | Presence of symptoms |
| JIS score | 2003 | 3334 Japanese patients | TNM (Japanese) | Child-Pugh | - |
| m-JIS score | 2006 | 42269 Japanese patients | TNM (Japanese) | Liver damage | - |
| Tokyo score | 2005 | 403 Japanese patients | Size, number | Bilirubin, albumin | - |
The BCLC staging system, which is recommended by AASLD and EASL, is used worldwide to plan treatment for HCC. In contrast, in Japan, the treatment algorithm in the Clinical Practice Guidelines for Hepatocellular Carcinoma is commonly used for selection of optimal treatment based on liver function and tumor conditions (Figure 1). BCLC system links stage stratification to a treatment strategy and recommends standard care for a given patient, whereas the Japanese guidelines are not directly associated with clinical tumor stage, such as the JIS score[22]. The another major difference between the treatment algorithm used in Japan and the BCLC system is the indication of hepatectomy for HCC with ≤ 3 lesions and a diameter ≤ 3 cm on Child-Pugh A/B. The BCLC system recommends liver transplantation or radiofrequency ablation (RFA) for HCC with 2 or 3 nodules and a diameter ≤ 3 cm. In contrast, the treatment algorithm in Japan recommends hepatectomy for HCC with ≤ 3 lesions if liver function is good, regardless of the tumor size. The recommended treatment strategy also differs for HCC with portal hypertension (Table 2). The BCLC system states that liver transplantation or RFA, instead of hepatectomy, is indicated in such patients, but the treatment algorithm in Japan advises that aggressive hepatectomy based on ICGR15 should be performed because the therapy must yield positive results[23].
| Tumor number | Tumor size (cm) | Child-Pugh class | Treatment | |
| BCLC system | Japanese guidelines | |||
| Single | 2 | A, B | Resection | 1 Resection |
| 2 Ablation | ||||
| 2.1-3 | A, B | 1 Resection | 1 Resection | |
| 2 Transplantation or ablation | 2 Ablation | |||
| 3.1-5 | A, B | 1 Resection | Resection | |
| 2 Transplantation | ||||
| 2 or 3 nodules | ≤ 3 | A, B | Transplantation or ablation | Resection or ablation |
| C | Palliative care | Transplantation | ||
| > 3 | A, B | Chemoembolization | 1 Resection | |
| 2 Chemoembolization | ||||
| 4 or more nodules | A, B | Chemoembolization | 1 Chemoembolization | |
| 2 Chemotherapy | ||||
| C | Palliative care | Palliative care | ||
Classical HCC is diagnosed based on CT images with early arterial enhancement and delayed washout (EASL criteria)[1,24]. Various guidelines have also adopted these criteria. Early HCC generally has stromal invasion in the portal region with remaining tumor[25] and has a macroscopically small nodular type with indistinct margins. Diagnostic imaging identifies this type as an ischemic mass. Prolongation of survival time by liver resection for early HCC is not significant and is limited due to the lead time bias[26]. This suggests that early HCC should be followed up without treatment based on the risk of a second primary cancer. This strategy is accepted according to the HCC management based on the consensus in the Japan Society of Hepatology[27].
The indication of hepatectomy for HCC is determined by the balance between liver function and tumor conditions. Excessive liver resection to completely remove lesions based on overestimation of hepatic functional reserve may cause hepatic failure, whereas minimal resection that does not correspond to the degree of tumor progression and focuses only on safety may increase the risk of early recurrence of HCC. Therefore, it is important to select an optimal approach that is appropriate for the degree of tumor progression based on the indication for hepatectomy. The major methods for preoperative assessment of liver function are the galactose tolerance test, 99mTc-GSA liver scintigraphy, and the ICG loading test. Makuuchi’s criteria are particularly useful for patients with chronic hepatitis or hepatic cirrhosis[14]. These criteria are based on three factors: ascites, serum bilirubin, and ICGR15. Patients who still have ascites after diuretic administration or those with a serum bilirubin level that is consistently > 2.0 mg/dL are not indicated for surgery. The patients with 1 < bilirubin level ≤ 2.0 mg/dL are indicated for limited liver resection. For eligible patients with serum bilirubin in the normal range of ≤ 1.0 mg/dL, the extent of resection is then determined based on ICGR15 as resection of 2/3 of the total liver volume (TLV) (e.g., right lobectomy) in patients with normal ICGR15 of < 10%; 1/3 of the TLV (e.g., left lobectomy) in those with ICGR15 of 10%-19%; and 1/6 of the TLV (Couinaud segmentectomy) in those with ICGR15 of 20%-29%. If ICGR15 is ≥ 30%, limited resection or enucleation should be applied. A surgical mortality rate of 0% has been reported in 1056 consecutive hepatic resections performed in accordance with these criteria[28].
