Published online Mar 27, 2015. doi: 10.4254/wjh.v7.i3.498
Peer-review started: July 16, 2014
First decision: August 28, 2014
Revised: September 6, 2014
Accepted: December 16, 2014
Article in press: December 16, 2014
Published online: March 27, 2015
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infections are the most common causes of chronic liver diseases and hepatocelluar carcinomas. Over the past few years, the liver-enriched microRNA-122 (miR-122) has been shown to differentially regulate viral replication of HBV and HCV. It is notable that the level of miR-122 is positively and negatively regulated by HCV and HBV, respectively. Consistent with the well-documented phenomenon that miR-122 promotes HCV accumulation, inhibition of miR-122 has been shown as an effective therapy for the treatment of HCV infection in both chimpanzees and humans. On the other hand, miR-122 is also known to block HBV replication, and HBV has recently been shown to inhibit miR-122 expression; such a reciprocal inhibition between miR-122 and HBV suggests an intriguing possibility that miR-122 replacement may represent a potential therapy for treatment of HBV infection. As HBV and HCV have shared transmission routes, dual infection is not an uncommon scenario, which is associated with more advanced liver disease than either HBV or HCV mono-infection. Thus, there is a clear need to further understand the interaction between HBV and HCV and to delineate the role of miR-122 in HBV/HCV dual infection in order to devise effective therapy. This review summarizes the current understanding of HBV/HCV dual infection, focusing on the pathobiological role and therapeutic potential of miR-122.
Core tip: This paper summarizes direct and indirect interactions between hepatitis B virus (HBV) and hepatitis C virus (HCV), and the pathobiological role and therapeutic potential of liver specific miR-122 in HBV/HCV dual infection.
- Citation: Song K, Han C, Dash S, Balart LA, Wu T. MiR-122 in hepatitis B virus and hepatitis C virus dual infection. World J Hepatol 2015; 7(3): 498-506
- URL: https://www.wjgnet.com/1948-5182/full/v7/i3/498.htm
- DOI: https://dx.doi.org/10.4254/wjh.v7.i3.498
Hepatitis B virus (HBV) and hepatitis C virus (HCV) infection are a major health problem globally. Approximately 350 million and 170 million people are infected with HBV and HCV worldwide, respectively[1]. Although the exact number of patients with HBV/HCV dual infection is unknown, about 2%-10% of patients with chronic HCV are found to be hepatitis B surface antigen (HBsAg) positive and 5%-20% of patients with chronic HBV are anti-HCV positive[2]. Several studies have reported that HBV/HCV dual infection accelerates the progression of chronic liver disease including fibrosis, cirrhosis and hepatocelluar carcinoma (HCC)[3,4].
Although HBV/HCV dual infection is not uncommon, it is rare to have both HBV and HCV actively replicating in the same patient[5]. In general, HBV DNA and HCV RNA levels are lower in dual infected patients compared to their mono-infected patients[4,6]. This is in accordance with the described phenomenon of “viral interference”, i.e., infection by a first virus results in resistance of cells to infection by a second virus[7]. Indeed, there is growing evidence of reciprocal inhibitions between HBV and HCV. These reciprocal inhibitions may be mediated by several mechanisms including direct interference between two viruses, indirect inhibition through host gene regulation, and innate/adaptive host immune responses. Recently, the liver-enriched microRNA (miRNA)-122 (miR-122) has been implicated in the regulation of both HBV and HCV infections.
MiRNAs are small noncoding RNAs which are implicated in various biological processes through destabilization or translational inhibition of protein encoding mRNAs. Several miRNAs have been shown to regulate the replication and life cycle of HBV and HCV, respectively[8,9]. Among these, the liver-specific miRNA, miR-122, is perhaps the best known miRNA implicated in HCV or HBV infection. Notably, although miR-122 has been shown to enhance HCV replication, it is also known to inhibit HBV replication. However, to date, the role of miR-122 in HBV/HCV dual infection has not yet been defined. This review summarizes the current understanding of the direct and indirect reciprocal interactions in HBV/HCV dual infection, and discusses the emerging role of miR-122 in this intricate process.
HBV/HCV dual infection can be classified into four types based on clinical features[2,10]: acute dual infection, occult HBV infection, and super-infection by either virus in patients with preexisting chronic hepatitis. Given the different modalities of HBV/HCV dual infection, its actual prevalence may be underestimated on the basis of the available clinical data. For instance, the predicted prevalence may vary depending on the relative sensitivity of both HBsAg and HBV DNA assay in case of occult HBV infection. Presently, the exact global prevalence of HBV/HCV dual infection is largely unknown.
The viral dominance and prevalence of HBV/HCV dual infection vary depending on the ethnicity and geographic region. Nguyen et al[11] analyzed 115 patients with HBV/HCV dual infection in multiethnic study and found that HBV viral dominance pattern is significantly higher in Asian patients than non-Asian patients (38% vs 10%; P = 0.02). In contrast, HCV viral dominance pattern is more common in North American and European patients than in Asian patients. Simultaneous infection of HBV and HCV is rare, and it is mostly found in the situations such as accidental needle-stick injury, blood transfusion and injection drug users[12,13]. Mimms et al[14] showed that delayed appearance of HBsAg and decreased alanine aminotransferase (ALT) levels in simultaneous dual infected patients compared to mono-infected patients, suggesting that HCV inhibits HBV activity. Occult HBV infection, defined as the presence of HBV DNA in the liver and/or in the serum from patients with undetectable HBsAg[15], has been frequently identified in patients with chronic HCV infection[16]. Several lines of evidences suggest that occult HBV infection may accelerate the progression of liver disease including HCC in chronic HCV infected patients[17-20]. For example, the prevalence of occult HBV infection is significantly higher (61.6% vs 36.3%) in HCV positive patients with HCC than non-HCC chronic HCV infected patients[17]. The incidence of HCC is also significantly higher (14% vs 1.4%) in chronic HCV patients with occult HBV compared to HCV patients without occult HBV[19]. HCV superinfection is common in areas where HBV is prevalent such as Asian countries[21,22]. Several studies have shown that HCV superinfection results in suppression of HBV replication and elimination of HBsAg[23,24]. However, acute HCV superinfection in patients with chronic hepatitis B is associated with worse prognosis in terms of higher incidence of cirrhosis and HCC compared to chronic hepatitis B alone[21]. Although HBV superinfection in patients with chronic hepatitis C is less common, several studies have shown that acute HBV superinfection leads to suppression of HCV replication. Sagnelli et al[25] showed that the patients who had been HCV RNA positive for at least 1 year before HBV superinfection became HCV RNA negative during HBV infection, but more severe clinical presentation (development of portosystemic encephalopathy, ascites or prothrombin activity lower than 25%) were observed in HBV superinfected patients[25,26].
Therefore, the evidence is compelling that HBV/HCV dual infection exhibits reciprocal inhibition of viral replication, yet more aggressive clinical course of liver disease and higher risk of HCC compared to HBV or HCV mono-infection.
Clinical and in vivo animal studies have shown that the HBV and HCV affect their replication each other. Patients with HBV/HCV dual infection show significantly lower titer of HCV RNA compared to patients with HCV mono-infection[27]. Decreased HBsAg and HBV DNA levels are seen in chronic hepatitis B patients co-infected with HCV[28]. Likewise, HCV super-infection results in significant reduction in the titer of serum HBsAg in chronic HBV-infected chimpanzee[29]. Conversely, in chronic HCV-infected chimpanzee, HBV infection is delayed and attenuated compared to HCV-negative chimpanzee[30]. These reciprocal inhibitions between HBV and HCV may account for various viral profiles including occult infection and viral dominance in HBV/HCV dual infection.
Studying the direct interaction between HBV and HCV requires appropriate in vitro and in vivo model systems. At present, only few cell systems are susceptible to hepatotropic viral infections, even in the case of HBV or HCV mono-infection. While human primary hepatocytes are susceptible to both HBV and HCV infection, they are restricted to infection with acute phase, due to their short life span and the loss of hepatocyte features during cell culture process[31]. As an alternative, heterologous overexpression of viral proteins has been utilized to study HBV and HCV viral protein interactions. Ectopic expression of HCV core protein has been shown to interrupt HBV X-protein (HBx)-mediated trans-activation of HBV genome through direct binding to HBx[32]. HCV core protein has also been shown to form complex with HBV polymerase, thereby inhibiting HBV transcription and viral encapsidation[32]. Recently, Bellecave et al[33] established an inducible HBV replicating Huh7 cell system that has consistent replicating subgenomic HCV RNA. In that system, tetracycline-induced HBV replication did not alter the replication of HCV, nor the subcellular localization of HCV proteins. Similar results were also observed in a newly established the HCC cell line, HLCZ01 (derived from HCC tissue of HCV-infected male patient; the HLCZ01 cells are susceptible to the entire life cycle of both HBV and HCV including virus entry, replication and viral particle production)[34]. In that system, HLCZ01 cells were infected with HBV for 10 d and then infected with HCV for another 6 d; following HCV infection, the intracellular HCV RNA gradually increased while the HBV DNA copies were not changed by HCV infection. Additionally, simultaneous infection of HLCZ01 cells by HBV and HCV did not affect either HBV or HCV replication[34]. Thus, the data on the direct interaction between HBV and HCV are rather conflicting; such an inconsistency may relate to different experimental conditions (such as cell system, viral genotypes, or duration of infection, etc.), although it points toward the possibility that the documented “viral interference” during HBV/HCV dual infection may be largely mediated by other mechanisms (such as indirect molecular interaction).
Infection with HBV or HCV alters host gene expression and cellular phenotypes through diverse mechanism such as integration into host genomes and viral-host protein interaction[35]. Several studies have reported that the gene expression profiles in patients with chronic HBV and HCV are different[36,37]. In patients with chronic hepatitis B, the genes related to pro-apoptotic signaling and DNA repair response are up-regulated, whereas the genes related to immune reaction, lipid metabolism and epidermal growth factor receptor signaling are up-regulated in patients with chronic hepatitis C[37]. While these differentially regulated genes may reflect the difference in the pathogenesis of chronic hepatitis B and C, it is possible that these host genes may mediate indirect molecular interaction between HBV and HCV in the setting of HBV/HCV dual infection.
HCV NS5A has been shown to activate PI3K-Akt pathway through direct binding with p85 regulatory subunit of PI3K[38]. HCV E2 has also been reported to activate PI3K-Akt pathway via interaction with CD81 and claudin-1; the resulting PI3K-Akt activation further enhances HCV infectivity[39]. Constitutively active Akt1 results in decreased HBV replication, while inhibition of PI3K-Akt promotes HBV RNA transcription and DNA replication in HepG2.2.15 cells[40]. These findings suggest that HCV may inhibit HBV replication through activating PI3K-Akt pathway in patients with HBV/HCV dual infection. In addition, several studies suggest that HCV core and NS2 proteins inhibit the transcription of HBV through interacting with nuclear receptor family and affecting other cellular transcription factors[41,42].