In portal venous invasion of HCC[29], the area supplied by the portal vein branches should be systemically removed as much as possible within the acceptable range of liver function. A new procedure of systematic subsegmentectomy has been developed to overcome the potential incompatibility between cure of cancer and preservation of liver function[30]. A study of survival after hepatectomy indicated a good prognosis in cases with a tumor diameter < 5 cm, a single lesion, capsule formation, no vascular invasion, serum albumin < 4.0 g/dL, and pathological TNM (pTNM) stage I or II. Of these parameters, pTNM stage is the most reliable prognostic factor[31]. A study of recurrence-free survival also identified the significant prognostic factors as the tumor stage, tumor size, number of tumors, and capsule formation, and also found that vascular invasion was a poor indicator of long-term survival[32]. Risk factors for early recurrence within 2 years postoperatively include non-anatomical resection, microscopic vascular invasion, and AFP ≥ 32 ng/mL[33]. A retrospective study showed that the cumulative survival rate was significantly greater after anatomical resection compared to that after non-anatomical resection, which suggests that the surgical technique can influence prognosis[34]. A future prospective study is required to clarify all of these findings.
Determination of the acceptable liver remnant volume after hepatectomy is an important task. In general, it is desirable to preserve the 20%-40% of the total liver volume (TLV) or the standard liver volume (SLV) in normal livers[35-42]. The MD Anderson group proposed that the smallest acceptable liver remnant volume is ≥ 20% of the SLV in cases without chronic underlying liver disease[36], with the validity of this proposal supported by an analysis of 301 consecutive patients after extended right lobectomy[43]. On the other hand, there was a mortality rate on postoperative day 60 of 4.7% in this literature cited. However, HCC often develops in livers with chronic hepatitis or hepatic cirrhosis, and major hepatectomy such as lobectomy may induce hepatic failure due to insufficient liver remnant volume. Portal vein embolization (PE) prevents hepatic failure since the portal vein branches in hepatectomy are blocked to induce compensatory hypertrophy in the remnant liver area[44]. PE can be applied to cases with ICGR15 < 10% and a ratio of nontumorous parenchymal volume of the resected liver to that of the whole liver (R2) ≥ 60%, and those with ICGR15≥ 10% - < 20% and R2 of 40%-60%[35]. Three-dimensional CT permits simple and accurate determination of the relative positions of major blood vessels and the tumor, resection ranges, and liver remnant volume[45].
In liver surgery, hepatic parenchymal transection is associated with increased intraoperative blood loss, postoperative hemorrhage, and early complications such as bile leakage and surgical site infection (SSI). In addition to hemostasis, new devices have been developed to stop bleeding from the resection margin, which allows performance of safer and more secure hepatic resection. The Pringle maneuver, which blocks hepatic inflow once by manual compression of the hepatoduodenal ligament to minimize blood loss during hepatic resection, is also widely used. Several randomized clinical trials (RCTs) have shown that the Pringle maneuver reduces blood loss without affecting liver function[46,47]. Hemihepatic vascular occlusion has also been applied when resection is limited to one lobe[48,49].
Bleeding from the hepatic vein occurs most commonly during hepatic resection. Intraoperative hemorrhage is positively associated with central venous pressure (CVP) and several RCTs have shown that a decrease of CVP to ≤ 5 cm H2O during hepatectomy reduces intraoperative blood loss and stabilizes hemodynamics[50,51]. In contrast, infra-hepatic inferior vena cava clamping with a low CVP has been shown not to reduce blood loss during hepatectomy[52], and thus the effects of low CVP require further study.
Hepatic parenchymal transection is performed using methods such as clamp crushing[53] and devices including the cavitron ultrasonic aspirator (CUSA)[54], Tissue Link[55], water jet scalpel[56], harmonic scalpel[57], floating ball[58], and LigaSure. In clamp crushing, a Pean clamp is used to ligate and resect remaining blood vessels after the hepatic parenchyma is crushed using the clamp. In RCTs, there were no differences in operating time, volume of blood loss, and incidence of postoperative complications between patients treated with clamp crushing and CUSA, but clamp crushing was superior in terms of complete appearance of landmark hepatic veins on the cut surface[53]. However, volume of blood loss and incidence of postoperative complications have also been reported to be lower using CUSA compared with clamp crushing[59]. A RCT comparing clamp crushing with Tissue Link found no differences in operating time, volume of blood loss and incidence of postoperative complications[60]. Another RCT showed the superiority of the LigaSure Vessel Sealing System for liver resection compared to vascular ligation based on clamp crushing[61], but a second RCT found no differences between these techniques[62].
Since 1990, hepatectomy for HCC has been performed with acceptable blood loss of approximately 500 ml at many high-volume medical centers[28,63-67]. Allogenic blood transfusion in the perioperative period should be avoided when possible because it is likely to promote cancer recurrence and to induce hyperbilirubinemia and hepatic failure, and lower hematocrit is also desirable for microcirculation in the liver[68]. Autologous blood transfusion avoids homogenous red blood cell transfusion and does not increase the frequency of cancer recurrence[69]. The use of fresh frozen plasma (FFP) has been recommended for supplement of coagulation factors, maintenance of an effective plasma volume, and volume substitution[70]. However, FFP transfusion has also been reported to have no effect on the post-hepatectomy course[71] and to be unnecessary in Child-Pugh class A cases with intraoperative blood loss of < 1000 mL and serum albumin levels > 2.4 g/dL on postoperative day (POD) 2[72].
Bile leakage is a complication that is specific to hepatectomy and may be intractable. A RCT of the efficacy of a bile leakage test on prevention of bile leakage from the liver resection margin showed no difference in the incidence of bile leakage between patients who did and did not receive the test[73], whereas another RCT found that the test was able to prevent bile leakage and complications after hepatic resection[74]. Thus, more cases are required to evaluate the utility of this test.
Other post-hepatectomy complications include hemorrhage and intra-abdominal abscess, and these conditions may be fatal if diagnosis is delayed. Intraperitoneal drain placement is required for monitoring and treatment of these complications, but the efficacy of elective hepatectomy with standardized drain placement has been questioned and the Centers for Disease Control and Prevention (CDC) guidelines state that such routine drain placement is not necessary: “If drainage is necessary, use a closed suction drain. Place a drain through a separate incision distant from the operative incision. Remove the drain as soon as possible”[75]. RCTs conducted in several countries on the need for drainage have also concluded that drain placement is not necessary[76-81]. Due to differences in the healthcare environment and health insurance system, drain placement has not been completely withdrawn in Japan, but early removal of drains has been recommended[82]. A RCT has also shown that subcutaneous drainage is not effective for prevention of SSI[83].
Immunity is weak after hepatectomy and this may result in hepatic failure and disseminated intravascular coagulation (DIC). A RCT of the efficacy of steroid administration for improvement of liver function after hepatectomy compared the post-hepatectomy liver function in patients treated with and without 500 mg/body hydrocortisone before hepatectomy[84]. Serum bilirubin levels were significantly lower in the steroid group on POD 2 compared with the non-steroid group and there were significant differences in serum bilirubin and prothrombin levels until POD 7, which shows the efficacy of steroid administration prior to hepatectomy. To unify the definition of post-hepatectomy liver failure (PHLF), in 2010 the International Study Group of Liver Surgery (ISGLS) proposed defining PHLF as an increased international normalized ratio (INR) and concomitant hyperbilirubinemia on or after POD 5[85]. PHLF seems to be the more efficient indicator comprehensively compared to 50-50 criteria[86] and MELD score because it is significantly associated with both of the incidence of post-hepatectomy complications and the post-hepatectomy mortality[87]. As for 50-50 criteria, it was not significantly related to the incidence of post-hepatectomy complications. As for MELD score, it revealed less strong association of the odds ratio (2.06) to the post-hepatectomy mortality.
In this article, we have described evidence-based techniques for hepatectomy and perioperative management of HCC. Improved assessment of liver function and development of surgical devices are likely to contribute to safe and effective hepatectomy and a good prognosis for patients.