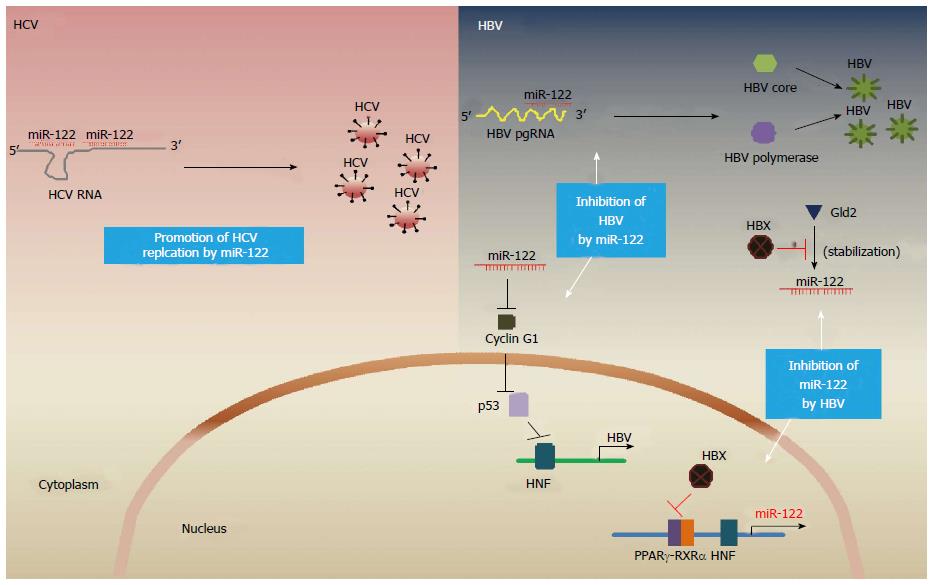
MiR-122 is implicated in diverse aspects of hepatic functions, including hepatic lipid and cholesterol metabolism and regulation of hepatitis C and B viruses[43]. On the subject of HCV regulation, miR-122 is known to positively regulate HCV replication through direct interaction with the 5’ UTR of the HCV genome. In contrast to the general phenomenon that miRNA-mRNA complex leads to either mRNA degradation or inhibition of translation, miR-122 is unique in that it stabilizes HCV viral RNA by protecting the 5’ terminus of the HCV genome from degradation by the host exonuclease, Xrn-1 and also stimulates HCV translation[44]. Given that the miR-122 promotes the accumulation of HCV, miR-122 represents an attractive therapeutic target for the treatment of HCV infection. Indeed, silencing of miR-122 has been shown to significantly inhibit HCV replication in cultured liver cells and chronically HCV-infected chimpanzee model[45,46]. A recent clinical study has shown that miravirsen, an antisense inhibitor of miR-122, significantly prolonged the reduction of HCV RNA in patients with chronic HCV genotype 1 infection[47].
On the subject of HBV regulation, miR-122 is known to inhibit the gene expression and replication of HBV. Chen et al[48] showed that miR-122 binds to highly conserved region of HBV pregenomic RNA, which is also a bicistronic mRNA encoding the HBV polymerase and core protein, thereby leading to inhibition of HBV gene expression and replication. MiR-122 also inhibits HBV replication by regulating the activity of p53 and its association with HBV enhancer via directly targeting of cyclin G1[49]. These findings suggest that liver-specific miR-122 is a critical regulator of viral replication in both HBV and HCV by either directly affecting viral RNA or modulating host gene expression.
The level of miRNAs is tightly controlled by transcriptional or post transcriptional regulation of biogenesis[50]. Although the expression of miR-122 is transcriptionally regulated by liver-enriched transcription factors including HNF4 and C/EBPα[51,52], it can be regulated by HBV and HCV infection. Recent studies have shown that the abundance of miR-122 is different in HCC patients with HBV versus HCV. The levels of miR-122 in patients from HBV-associated HCC is significantly lower compared to HCV-associated HCC[53]. Down-regulation of miR-122 is observed in a stable HBV-expressing cell line, HepG2.2.15, compared to its parental cell line, HepG2[54,55]. In addition, HBV infection also decreases the levels of miR-122 in human hepatocyte and HepaRG cells[56]. Recent evidence suggests that HBx is an important negative regulator of miR-122 expression, highlighted by the fact that HBx binds to peroxisome proliferator activated receptor gamma and inhibits the transcription of miR-122[56]. A separate study shows that HBx decreases miR-122 level post-transcriptionally through downregulation of Germline development 2[55].
Acute HCV infection has been reported to increase the level of miR-122 in cultured cells and chimpanzee models. In HCV infected Huh7.5.1 cells, the level of miR-122 increased at early time points (at day 19 post-infection), then decreased quickly and reached minimum levels at late time points (at day 32 post-infection)[57]. In a chimpanzee model, inoculation of HCV genotype 1 resulted in increased expression of miR-122 at the first 4 wk (rapidly increasing HCV viral titer), followed by declined levels of miR-122 at 10-14 wk (with elevation of serum ALT)[58].
The above findings provide evidence for reciprocal interactions between miR-122 and HBV/HCV (summarized in Figure 1). It is possible that the dominant virus in HBV/HCV dual infection may affect the level of miR-122 and thereby influence the replication of the other virus, although details of miR-122 and HBV/HCV interactions remain to be further defined.