P- Reviewer: Han HS, Kokudo N S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3249] [Cited by in F6Publishing: 3477] [Article Influence: 289.8] [Reference Citation Analysis (3)] |
| 2. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5972] [Cited by in F6Publishing: 6338] [Article Influence: 487.5] [Reference Citation Analysis (1)] |
| 3. | Kokudo N, Makuuchi M. Evidence-based clinical practice guidelines for hepatocellular carcinoma in Japan: the J-HCC guidelines. J Gastroenterol. 2009;44 Suppl 19:119-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Makuuchi M, Kokudo N, Arii S, Futagawa S, Kaneko S, Kawasaki S, Matsuyama Y, Okazaki M, Okita K, Omata M. Development of evidence-based clinical guidelines for the diagnosis and treatment of hepatocellular carcinoma in Japan. Hepatol Res. 2008;38:37-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 216] [Cited by in F6Publishing: 226] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Hepatology JSo. Clinical practice guidelines for hepatocellular carcinoma. Tokyo, Japan: Kanehara 2013; . [Cited in This Article: ] |
| 6. | Kokudo N, Sasaki Y, Nakayama T, Makuuchi M. Dissemination of evidence-based clinical practice guidelines for hepatocellular carcinoma among Japanese hepatologists, liver surgeons and primary care physicians. Gut. 2007;56:1020-1021. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Sobin LH, Compton CC. TNM seventh edition: what’s new, what’s changed: communication from the International Union Against Cancer and the American Joint Committee on Cancer. Cancer. 2010;116:5336-5339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 354] [Cited by in F6Publishing: 384] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 8. | Vauthey JN, Lauwers GY, Esnaola NF, Do KA, Belghiti J, Mirza N, Curley SA, Ellis LM, Regimbeau JM, Rashid A. Simplified staging for hepatocellular carcinoma. J Clin Oncol. 2002;20:1527-1536. [PubMed] [Cited in This Article: ] |
| 9. | Japan LCSGo. General rules for the clinical and pathological study of primary liver cancer. 3rd ed. Tokyo: Kanehara 2010; . [Cited in This Article: ] |
| 10. | Minagawa M, Ikai I, Matsuyama Y, Yamaoka Y, Makuuchi M. Staging of hepatocellular carcinoma: assessment of the Japanese TNM and AJCC/UICC TNM systems in a cohort of 13,772 patients in Japan. Ann Surg. 2007;245:909-922. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 263] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 11. | Child CG. The liver and portal hypertension. 3rd. MPCS. Philadelphia: W.B. Saunders 1964; . [Cited in This Article: ] |
| 12. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] [Cited in This Article: ] |
| 13. | Lau H, Man K, Fan ST, Yu WC, Lo CM, Wong J. Evaluation of preoperative hepatic function in patients with hepatocellular carcinoma undergoing hepatectomy. Br J Surg. 1997;84:1255-1259. [PubMed] [Cited in This Article: ] |
| 14. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [PubMed] [Cited in This Article: ] |
| 15. | Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, Makuuchi M, Kojiro M, Ichida T, Arii S. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884-892. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 16. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3462] [Cited by in F6Publishing: 3432] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 17. | Okuda K, Ohtsuki T, Obata H, Tomimatsu M, Okazaki N, Hasegawa H, Nakajima Y, Ohnishi K. Natural history of hepatocellular carcinoma and prognosis in relation to treatment. Study of 850 patients. Cancer. 1985;56:918-928. [PubMed] [Cited in This Article: ] |
| 18. | A new prognostic system for hepatocellular carcinoma: a retrospective study of 435 patients: the Cancer of the Liver Italian Program (CLIP) investigators. Hepatology. 1998;28:751-755. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 908] [Cited by in F6Publishing: 933] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 19. | Leung TW, Tang AM, Zee B, Lau WY, Lai PB, Leung KL, Lau JT, Yu SC, Johnson PJ. Construction of the Chinese University Prognostic Index for hepatocellular carcinoma and comparison with the TNM staging system, the Okuda staging system, and the Cancer of the Liver Italian Program staging system: a study based on 926 patients. Cancer. 2002;94:1760-1769. [PubMed] [Cited in This Article: ] |
| 20. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 259] [Cited by in F6Publishing: 258] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 21. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 191] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 22. | Takayama T. Hepatocellular carcinoma. Malignant liver tumors: current and emerging therapies. 3rd ed. London: Wiley-Blackwell 2010; 317-323. [Cited in This Article: ] |
| 23. | Ishizawa T, Hasegawa K, Aoki T, Takahashi M, Inoue Y, Sano K, Imamura H, Sugawara Y, Kokudo N, Makuuchi M. Neither multiple tumors nor portal hypertension are surgical contraindications for hepatocellular carcinoma. Gastroenterology. 2008;134:1908-1916. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 513] [Cited by in F6Publishing: 543] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 24. | Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42:1208-1236. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4333] [Cited by in F6Publishing: 4404] [Article Influence: 231.8] [Reference Citation Analysis (0)] |
| 25. | Pathologic diagnosis of early hepatocellular carcinoma: a report of the international consensus group for hepatocellular neoplasia. Hepatology. 2009;49:658-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 610] [Cited by in F6Publishing: 554] [Article Influence: 36.9] [Reference Citation Analysis (1)] |
| 26. | Midorikawa Y, Takayama T, Shimada K, Nakayama H, Higaki T, Moriguchi M, Nara S, Tsuji S, Tanaka M. Marginal survival benefit in the treatment of early hepatocellular carcinoma. J Hepatol. 2013;58:306-311. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 549] [Cited by in F6Publishing: 630] [Article Influence: 48.5] [Reference Citation Analysis (0)] |
| 28. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 597] [Cited by in F6Publishing: 585] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 29. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Itoh A. Proposal of objective morphological classification system for hepatocellular carcinoma using preoperative multiphase computed tomography. J Gastroenterol. 2014;49:1430-1437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161:346-350. [PubMed] [Cited in This Article: ] |
| 31. | Poon RT, Ng IO, Fan ST, Lai EC, Lo CM, Liu CL, Wong J. Clinicopathologic features of long-term survivors and disease-free survivors after resection of hepatocellular carcinoma: a study of a prospective cohort. J Clin Oncol. 2001;19:3037-3044. [PubMed] [Cited in This Article: ] |
| 32. | Arii S, Tanaka J, Yamazoe Y, Minematsu S, Morino T, Fujita K, Maetani S, Tobe T. Predictive factors for intrahepatic recurrence of hepatocellular carcinoma after partial hepatectomy. Cancer. 1992;69:913-919. [PubMed] [Cited in This Article: ] |
| 33. | Imamura H, Matsuyama Y, Tanaka E, Ohkubo T, Hasegawa K, Miyagawa S, Sugawara Y, Minagawa M, Takayama T, Kawasaki S. Risk factors contributing to early and late phase intrahepatic recurrence of hepatocellular carcinoma after hepatectomy. J Hepatol. 2003;38:200-207. [PubMed] [Cited in This Article: ] |
| 34. | Hasegawa K, Kokudo N, Imamura H, Matsuyama Y, Aoki T, Minagawa M, Sano K, Sugawara Y, Takayama T, Makuuchi M. Prognostic impact of anatomic resection for hepatocellular carcinoma. Ann Surg. 2005;242:252-259. [PubMed] [Cited in This Article: ] |
| 35. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 198] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 36. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-680; discussion 680-681. [PubMed] [Cited in This Article: ] |