Since HBV/HCV dual infection is heterogeneous, currently there is no standard therapy for the treatment of HBV-HCV dual infected patients. Although interferon-α (IFN-α) is the only treatment option effective for both viruses, early treatment trials showed that HBV/HCV dual infected patients were less responsive to IFN than HCV mono-infective patients[59]. Therefore, it is very important to identify virological profiles including viral dominances in HBV/HCV dual infection to guide treatment. A previous study report that approximately 70% of dual infected patients have active HCV, whereas 38% of patients have active HBV[60]. Multiple studies have evaluated the efficacy of pegylated interferon-alpha (Peg-IFN-α) and ribavirin on HBV/HCV dual infection with active HCV infection. Potthoff et al[61] administered Peg-IFN-α/ribavirin to 19 patients with chronic hepatitis C and positive HBsAg for 24 wk; sustained virologic response (SVR) was achieved in 74% patients (14/19); although HBsAg and HBeAg status remained unchanged, HBV-DNA became negative in two of six HBV-DNA positive patients; however, four of the 13 patients (31%) with HBV-DNA negative patients became positive after clearance of HCV. Liu et al[62] conducted a larger multicenter clinical trial and found that HCV SVR was 72.2% in dually infected patients vs 77.3% in mono-infected patients with genotype 1 infection. For patients with genotype 2/3, SVR were 82.8% and 84%, respectively. Notably, 11.2% (18/161) of the dually infected patients showed HBsAg clearance and serum HBV-DNA became undetectable in 55.9% (38/68) of patients at the end of the follow-up period. In contrast, 36.3% (28/77) dually infected patients whose pretreatment serum HBV DNA was undetectable showed reappearance of HBV DNA. A separate study has demonstrated the effectiveness of IFN and lamivudin in dually infected patients with active HBV[63]. In that study, eight patients received IFN plus lamivudin for 12 mo and followed by 6 mo of lamivudin alone; HBV DNA and HBeAg clearance were observed in 3/8 patients and serum HCV RNA became negative in 4/8 patients. These results suggest that targeting dominant virus through combined IFN plus ribavirin or IFN plus nucleoside/nucleotide analog might be effective in HBV/HCV dual infection.
Studies have shown that SVR to IFN-based therapy is associated with single-nucleotide polymorphisms near the IL28B gene such as rs12979860, rs12980275 and rs8099917. The IL-28B rs12979860 CC, rs12980275 AA and rs8099917 TT genotypes are more frequently found in chronic hepatitis C patients (genotype 1) in SVR group than in null virological response (NVR)[64-66]. In case of genotype 2/3, only IL-28B rs12979860 CC genotype is associated with SVR to Peg-INF-α/ribavirin therapy, but not rs12980275 and rs8099917 genotypes[67]. These genotypes are also closely associated with SVR to IFN therapy in patients with HBV infection[68,69]. Consistent with their role in mono-infection, IL-28B genotypes are also associated with SVR to Peg-INF-α/ribavirin therapy in HBV/HCV dual infection. Guo et al[70] showed that IL-28B rs12979860 CC and rs8099917 TT genotypes are frequently found in SVR group than NVR in dual infected patients treated with Peg-INF-α/ribavirin. Interestingly, the reactivation rate of HBV DNA was significantly lower in IL28B rs8099917 TT genotypes than their TG + GG genotypes (TT vs TG + GG; 13.9% vs 41.7%, P = 0.005)[70]. Therefore, these results suggest that IL28B gene polymorphism can be used to predict HBV reactivation as well as IFN response in HBV/HCV dual infection.
Several recent studies suggest that miR-122 levels might be related to the IL28B genotype. Su et al[71] found that IL28B rs8099917 TT genotypes had significantly high pre-treatment miR-122 levels in serum with strong response to treatment than the IL28B GT or GG genotypes. Conversely, low levels of pre-treatment miR-122 have been observed in chronic HCV patients who had no virological response during Peg-IFN-α/ribavirin therapy[71,72]. Hao et al[73] showed that IFN-α treatment induced a marked decrease of miR-122 in hepatocyte through sequestration by the IFN-stimulated gene, NT5C3, and thereby negatively affects the anti-HBV efficiency of IFN-α. These findings suggest that the level of miR-122 is regulated by IFN-stimulated genes and is closely related to interferon response in patients with HBV or HCV. Given that Peg-IFN-α/ribavirin is also used as therapeutic drugs for dual infected patients with active HCV, pre- and post-treatment miR-122 levels should be measured in various types of dual infected patients; the data may not only help predict therapeutic response, but also provide insights into viral interaction and reactivation.
As stated in the preceding section, targeting miR-122 using anti-sense nucleic acid is highly effective for the treatment of chronic HCV infection[45-47]. This approach has several advantages, including effectiveness on all HCV genotypes (because of highly conserved miR-122 binding sites across HCV genotypes[74]), no evidence of viral resistance, and lack of significant side effect[46]. However, as mentioned above, miR-122 has dual function on HBV and HCV replication and it may play a role in reciprocal viral inhibition between these two viruses. Thus, silencing of miR-122 might not be suitable for the treatment of HBV/HCV dual infected patients, even in dual infected patients with dominant HCV, as inhibition of miR-122 may cause reactivation of HBV. Careful testing and screening for HBV is required before initiation of miR-122 inhibitor in patients with positive HCV serology.
On the other hand, evidence also suggests the possibility of miR-122 replacement therapy for the treatment of HBV or even late stage of HCV-associated diseases. Several studies have shown that hepatic level of miR-122 was significantly decreased in the late stage of fibrosis and HCC[75,76], suggesting that miR-122 may inhibit hepatic fibrogenesis and carcinogenesis; these observations argue for miR-122 replacement therapy in late stage of HCV-associated liver disease. Nguyen et al[11] found that 83% of HBV-dominant dual infected patients had complete dominance (i.e., with negative HCV RNA). For these cases, it remains to be determined whether miR-122 replacement therapy can be strategized to curtail HBV and improve HBV-associated pathology; should this be attempted, it would be critical to continually monitor HCV replication given the possibility of HCV reactivation. Thus, whether or when to inhibit or enhance miR-122 for therapy requires careful consideration of the context of the viral infections and the stage of the liver diseases.
Given the enormous promise of direct-acting antiviral (DAA) drugs (including NS3/4A protease inhibitors, NS5A and NS5B polymerase inhibitors) for successful interferon-free treatment of HCV across multiple genotypes[77-79], further investigation is warranted to determine whether DAA drugs could be effectively utilized for optimal antiviral therapy in HCV/HBV dual infected patients and whether their antiviral efficacy is dependent upon miR-122.
HBV and HCV dual infection is associated with more advanced liver disease than either mono-infection. The modalities and viral dominance are highly variable in patient with HBV-HCV dual infection. Recent clinical and experimental studies have shown reciprocal inhibition between HBV and HCV. However, until now the underlying mechanisms of these inhibitions are poorly understood because of the complicated viral profiles of HBV/HCV dual infection and the lack of optimal experimental models. A better understanding of the dynamic and intricate interactions between HBV and HCV is needed prior to consideration of treatment of HBV/HCV dual infections. The host genes regulated by HBV and HCV viruses have been recognized as important players that mediate the reciprocal inhibition between HBV and HCV and regulate the pathogenesis of viral hepatitis. Recent studies have shown that miR-122 is a crucial host gene that differentially regulates the replication of both HBV and HCV. Conversely, the expression of miR-122 is regulated differently by these two viruses. Although silencing of miR-122 is an attractive therapy against HCV infection, this approach might not be suitable for the treatment of HBV/HCV dual infection, given the potential concern for HBV reactivation. Further studies are needed to better understand the mechanisms for miR-122-mediated hepatotropic viral replication and to devise the optimal regimen for treatment of HBV/HCV dual infection.
P- Reviewer: Bare P, Datta S, Yao SK S- Editor: Gong XM L- Editor: A E- Editor: Wu HL
| 1. | Liu Z, Hou J. Hepatitis B virus (HBV) and hepatitis C virus (HCV) dual infection. Int J Med Sci. 2006;3:57-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 55] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Chu CJ, Lee SD. Hepatitis B virus/hepatitis C virus coinfection: epidemiology, clinical features, viral interactions and treatment. J Gastroenterol Hepatol. 2008;23:512-520. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 3. | Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, Seigneurin JM, Buffet C, Dhumeaux D. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 260] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 4. | Sagnelli E, Coppola N, Scolastico C, Filippini P, Santantonio T, Stroffolini T, Piccinino F. Virologic and clinical expressions of reciprocal inhibitory effect of hepatitis B, C, and delta viruses in patients with chronic hepatitis. Hepatology. 2000;32:1106-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 165] [Cited by in F6Publishing: 174] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Aghemo A, Colombo M. Treatment of patients with dual hepatitis B and C: a step in the right direction. Gut. 2014;63:380-381. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Jardi R, Rodriguez F, Buti M, Costa X, Cotrina M, Galimany R, Esteban R, Guardia J. Role of hepatitis B, C, and D viruses in dual and triple infection: influence of viral genotypes and hepatitis B precore and basal core promoter mutations on viral replicative interference. Hepatology. 2001;34:404-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 177] [Cited by in F6Publishing: 184] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 7. | Dianzani F. Viral interference and interferon. Ric Clin Lab. 1975;5:196-213. [PubMed] [Cited in This Article: ] |
| 8. | Zhang X, Hou J, Lu M. Regulation of hepatitis B virus replication by epigenetic mechanisms and microRNAs. Front Genet. 2013;4:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Singaravelu R, Russell RS, Tyrrell DL, Pezacki JP. Hepatitis C virus and microRNAs: miRed in a host of possibilities. Curr Opin Virol. 2014;7:1-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Crockett SD, Keeffe EB. Natural history and treatment of hepatitis B virus and hepatitis C virus coinfection. Ann Clin Microbiol Antimicrob. 2005;4:13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Nguyen LH, Ko S, Wong SS, Tran PS, Trinh HN, Garcia RT, Ahmed A, Lutchman GA, Keeffe EB, Nguyen MH. Ethnic differences in viral dominance patterns in patients with hepatitis B virus and hepatitis C virus dual infection. Hepatology. 2011;53:1839-1845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Rodríguez M, Navascués CA, Martínez A, Suárez A, Sotorrío NG, Cimadevilla R, Linares A, Pérez R, Rodrigo L. Hepatitis C virus infection in patients with acute hepatitis B. Infection. 1992;20:316-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 13. | Liaw YF, Chu CM, Chang-Chien CS, Wui CS. Simultaneous acute infections with hepatitis non-A, non-B, and B viruses. Dig Dis Sci. 1982;27:762-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Mimms LT, Mosley JW, Hollinger FB, Aach RD, Stevens CE, Cunningham M, Vallari DV, Barbosa LH, Nemo GJ. Effect of concurrent acute infection with hepatitis C virus on acute hepatitis B virus infection. BMJ. 1993;307:1095-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Huang X, Hollinger FB. Occult hepatitis B virus infection and hepatocellular carcinoma: a systematic review. J Viral Hepat. 2014;21:153-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 16. | Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 474] [Cited by in F6Publishing: 491] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 17. | Pollicino T, Squadrito G, Cerenzia G, Cacciola I, Raffa G, Craxi A, Farinati F, Missale G, Smedile A, Tiribelli C. Hepatitis B virus maintains its pro-oncogenic properties in the case of occult HBV infection. Gastroenterology. 2004;126:102-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 314] [Cited by in F6Publishing: 308] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Tanaka T, Inoue K, Hayashi Y, Abe A, Tsukiyama-Kohara K, Nuriya H, Aoki Y, Kawaguchi R, Kubota K, Yoshiba M. Virological significance of low-level hepatitis B virus infection in patients with hepatitis C virus associated liver disease. J Med Virol. 2004;72:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Squadrito G, Pollicino T, Cacciola I, Caccamo G, Villari D, La Masa T, Restuccia T, Cucinotta E, Scisca C, Magazzu D. Occult hepatitis B virus infection is associated with the development of hepatocellular carcinoma in chronic hepatitis C patients. Cancer. 2006;106:1326-1330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 112] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 20. | Matsuoka S, Nirei K, Tamura A, Nakamura H, Matsumura H, Oshiro S, Arakawa Y, Yamagami H, Tanaka N, Moriyama M. Influence of occult hepatitis B virus coinfection on the incidence of fibrosis and hepatocellular carcinoma in chronic hepatitis C. Intervirology. 2008;51:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Liaw YF, Chen YC, Sheen IS, Chien RN, Yeh CT, Chu CM. Impact of acute hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. Gastroenterology. 2004;126:1024-1029. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 151] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Liaw YF. Hepatitis C virus superinfection in patients with chronic hepatitis B virus infection. J Gastroenterol. 2002;37 Suppl 13:65-68. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Liaw YF, Lin SM, Sheen IS, Chu CM. Acute hepatitis C virus superinfection followed by spontaneous HBeAg seroconversion and HBsAg elimination. Infection. 1991;19:250-251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Sagnelli E, Coppola N, Marrocco C, Onofrio M, Sagnelli C, Coviello G, Scolastico C, Filippini P. Hepatitis C virus superinfection in hepatitis B virus chronic carriers: a reciprocal viral interaction and a variable clinical course. J Clin Virol. 2006;35:317-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Sagnelli E, Coppola N, Pisaturo M, Masiello A, Tonziello G, Sagnelli C, Messina V, Filippini P. HBV superinfection in HCV chronic carriers: a disease that is frequently severe but associated with the eradication of HCV. Hepatology. 2009;49:1090-1097. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 76] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Sagnelli E, Coppola N, Messina V, Di Caprio D, Marrocco C, Marotta A, Onofrio M, Scolastico C, Filippini P. HBV superinfection in hepatitis C virus chronic carriers, viral interaction, and clinical course. Hepatology. 2002;36:1285-1291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 106] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 27. | Bini EJ, Perumalswami PV. Hepatitis B virus infection among American patients with chronic hepatitis C virus infection: prevalence, racial/ethnic differences, and viral interactions. Hepatology. 2010;51:759-766. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Chu CM, Yeh CT, Liaw YF. Low-level viremia and intracellular expression of hepatitis B surface antigen (HBsAg) in HBsAg carriers with concurrent hepatitis C virus infection. J Clin Microbiol. 1998;36:2084-2086. [PubMed] [Cited in This Article: ] |
| 29. | Bradley DW, Maynard JE, McCaustland KA, Murphy BL, Cook EH, Ebert JW. Non-A, non-B hepatitis in chimpanzees: interference with acute hepatitis A virus and chronic hepatitis B virus infections. J Med Virol. 1983;11:207-213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wieland SF, Asabe S, Engle RE, Purcell RH, Chisari FV. Limited hepatitis B virus replication space in the chronically hepatitis C virus-infected liver. J Virol. 2014;88:5184-5188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Zhou M, Zhao F, Li J, Cheng Z, Tian X, Zhi X, Huang Y, Hu K. Long-term maintenance of human fetal hepatocytes and prolonged susceptibility to HBV infection by co-culture with non-parenchymal cells. J Virol Methods. 2014;195:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Chen SY, Kao CF, Chen CM, Shih CM, Hsu MJ, Chao CH, Wang SH, You LR, Lee YH. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591-607. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 128] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 33. | Bellecave P, Gouttenoire J, Gajer M, Brass V, Koutsoudakis G, Blum HE, Bartenschlager R, Nassal M, Moradpour D. Hepatitis B and C virus coinfection: a novel model system reveals the absence of direct viral interference. Hepatology. 2009;50:46-55. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 123] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 34. | Yang D, Zuo C, Wang X, Meng X, Xue B, Liu N, Yu R, Qin Y, Gao Y, Wang Q. Complete replication of hepatitis B virus and hepatitis C virus in a newly developed hepatoma cell line. Proc Natl Acad Sci USA. 2014;111:E1264-E1273. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 35. | Anzola M. Hepatocellular carcinoma: role of hepatitis B and hepatitis C viruses proteins in hepatocarcinogenesis. J Viral Hepat. 2004;11:383-393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 36. | Ueda T, Honda M, Horimoto K, Aburatani S, Saito S, Yamashita T, Sakai Y, Nakamura M, Takatori H, Sunagozaka H. Gene expression profiling of hepatitis B- and hepatitis C-related hepatocellular carcinoma using graphical Gaussian modeling. Genomics. 2013;101:238-248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 37. | Honda M, Yamashita T, Ueda T, Takatori H, Nishino R, Kaneko S. Different signaling pathways in the livers of patients with chronic hepatitis B or chronic hepatitis C. Hepatology. 2006;44:1122-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 38. | Street A, Macdonald A, Crowder K, Harris M. The Hepatitis C virus NS5A protein activates a phosphoinositide 3-kinase-dependent survival signaling cascade. J Biol Chem. 2004;279:12232-12241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 181] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 39. | Liu Z, Tian Y, Machida K, Lai MM, Luo G, Foung SK, Ou JH. Transient activation of the PI3K-AKT pathway by hepatitis C virus to enhance viral entry. J Biol Chem. 2012;287:41922-41930. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 40. | Guo H, Zhou T, Jiang D, Cuconati A, Xiao GH, Block TM, Guo JT. Regulation of hepatitis B virus replication by the phosphatidylinositol 3-kinase-akt signal transduction pathway. J Virol. 2007;81:10072-10080. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Schüttler CG, Fiedler N, Schmidt K, Repp R, Gerlich WH, Schaefer S. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J Hepatol. 2002;37:855-862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 42. | Dumoulin FL, von dem Bussche A, Li J, Khamzina L, Wands JR, Sauerbruch T, Spengler U. Hepatitis C virus NS2 protein inhibits gene expression from different cellular and viral promoters in hepatic and nonhepatic cell lines. Virology. 2003;305:260-266. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 43. | Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1612] [Cited by in F6Publishing: 1588] [Article Influence: 88.2] [Reference Citation Analysis (0)] |
| 44. | Li Y, Masaki T, Yamane D, McGivern DR, Lemon SM. Competing and noncompeting activities of miR-122 and the 5’ exonuclease Xrn1 in regulation of hepatitis C virus replication. Proc Natl Acad Sci USA. 2013;110:1881-1886. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 45. | Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1993] [Cited by in F6Publishing: 1929] [Article Influence: 101.5] [Reference Citation Analysis (0)] |
| 46. | Lanford RE, Hildebrandt-Eriksen ES, Petri A, Persson R, Lindow M, Munk ME, Kauppinen S, Ørum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198-201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1284] [Cited by in F6Publishing: 1262] [Article Influence: 84.1] [Reference Citation Analysis (0)] |
| 47. | Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685-1694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1665] [Cited by in F6Publishing: 1627] [Article Influence: 147.9] [Reference Citation Analysis (0)] |
| 48. | Chen Y, Shen A, Rider PJ, Yu Y, Wu K, Mu Y, Hao Q, Liu Y, Gong H, Zhu Y. A liver-specific microRNA binds to a highly conserved RNA sequence of hepatitis B virus and negatively regulates viral gene expression and replication. FASEB J. 2011;25:4511-4521. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 141] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 49. | Wang S, Qiu L, Yan X, Jin W, Wang Y, Chen L, Wu E, Ye X, Gao GF, Wang F. Loss of microRNA 122 expression in patients with hepatitis B enhances hepatitis B virus replication through cyclin G(1) -modulated P53 activity. Hepatology. 2012;55:730-741. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 196] [Cited by in F6Publishing: 201] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 50. | Siomi H, Siomi MC. Posttranscriptional regulation of microRNA biogenesis in animals. Mol Cell. 2010;38:323-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 442] [Cited by in F6Publishing: 434] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 51. | Li ZY, Xi Y, Zhu WN, Zeng C, Zhang ZQ, Guo ZC, Hao DL, Liu G, Feng L, Chen HZ. Positive regulation of hepatic miR-122 expression by HNF4α. J Hepatol. 2011;55:602-611. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 52. | Xu H, He JH, Xiao ZD, Zhang QQ, Chen YQ, Zhou H, Qu LH. Liver-enriched transcription factors regulate microRNA-122 that targets CUTL1 during liver development. Hepatology. 2010;52:1431-1442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 204] [Cited by in F6Publishing: 217] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 53. | Spaniel C, Honda M, Selitsky SR, Yamane D, Shimakami T, Kaneko S, Lanford RE, Lemon SM. microRNA-122 abundance in hepatocellular carcinoma and non-tumor liver tissue from Japanese patients with persistent HCV versus HBV infection. PLoS One. 2013;8:e76867. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 54. | Jung CJ, Iyengar S, Blahnik KR, Ajuha TP, Jiang JX, Farnham PJ, Zern M. Epigenetic modulation of miR-122 facilitates human embryonic stem cell self-renewal and hepatocellular carcinoma proliferation. PLoS One. 2011;6:e27740. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 55. | Peng F, Xiao X, Jiang Y, Luo K, Tian Y, Peng M, Zhang M, Xu Y, Gong G. HBx down-regulated Gld2 plays a critical role in HBV-related dysregulation of miR-122. PLoS One. 2014;9:e92998. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 56. | Song K, Han C, Zhang J, Lu D, Dash S, Feitelson M, Lim K, Wu T. Epigenetic regulation of MicroRNA-122 by peroxisome proliferator activated receptor-gamma and hepatitis b virus X protein in hepatocellular carcinoma cells. Hepatology. 2013;58:1681-1692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 57. | Li S, Xing X, Yang Q, Xu H, He J, Chen Z, Zhu H. The effects of hepatitis C virus core protein on the expression of miR-122 in vitro. Virol J. 2013;10:98. [PubMed] [Cited in This Article: ] |
| 58. | Choi Y, Dienes HP, Krawczynski K. Kinetics of miR-122 expression in the liver during acute HCV infection. PLoS One. 2013;8:e76501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 59. | Villa E, Grottola A, Buttafoco P, Colantoni A, Bagni A, Ferretti I, Cremonini C, Bertani H, Manenti F. High doses of alpha-interferon are required in chronic hepatitis due to coinfection with hepatitis B virus and hepatitis C virus: long term results of a prospective randomized trial. Am J Gastroenterol. 2001;96:2973-2977. [PubMed] [Cited in This Article: ] |
| 60. | Raimondo G, Brunetto MR, Pontisso P, Smedile A, Maina AM, Saitta C, Squadrito G, Tono N. Longitudinal evaluation reveals a complex spectrum of virological profiles in hepatitis B virus/hepatitis C virus-coinfected patients. Hepatology. 2006;43:100-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 61. | Potthoff A, Wedemeyer H, Boecher WO, Berg T, Zeuzem S, Arnold J, Spengler U, Gruengreiff K, Kaeser T, Schuchmann M. The HEP-NET B/C co-infection trial: A prospective multicenter study to investigate the efficacy of pegylated interferon-alpha2b and ribavirin in patients with HBV/HCV co-infection. J Hepatol. 2008;49:688-694. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 62. | Liu CJ, Chuang WL, Lee CM, Yu ML, Lu SN, Wu SS, Liao LY, Chen CL, Kuo HT, Chao YC. Peginterferon alfa-2a plus ribavirin for the treatment of dual chronic infection with hepatitis B and C viruses. Gastroenterology. 2009;136:496-504.e3. [PubMed] [Cited in This Article: ] |
| 63. | Marrone A, Zampino R, D’Onofrio M, Ricciotti R, Ruggiero G, Utili R. Combined interferon plus lamivudine treatment in young patients with dual HBV (HBeAg positive) and HCV chronic infection. J Hepatol. 2004;41:1064-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 64. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [PubMed] [Cited in This Article: ] |
| 65. | Venegas M, Villanueva RA, González K, Brahm J. IL28B polymorphisms associated with therapy response in Chilean chronic hepatitis C patients. World J Gastroenterol. 2011;17:3636-3639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Rauch A, Kutalik Z, Descombes P, Cai T, Di Iulio J, Mueller T, Bochud M, Battegay M, Bernasconi E, Borovicka J. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology. 2010;138:1338-1345, 1345-1347. [PubMed] [Cited in This Article: ] |
| 67. | Sarrazin C, Susser S, Doehring A, Lange CM, Müller T, Schlecker C, Herrmann E, Lötsch J, Berg T. Importance of IL28B gene polymorphisms in hepatitis C virus genotype 2 and 3 infected patients. J Hepatol. 2011;54:415-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 173] [Cited by in F6Publishing: 186] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 68. | Sonneveld MJ, Wong VW, Woltman AM, Wong GL, Cakaloglu Y, Zeuzem S, Buster EH, Uitterlinden AG, Hansen BE, Chan HL. Polymorphisms near IL28B and serologic response to peginterferon in HBeAg-positive patients with chronic hepatitis B. Gastroenterology. 2012;142:513-520.e1. [PubMed] [Cited in This Article: ] |
| 69. | Lampertico P, Viganò M, Cheroni C, Facchetti F, Invernizzi F, Valveri V, Soffredini R, Abrignani S, De Francesco R, Colombo M. IL28B polymorphisms predict interferon-related hepatitis B surface antigen seroclearance in genotype D hepatitis B e antigen-negative patients with chronic hepatitis B. Hepatology. 2013;57:890-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 121] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 70. | Guo X, Yang G, Yuan J, Ruan P, Zhang M, Chen X, Zhou B. Genetic variation in interleukin 28B and response to antiviral therapy in patients with dual chronic infection with hepatitis B and C viruses. PLoS One. 2013;8:e77911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 71. | Su TH, Liu CH, Liu CJ, Chen CL, Ting TT, Tseng TC, Chen PJ, Kao JH, Chen DS. Serum microRNA-122 level correlates with virologic responses to pegylated interferon therapy in chronic hepatitis C. Proc Natl Acad Sci USA. 2013;110:7844-7849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 72. | Sarasin-Filipowicz M, Krol J, Markiewicz I, Heim MH, Filipowicz W. Decreased levels of microRNA miR-122 in individuals with hepatitis C responding poorly to interferon therapy. Nat Med. 2009;15:31-33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 254] [Cited by in F6Publishing: 251] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 73. | Hao J, Jin W, Li X, Wang S, Zhang X, Fan H, Li C, Chen L, Gao B, Liu G. Inhibition of alpha interferon (IFN-α)-induced microRNA-122 negatively affects the anti-hepatitis B virus efficiency of IFN-α. J Virol. 2013;87:137-147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 74. | Li YP, Gottwein JM, Scheel TK, Jensen TB, Bukh J. MicroRNA-122 antagonism against hepatitis C virus genotypes 1-6 and reduced efficacy by host RNA insertion or mutations in the HCV 5’ UTR. Proc Natl Acad Sci USA. 2011;108:4991-4996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 146] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 75. | Trebicka J, Anadol E, Elfimova N, Strack I, Roggendorf M, Viazov S, Wedemeyer I, Drebber U, Rockstroh J, Sauerbruch T. Hepatic and serum levels of miR-122 after chronic HCV-induced fibrosis. J Hepatol. 2013;58:234-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 139] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 76. | Tsai WC, Hsu PW, Lai TC, Chau GY, Lin CW, Chen CM, Lin CD, Liao YL, Wang JL, Chau YP. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology. 2009;49:1571-1582. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 450] [Cited by in F6Publishing: 465] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 77. | Stedman C. Sofosbuvir, a NS5B polymerase inhibitor in the treatment of hepatitis C: a review of its clinical potential. Therap Adv Gastroenterol. 2014;7:131-140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 78. | Lok AS, Gardiner DF, Lawitz E, Martorell C, Everson GT, Ghalib R, Reindollar R, Rustgi V, McPhee F, Wind-Rotolo M. Preliminary study of two antiviral agents for hepatitis C genotype 1. N Engl J Med. 2012;366:216-224. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 478] [Cited by in F6Publishing: 471] [Article Influence: 39.3] [Reference Citation Analysis (0)] |
| 79. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 284] [Cited by in F6Publishing: 303] [Article Influence: 25.3] [Reference Citation Analysis (0)] |