| 37. | Schindl MJ, Redhead DN, Fearon KC, Garden OJ, Wigmore SJ. The value of residual liver volume as a predictor of hepatic dysfunction and infection after major liver resection. Gut. 2005;54:289-296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 397] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 38. | Nagino M, Kamiya J, Nishio H, Ebata T, Arai T, Nimura Y. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 386] [Cited by in F6Publishing: 364] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 39. | Sano T, Shimada K, Sakamoto Y, Yamamoto J, Yamasaki S, Kosuge T. One hundred two consecutive hepatobiliary resections for perihilar cholangiocarcinoma with zero mortality. Ann Surg. 2006;244:240-247. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 187] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 40. | Ferrero A, Viganò L, Polastri R, Muratore A, Eminefendic H, Regge D, Capussotti L. Postoperative liver dysfunction and future remnant liver: where is the limit? Results of a prospective study. World J Surg. 2007;31:1643-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 158] [Cited by in F6Publishing: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 41. | Giraudo G, Greget M, Oussoultzoglou E, Rosso E, Bachellier P, Jaeck D. Preoperative contralateral portal vein embolization before major hepatic resection is a safe and efficient procedure: a large single institution experience. Surgery. 2008;143:476-482. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Shimada K, Nara S, Esaki M, Sakamoto Y, Kosuge T, Hiraoka N. Extended right hemihepatectomy for gallbladder carcinoma involving the hepatic hilum. Br J Surg. 2011;98:117-123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 43. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 339] [Cited by in F6Publishing: 336] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 44. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] [Cited in This Article: ] |
| 45. | Saito S, Yamanaka J, Miura K, Nakao N, Nagao T, Sugimoto T, Hirano T, Kuroda N, Iimuro Y, Fujimoto J. A novel 3D hepatectomy simulation based on liver circulation: application to liver resection and transplantation. Hepatology. 2005;41:1297-1304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 46. | Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg. 1997;226:704-711; discussion 711-713. [PubMed] [Cited in This Article: ] |
| 47. | Scatton O, Zalinski S, Jegou D, Compagnon P, Lesurtel M, Belghiti J, Boudjema K, Lentschener C, Soubrane O. Randomized clinical trial of ischaemic preconditioning in major liver resection with intermittent Pringle manoeuvre. Br J Surg. 2011;98:1236-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 48. | Makuuchi M, Mori T, Gunvén P, Yamazaki S, Hasegawa H. Safety of hemihepatic vascular occlusion during resection of the liver. Surg Gynecol Obstet. 1987;164:155-158. [PubMed] [Cited in This Article: ] |
| 49. | Fu SY, Lau WY, Li GG, Tang QH, Li AJ, Pan ZY, Huang G, Yin L, Wu MC, Lai EC. A prospective randomized controlled trial to compare Pringle maneuver, hemihepatic vascular inflow occlusion, and main portal vein inflow occlusion in partial hepatectomy. Am J Surg. 2011;201:62-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Rahbari NN, Koch M, Zimmermann JB, Elbers H, Bruckner T, Contin P, Reissfelder C, Schmidt T, Weigand MA, Martin E. Infrahepatic inferior vena cava clamping for reduction of central venous pressure and blood loss during hepatic resection: a randomized controlled trial. Ann Surg. 2011;253:1102-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 51. | Ryu HG, Nahm FS, Sohn HM, Jeong EJ, Jung CW. Low central venous pressure with milrinone during living donor hepatectomy. Am J Transplant. 2010;10:877-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 52. | Kato M, Kubota K, Kita J, Shimoda M, Rokkaku K, Sawada T. Effect of infra-hepatic inferior vena cava clamping on bleeding during hepatic dissection: a prospective, randomized, controlled study. World J Surg. 2008;32:1082-1087. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 53. | Takayama T, Makuuchi M, Kubota K, Harihara Y, Hui AM, Sano K, Ijichi M, Hasegawa K. Randomized comparison of ultrasonic vs clamp transection of the liver. Arch Surg. 2001;136:922-928. [PubMed] [Cited in This Article: ] |
| 54. | Hodgson WJ, Morgan J, Byrne D, DelGuercio LR. Hepatic resections for primary and metastatic tumors using the ultrasonic surgical dissector. Am J Surg. 1992;163:246-250. [PubMed] [Cited in This Article: ] |
| 55. | Nissen NN, Grewal N, Lee J, Nawabi A, Korman J. Completely laparoscopic nonanatomic hepatic resection using saline-cooled cautery and hydrodissection. Am Surg. 2007;73:987-990. [PubMed] [Cited in This Article: ] |
| 56. | Papachristou DN, Barters R. Resection of the liver with a water jet. Br J Surg. 1982;69:93-94. [PubMed] [Cited in This Article: ] |
| 57. | Cherqui D, Husson E, Hammoud R, Malassagne B, Stéphan F, Bensaid S, Rotman N, Fagniez PL. Laparoscopic liver resections: a feasibility study in 30 patients. Ann Surg. 2000;232:753-762. [PubMed] [Cited in This Article: ] |
| 58. | Sakamoto Y, Yamamoto J, Kokudo N, Seki M, Kosuge T, Yamaguchi T, Muto T, Makuuchi M. Bloodless liver resection using the monopolar floating ball plus ligasure diathermy: preliminary results of 16 liver resections. World J Surg. 2004;28:166-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 76] [Cited by in F6Publishing: 73] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 59. | Fan ST, Lai EC, Lo CM, Chu KM, Liu CL, Wong J. Hepatectomy with an ultrasonic dissector for hepatocellular carcinoma. Br J Surg. 1996;83:117-120. [PubMed] [Cited in This Article: ] |
| 60. | Arita J, Hasegawa K, Kokudo N, Sano K, Sugawara Y, Makuuchi M. Randomized clinical trial of the effect of a saline-linked radiofrequency coagulator on blood loss during hepatic resection. Br J Surg. 2005;92:954-959. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 61. | Saiura A, Yamamoto J, Koga R, Sakamoto Y, Kokudo N, Seki M, Yamaguchi T, Yamaguchi T, Muto T, Makuuchi M. Usefulness of LigaSure for liver resection: analysis by randomized clinical trial. Am J Surg. 2006;192:41-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 115] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 62. | Ikeda M, Hasegawa K, Sano K, Imamura H, Beck Y, Sugawara Y, Kokudo N, Makuuchi M. The vessel sealing system (LigaSure) in hepatic resection: a randomized controlled trial. Ann Surg. 2009;250:199-203. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 100] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 63. | Grazi GL, Ercolani G, Pierangeli F, Del Gaudio M, Cescon M, Cavallari A, Mazziotti A. Improved results of liver resection for hepatocellular carcinoma on cirrhosis give the procedure added value. Ann Surg. 2001;234:71-78. [PubMed] [Cited in This Article: ] |
| 64. | Jarnagin WR, Gonen M, Fong Y, DeMatteo RP, Ben-Porat L, Little S, Corvera C, Weber S, Blumgart LH. Improvement in perioperative outcome after hepatic resection: analysis of 1,803 consecutive cases over the past decade. Ann Surg. 2002;236:397-406; discussion 406-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 382] [Reference Citation Analysis (0)] |
| 65. | Poon RT, Fan ST, Lo CM, Liu CL, Lam CM, Yuen WK, Yeung C, Wong J. Improving perioperative outcome expands the role of hepatectomy in management of benign and malignant hepatobiliary diseases: analysis of 1222 consecutive patients from a prospective database. Ann Surg. 2004;240:698-708; discussion 708-710. [PubMed] [Cited in This Article: ] |
| 66. | Sima CS, Jarnagin WR, Fong Y, Elkin E, Fischer M, Wuest D, D’Angelica M, DeMatteo RP, Blumgart LH, Gönen M. Predicting the risk of perioperative transfusion for patients undergoing elective hepatectomy. Ann Surg. 2009;250:914-921. [PubMed] [Cited in This Article: ] |
| 67. | Aramaki O, Takayama T, Higaki T, Nakayama H, Ohkubo T, Midorikawa Y, Moriguchi M, Matsuyama Y. Decreased blood loss reduces postoperative complications in resection for hepatocellular carcinoma. J Hepatobiliary Pancreat Sci. 2014;21:585-591. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 68. | Katz SC, Shia J, Liau KH, Gonen M, Ruo L, Jarnagin WR, Fong Y, D’Angelica MI, Blumgart LH, Dematteo RP. Operative blood loss independently predicts recurrence and survival after resection of hepatocellular carcinoma. Ann Surg. 2009;249:617-623. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 307] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 69. | Ishizawa T, Hasegawa K, Tsuno NH, Tanaka M, Mise Y, Aoki T, Imamura H, Beck Y, Sugawara Y, Makuuchi M. Predeposit autologous plasma donation in liver resection for hepatocellular carcinoma: toward allogenic blood-free operations. J Am Coll Surg. 2009;209:206-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Makuuchi M, Takayama T, Gunvén P, Kosuge T, Yamazaki S, Hasegawa H. Restrictive versus liberal blood transfusion policy for hepatectomies in cirrhotic patients. World J Surg. 1989;13:644-648. [PubMed] [Cited in This Article: ] |
| 71. | Tomimaru Y, Wada H, Marubashi S, Kobayashi S, Eguchi H, Takeda Y, Tanemura M, Noda T, Umeshita K, Doki Y. Fresh frozen plasma transfusion does not affect outcomes following hepatic resection for hepatocellular carcinoma. World J Gastroenterol. 2010;16:5603-5610. [PubMed] [Cited in This Article: ] |
| 72. | Yamazaki S, Takayama T, Kimura Y, Moriguchi M, Higaki T, Nakayama H, Fujii M, Makuuchi M. Transfusion criteria for fresh frozen plasma in liver resection: a 3 + 3 cohort expansion study. Arch Surg. 2011;146:1293-1299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 73. | Ijichi M, Takayama T, Toyoda H, Sano K, Kubota K, Makuuchi M. Randomized trial of the usefulness of a bile leakage test during hepatic resection. Arch Surg. 2000;135:1395-1400. [PubMed] [Cited in This Article: ] |
| 74. | Liu Z, Jin H, Li Y, Gu Y, Zhai C. Randomized controlled trial of the intraoperative bile leakage test in preventing bile leakage after hepatic resection. Dig Surg. 2012;29:510-515. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 75. | Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250-278; quiz 279-280. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2944] [Cited by in F6Publishing: 2721] [Article Influence: 108.8] [Reference Citation Analysis (0)] |
| 76. | Liu CL, Fan ST, Lo CM, Wong Y, Ng IO, Lam CM, Poon RT, Wong J. Abdominal drainage after hepatic resection is contraindicated in patients with chronic liver diseases. Ann Surg. 2004;239:194-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 77. | Sun HC, Qin LX, Lu L, Wang L, Ye QH, Ren N, Fan J, Tang ZY. Randomized clinical trial of the effects of abdominal drainage after elective hepatectomy using the crushing clamp method. Br J Surg. 2006;93:422-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 78. | Belghiti J, Kabbej M, Sauvanet A, Vilgrain V, Panis Y, Fekete F. Drainage after elective hepatic resection. A randomized trial. Ann Surg. 1993;218:748-753. [PubMed] [Cited in This Article: ] |
| 79. | Franco D, Karaa A, Meakins JL, Borgonovo G, Smadja C, Grange D. Hepatectomy without abdominal drainage. Results of a prospective study in 61 patients. Ann Surg. 1989;210:748-750. [PubMed] [Cited in This Article: ] |
| 80. | Burt BM, Brown K, Jarnagin W, DeMatteo R, Blumgart LH, Fong Y. An audit of results of a no-drainage practice policy after hepatectomy. Am J Surg. 2002;184:441-445. [PubMed] [Cited in This Article: ] |
| 81. | Lu L, Sun HC, Qin LX, Wang L, Ye QH, Ren N, Fan J, Tang ZY. Abdominal drainage was unnecessary after hepatectomy using the conventional clamp crushing technique. J Gastrointest Surg. 2006;10:302-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 82. | Yamazaki S, Takayama T, Moriguchi M, Mitsuka Y, Okada S, Midorikawa Y, Nakayama H, Higaki T. Criteria for drain removal following liver resection. Br J Surg. 2012;99:1584-1590. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 83. | Nakayama H, Takayama T, Okubo T, Higaki T, Midorikawa Y, Moriguchi M, Aramaki O, Yamazaki S. Subcutaneous drainage to prevent wound infection in liver resection: a randomized controlled trial. J Hepatobiliary Pancreat Sci. 2014;21:509-517. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 84. | Hayashi Y, Takayama T, Yamazaki S, Moriguchi M, Ohkubo T, Nakayama H, Higaki T. Validation of perioperative steroids administration in liver resection: a randomized controlled trial. Ann Surg. 2011;253:50-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Rahbari NN, Garden OJ, Padbury R, Brooke-Smith M, Crawford M, Adam R, Koch M, Makuuchi M, Dematteo RP, Christophi C. Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149:713-724. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1224] [Cited by in F6Publishing: 1478] [Article Influence: 113.7] [Reference Citation Analysis (0)] |
| 86. | Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, Durand F. The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824-828, discussion 824-828. [PubMed] [Cited in This Article: ] |
| 87. | Rahbari NN, Reissfelder C, Koch M, Elbers H, Striebel F, Büchler MW, Weitz J. The predictive value of postoperative clinical risk scores for outcome after hepatic resection: a validation analysis in 807 patients. Ann Surg Oncol. 2011;18:3640-3649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |