Published online Mar 18, 2017. doi: 10.4254/wjh.v9.i8.409
Peer-review started: October 10, 2016
First decision: November 11, 2016
Revised: December 27, 2016
Accepted: January 11, 2017
Article in press: January 14, 2017
Published online: March 18, 2017
Changes in liver structure are an important issue in chronic hepatopathies. Until the end of the 20th century, these changes could only be determined by histological analyses of a liver specimen obtained via biopsy. The well-known limitations of this technique (i.e., pain, bleeding and the need for sedation) have precluded its routine use in follow-up of patients with liver diseases. However, the introduction of non-invasive technologies, such as ultrasound and magnetic resonance imaging, for measurement of liver stiffness as an indirect marker of fibroses has changed this situation. Today, several non-invasive tools are available to physicians to estimate the degree of liver fibrosis by analysing liver stiffness. This review describes the currently available tools for liver stiffness determination that are applicable to follow-up of liver fibrosis/cirrhosis with established clinical use in children, and discusses their features in comparison to the “historical” tools.
Core tip: Non-invasive liver stiffness measurement is a new and helpful tool for assessing liver fibroses in children, but it cannot yet replace liver biopsy.
- Citation: Engelmann G, Quader J, Teufel U, Schenk JP. Limitations and opportunities of non-invasive liver stiffness measurement in children. World J Hepatol 2017; 9(8): 409-417
- URL: https://www.wjgnet.com/1948-5182/full/v9/i8/409.htm
- DOI: https://dx.doi.org/10.4254/wjh.v9.i8.409
Until the end of the 20th century structural changes of the liver could only be determined by histological analyses of a liver specimen obtained by percutaneous liver biopsy. The well-known limitations of this technique (i.e., pain, bleeding and the need for sedation), however, precluded its routine use in follow-up of patients with liver diseases, and it has only been used routinely in studies[1]. The introduction of non-invasive imaging technologies, such as ultrasound and magnetic resonance imaging, has changed this situation, allowing for measurement of liver stiffness as an indirect marker of fibroses. Today, several non-invasive tools are available to physicians to estimate the degree of liver fibrosis by analysing liver stiffness.
This review will describe the currently available tools for liver stiffness determination that are applicable to follow-up of liver fibrosis/cirrhosis with established clinical use in paediatric patients (children between 0 and 18-year-old), and discusses their features in comparison to the “historical” tools.
Liver fibrosis is a dynamic reaction of the healthy liver towards chronic cell injury[2]. It is frequently observed in patients with chronic liver disease, regardless of aetiology[3] and patient age. Structural changes of liver architecture usually appear slowly, within years or decades, and accompanied by a continual development from low-grade fibrosis to liver cirrhosis. Liver cirrhosis, itself, represents the end-stage of fibrotic liver diseases.
Development of fibrosis leads to an increase in liver stiffness, detectable by non-invasive methods. Progression from liver fibrosis to cirrhosis may be preventable, if the fibrosis is detected early in the course. Examples of preventable fibrosing liver diseases are hepatitis B or hepatitis C infections[4,5], liver transplantation[6] or Wilson’s disease. For other fibrosis aetiologies, a close follow-up is recommended to detect changes in liver structure in a timely manner and to determine the disease course. This holds true for post-liver transplant patients and patients with autoimmune liver diseases. Today, histology is the gold standard for the diagnosis of liver fibrosis.
Liver biopsy remains the method of choice for clarification of the aetiology of hepatopathies. It has the advantage of obtaining direct information, not only on the degree of fibrosis but also on the presence of inflammation, necrosis, steatosis, and iron or copper deposits. However, the histopathologic examination of a liver specimen also has limitations. Studies have clearly indicated that liver biopsy is prone to sampling errors and may underestimate the amount of liver fibrosis. As such, cirrhosis could be missed on a percutaneous liver biopsy, reportedly affecting an estimated 30% of cases[7,8]. Liver biopsy has further technical limitations. There is a small risk of clinically relevant bleeding (0.3%) and mortality due to the intervention, shown to affect 0.04%-0.07% in a large case series[9]. In a paediatric series, major complications occurred in 1.5% and minor complications in 25% of 275 liver biopsies[10]. Another drawback of this method is the size of the specimen obtained[8]. A single liver biopsy reportedly has a 20%-30% chance of missing the relevant area of interest, thereby underestimating liver diseases[11]. Paediatric patients have an additional risk due to the need of sedation for the biopsy procedure. Therefore, in clinical practice liver histology is almost exclusively used for diagnoses and only in certain settings, such as liver transplantation, and for therapy control[1,12].
On the other hand, liver biopsy has some clear advantages. A recent study of a cohort of patients with either histologically-proven non-alcoholic steatohepatitis (NASH) or non-alcoholic fatty liver disease (NAFLD) showed that outcome (i.e., death, liver transplantation or severe liver disease) was directly dependent upon the degree of fibroses[13]. Another recent study by Mann et al[14] demonstrated an association of portal inflammation, metabolic syndrome and fibrosis in 430 obese children. These findings support the current tenet that portal inflammation and exact degree of fibrosis are best determined by liver biopsy.
The liver biopsy specimen is recommended to have length of at least 10 mm and width of at least 1 mm (obtained with > 18 gauge needle)[15]. Several histological scoring systems have been established for grading (necroinflammatory activity) and staging (fibrosis) of structural liver damage in patients[16]. The Desmet score[17] is used to evaluate adult hepatitis C patients, and the METAVIR[18,19] and Ishak score[20] are used in cases of chronic viral hepatitis (B and C). The SSS-score of Chevallier[21] was developed to quantify fibroses irrespective of the underlying disease. Some of these scores have been evaluated in children (Table 1), and a detailed break-down of each (in children and adults) is provided below: (1) the METAVIR score[18] assesses fibrosis qualitatively on a 0-4 scale, with F0 indicating absence of fibrosis, F1 indicating portal fibrosis without septa, F2 indicating portal fibrosis with a few septa, F3 indicating architectural distortion with numerous septa without cirrhosis, and F4 indicating cirrhosis. This score has been used to evaluate adult patients with hepatitis B and C[19] and paediatric patients after liver transplantation[22], biliary atresia[23], intestinal failure[24] and total parenteral nutrition[25]; (2) the grading score of Ishak et al[20] assesses fibrosis qualitatively on a 0-6 scale. The Ishak score has been used in paediatric populations with various liver diseases, and including children after liver transplantation[26] or cardiovascular surgery[27]; (3) the grading score of Desmet et al[17] assesses fibrosis qualitatively on a 0-4 scale, with F0 indicating absence of fibrosis, F1 indicating portal fibrosis, F2 indicating fibrosis with septa without distortion of the liver architecture, F3 indicating septal fibrosis with severe distortion of the liver architecture, and F4 indicating cirrhosis. It has been used to evaluate adult patients with chronic hepatitis C[28]; and (4) the semi-quantitative severity score of Chevalier et al[21] has been used in children[29] and adults with hepatitis B[30] and C[31].
| Scoring system | Staging | Evaluated in adults with | Evaluated in children with |
| METAVIR | F0-F4 | Hepatitis B and C | Biliary atresia, intestinal failure, total parenteral nutrition and post-liver transplantation |
| Ishak | F0-F6 | Hepatitis B and C | Post-liver transplantation and after cardiovascular surgery |
| Desmet | F0-F4 | Hepatitis C | No |
| SSS-score | 0- > 15 | Hepatitis B and C | Hepatitis B |
Numerous attempts have been made to determine liver fibroses by non-invasive means. One of the oldest is measurement of serum aminotransferases, which remains the most widely used, and convenient, tool to measure liver cell integrity. Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) are inexpensive laboratory values. They can be easily obtained from a patient and are stable in serum specimen. ALT, especially, is highly liver specific.
Unfortunately, aminotransferases poorly reflect the stage of liver fibrosis or cirrhosis. If they are elevated, a more detailed examination of the liver is obligate. But, ALT and AST may even be normal or only slightly elevated in fibrotic or cirrhotic liver diseases. The positive predictive value of aminotransferases for NAFLD or NASH is low. In a series of 222 patients with histologically-proven NAFLD, 37% of the patients with advanced fibrosis or NASH presented with normal ALT levels. This phenomenon was also recently demonstrated in children, in a study of paediatric cases of NAFLD conducted by Molleston et al[32].
Aminotransferases may serve as a first screening tool for detection of fibrosis, but even normal levels of aminotransferases do not exclude severe liver disease with changes in liver structure. Some of the techniques that have been developed to identify NAFLD in adult patients have been tested in children, including the AST to platelet ratio index (APRI) score, the NAFLD fibrosis score[33] and the Fibrosis-4 index score. Yet, recent data have indicated that only the APRI score and the paediatric NAFLD fibrosis score reliably reflect fibrotic changes of the liver. Alkhouri et al[34] have developed and published a new paediatric NAFLD fibrosis score based on a model using ALT, alkaline phosphatase, platelet counts and gamma-glutamyl transferase, and demonstrated its predictive ability of fibroses as good.
Collectively, these tests are reliable in detecting severe fibrosis or cirrhosis (grade 2 or greater for the Desmet score). Thus, while they can reliably show if the patient suffers from a change in liver structure they cannot reliably predict the exact degree of fibrosis.
Transient elastography (TE) is a technique based on the measurement of the velocity of a shear wave that is induced to the liver by a mechanical impulse. To apply that impulse to the liver, the probe has to be pressed onto the skin with a certain force, and the thoracic wall prevents the liver from being compressed by the probe. Therefore, TE can only be measured reliably in the right lobe of the liver and not in other organs or in other parts of the liver.
The velocity of the shear wave is directly proportional to the stiffness of the liver. Stiffness mainly depends on the amount of fibrotic material in the liver. Therefore, liver elasticity is measured in kilopascal (kPa) and liver stiffness increases with liver fibrosis. The probe is placed in the 7th or 8th intercostal space in the right ventral axillary line. The patient lies in supine position, with the right arm in maximal abduction. This technique has been described in detail elsewhere[35]. A mechanical impulse of 50 Hz induces an elastic shear wave that passes through the liver tissue. The speed of this wave is measured via ultrasound. For more detailed information on the basic physical principle, the Young Modules, see Frulio et al[36].
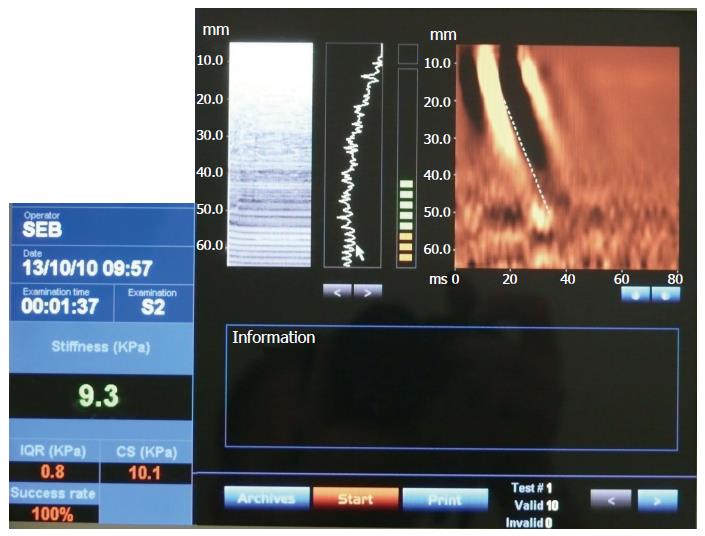
TE reliably detects liver fibroses, as demonstrated in numerous studies and meta-analyses comparing the technology to liver biopsy[35-42]. The median liver stiffness in adults varies between 4.4 and 5.5[43,44]. In addition, there is evidence that stiffness is greater in males, increases with body mass index in adult patients, and tends to increase with age but not to a statistically significant extent[44]. In children, the median liver stiffness significantly rises with age, starting with 4.4 in preschool children and rising to 5.1 in pubertal children. Liver stiffness in children has also been shown to differ according to sex, with girls showing significantly less (4.7) than boys (5.6)[45]. In split liver transplants of left liver, which is the main transplantation technique used in infants, toddlers and preschool children, liver stiffness measurement cannot be used because it is technically performable only in the right liver lobe (as detailed above). A clinical example of TE use in a paediatric patient is presented in Figure 1.
Introduction of the small TE-probe that is also suitable for use with infants and very young children has made TE possible for every age group. But liver stiffness measurement can only be performed in a patient that is laying calmly in supine position. This is usually not an attainable state in toddlers without sedation. Therefore, the problem of invalid liver stiffness measurement due to moving and crying of the patients makes this method questionable in infants.
Another general drawback of this method is the price. The technique is reliant on hardware that ultrasound machines do not come equipped with normally. Therefore, an extra-device is required to accompany the ultrasound machine and this produces extra-costs of more than 50000 Euros. Finally, the capacity for integrated measurement in B-mode ultrasound images is not yet available.
Findings from a recent Cochrane analysis of adult patients with alcoholic liver disease led to the recommendation of TE as a useful tool to exclude fibroses and, in cases of liver stiffness measurement above 12.5 kPa, to suggest cirrhosis. These data, however, still have to be confirmed in further studies[46], especially for their applicability to the paediatric age group. It is well accepted that TE enables the investigator to clearly exclude severe changes in liver architecture, but it remains a matter of debate whether TE can also enable clear staging of fibrosis. As such, TE is routinely used to assess liver fibrosis in adult patients with chronic hepatitis C, and this use is confirmed in the EASL Clinical Practice Guidelines 2011[47]. With the increasing application of TE in children with viral hepatitis, however, TE has the capability to gain more relevance for detection of liver fibrosis.
Acoustic radiation force impulse (ARFI) is a point shear wave elastography that measures tissue elasticity independent of an external mechanical impulse to the tissue. Therefore, this method is not only useful for liver stiffness measurement but also for determination of changes in stiffness of the spleen[48], testis[49], thyroid[50], breast[51], placenta[52], pancreas in chronic pancreatitis[53] and transplanted kidney[54]. The technique is based on an acoustic impulse and measurement of the speed of the shear wave induced by it; results are displayed in m/s. The stiffer the organ, the faster the shear wave.
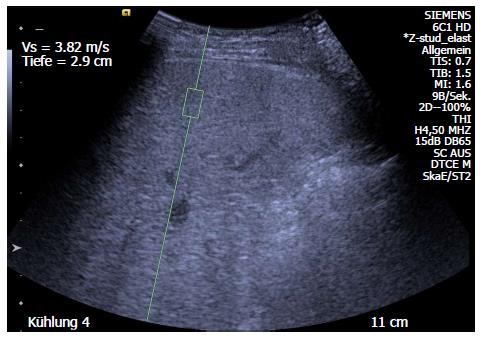
The ARFI method has two advantages. First, it can be performed by an additional technical tool for a high-end ultrasound system, providing integrated B-mode images. Second, the tissue is not compressed by the probe, as in TE. Compression itself causes changes in stiffness, and this feature of ARFI enables measurement of stiffness in numerous tissues. Many studies have shown the reliability and reproducibility of this technique in adult patients[55] and in children[56]. The correlation of ARFI and fibroses is in a good range[57], comparable to that of TE[58], and control-values have been established for children[56,59] and adults[36] (Table 2). Moreover, ARFI was demonstrated as effective in paediatric patient groups with biliary atresia or severe fibrosis[60,61] and in follow-up after liver transplantation[62]. A clinical example of ARFI use in a paediatric patient is presented in Figure 2.
Children with biliary atresia could gain particular benefit from non-invasive examinations for assessment of timing of liver transplantation after kasai-portoenterostomy[63,64]. According to METAVIR or SSS-score, ARFI shows overlap of shear wave velocity values in different fibrosis stages, as shown in the study by Hanquinet et al[65]. ARFI might offer diagnostic advantages over B-mode imaging in terms of combining stiffness measurement with sonomorphological parameters as the qualitative sonomorphological aspect becomes quantitative[61]. This makes comparison in patients easier.
Similar to TE, increased application of ARFI in children could lead to an implementation of this type of measurement in the routine clinical work flow, especially for patients with specific paediatric diseases, such as cystic fibrosis or biliary atresia.
Real-time tissue elastography (RTE) examinations can be performed with an ultrasound device and a standard linear transducer[66]. The RTE software captures images of tissue motions caused by heartbeats or respiration. These images are then transferred into colour-coded plane and the system calculates a histogram of strain elasticity values of the matrix in arbitrary units (a.u.), ranging from 0 to 255[67]. The method can be performed without extra-hardware, but data on the value of this method in children are scarce. Morikawa et al[67] analysed RTE in 101 adult patients with hepatitis c and found a good correlation of the RTE values with the histologic grading of fibroses. In contrast, data obtained from children in another study[68] showed only a moderate correlation, and it was concluded that RTE could not be recommended for a clear differentiation of fibrosis stages while the difference between stage IV fibrosis and normal liver tissue or stage I fibrosis was significant.
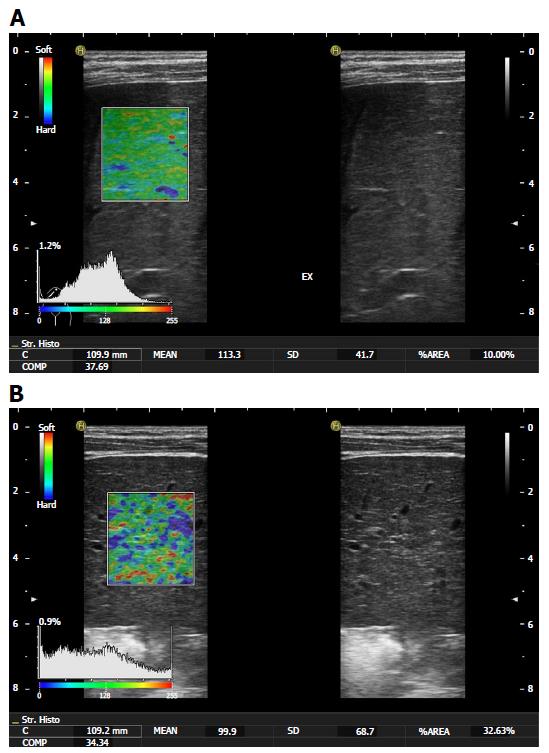
Other studies of adult patients[69] have concentrated on the elastic or fibrosis index values, which have not been adequately studied in the paediatric age group. In a meta-analysis of RTE conducted by Kobayashi et al[70], the authors concluded that RTE has low accuracy for detecting any stage of fibrosis. Today, we would not recommend the use of single statistical parameters as the mean elasticity value of strain histogram or %AREA in children alone to predict the histological fibrosis stage. Differentiation of high fibrosis stages to normal tissue is possible, but application in young infants can be difficult. Clinical examples of RTE use in two paediatric patients are presented in Figure 3.
Further studies on the use of the elastic index in paediatric patients should be conducted. High fibrosis stages can be differentiated from low fibrosis stages, but no clinical recommendations exist as of yet.
MR-elastography (MRE) is an elastography technique using an acoustic impulse to produce a shear wave. The impulse is produced by an audio subwoofer and subsequently transmitted to the liver via a connecting-tube that is placed on the skin of the patient. Then, the shear wave induced by this acoustic impulse is measured and stiffness is calculated in kPa[71]. Studies of MRE in adult patients with hepatitis C have shown good relation of MRE-measured liver stiffness, as compared to Child-Pugh score[72]. In another study of adult patients with cystic fibrosis[73] the liver stiffness measurement was shown to correlate well with serum levels of aminotransferases and also with ultrasound findings, but there were insufficient data to make any conclusions regarding histopathologic changes.
A new and promising application of MRE involves the differentiation of NASH from NAFLD. Both diseases can occur in obese patients, but there is yet no non-invasive method capable of distinguishing between the two. Patients with NASH develop cirrhosis in 10% of cases, while patients with NAFLD do not. Neither aminotransferases[32] nor ultrasound can differentiate these two diseases. Recent studies have suggested that MRE might be able to reliably determine the presence of NASH in an obese patient[74]. Future studies may prove that MRE, therefore, is useful, even in clinical analysis of obese patients, for defining relevant end-points.
ARFI does not replace liver biopsy for staging of liver fibroses or cirrhosis, neither do TE, RTE or MRE[75,76]. The limitations of these non-invasive techniques are low specificity and high cost, the latter being especially relevant for TE.
Liver structure changes can be excluded by each of these non-invasive techniques, with an acceptable sensitivity but an unacceptable low specificity. TE, ARFI and MRE have the potential to exclude severe liver structure changes. For RTE, however, the data are conflicting and do not support a recommendation; certainly, further studies are necessary. For diagnosing liver disease, none of these non-invasive techniques is useful. But, in many patients, the ethology is quite clear due to readily assessable clinical or laboratory aspects, such as the presence of obesity, chronic viral hepatitis or alpha-1 antitrypsin deficiency. In cases of the patient being post-liver transplantation or with an already-obtained liver biopsy, the analysis of liver structure changes is of greater importance.
A possible diagnostic approach to patients with liver disease in 2016 is to first perform clinical examinations to obtain anthropometric data, ultrasound images and standard laboratory measures. If then there is evidence for liver disease, ARFI or TE should be performed. If those findings then suggest liver structure changes, a biopsy should be obtained in any case. If the findings suggest normal liver structure, the biopsy may be delayed and further laboratory studies may be performed first. If there is no change in aminotransferase levels after 6 mo, a liver biopsy should be performed. Non-invasive liver stiffness measurement can be used for follow-up after liver biopsy if the stage of fibrosis has been determined based on histopathological criteria[77].
In patients with obesity, MRE possibly offers a new approach by which to define patients at risk for NASH or even to diagnose NASH in obese patients. Therefore, in the setting of an obese patient, MRE presents a real advantage over the classical methods of hepatology and future studies will show if this promising technique is suited to becoming part of the routine diagnostic workup in obese patients early in their clinical course and also in follow-up.
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Balsano C, Cordeiro A, Riordan JD S- Editor: Song XX L- Editor: A E- Editor: Li D
| 1. | Yalamanchili K, Saadeh S, Klintmalm GB, Jennings LW, Davis GL. Nonalcoholic fatty liver disease after liver transplantation for cryptogenic cirrhosis or nonalcoholic fatty liver disease. Liver Transpl. 2010;16:431-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 171] [Cited by in F6Publishing: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 2. | Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887-1901. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 435] [Cited by in F6Publishing: 462] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 3. | Tanner MS. Mechanisms of liver injury relevant to pediatric hepatology. Crit Rev Clin Lab Sci. 2002;39:1-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Manns M, Pol S, Jacobson IM, Marcellin P, Gordon SC, Peng CY, Chang TT, Everson GT, Heo J, Gerken G. All-oral daclatasvir plus asunaprevir for hepatitis C virus genotype 1b: a multinational, phase 3, multicohort study. Lancet. 2014;384:1597-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 289] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 5. | Bernuth S, Yagmur E, Schuppan D, Sprinzl MF, Zimmermann A, Schad A, Kittner JM, Weyer V, Knapstein J, Schattenberg JM. Early changes in dynamic biomarkers of liver fibrosis in hepatitis C virus-infected patients treated with sofosbuvir. Dig Liver Dis. 2016;48:291-297. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Crespo G, Castro-Narro G, García-Juárez I, Benítez C, Ruiz P, Sastre L, Colmenero J, Miquel R, Sánchez-Fueyo A, Forns X. Usefulness of liver stiffness measurement during acute cellular rejection in liver transplantation. Liver Transpl. 2016;22:298-304. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Poniachik J, Bernstein DE, Reddy KR, Jeffers LJ, Coelho-Little ME, Civantos F, Schiff ER. The role of laparoscopy in the diagnosis of cirrhosis. Gastrointest Endosc. 1996;43:568-571. [PubMed] [Cited in This Article: ] |
| 8. | Maharaj B, Maharaj RJ, Leary WP, Cooppan RM, Naran AD, Pirie D, Pudifin DJ. Sampling variability and its influence on the diagnostic yield of percutaneous needle biopsy of the liver. Lancet. 1986;1:523-525. [PubMed] [Cited in This Article: ] |
| 9. | Atwell TD, Smith RL, Hesley GK, Callstrom MR, Schleck CD, Harmsen WS, Charboneau JW, Welch TJ. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. AJR Am J Roentgenol. 2010;194:784-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 10. | Westheim BH, Østensen AB, Aagenæs I, Sanengen T, Almaas R. Evaluation of risk factors for bleeding after liver biopsy in children. J Pediatr Gastroenterol Nutr. 2012;55:82-87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Poynard T, Munteanu M, Imbert-Bismut F, Charlotte F, Thabut D, Le Calvez S, Messous D, Thibault V, Benhamou Y, Moussalli J. Prospective analysis of discordant results between biochemical markers and biopsy in patients with chronic hepatitis C. Clin Chem. 2004;50:1344-1355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 252] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 12. | Briem-Richter A, Ganschow R, Sornsakrin M, Brinkert F, Schirmer J, Schaefer H, Grabhorn E. Liver allograft pathology in healthy pediatric liver transplant recipients. Pediatr Transplant. 2013;17:543-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Keach JC, Lafferty HD, Stahler A. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology. 2015;149:389-397.e10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1701] [Cited by in F6Publishing: 1920] [Article Influence: 213.3] [Reference Citation Analysis (0)] |
| 14. | Mann JP, De Vito R, Mosca A, Alisi A, Armstrong MJ, Raponi M, Baumann U, Nobili V. Portal inflammation is independently associated with fibrosis and metabolic syndrome in pediatric nonalcoholic fatty liver disease. Hepatology. 2016;63:745-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 15. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [PubMed] [Cited in This Article: ] |
| 16. | Cholongitas E, Senzolo M, Standish R, Marelli L, Quaglia A, Patch D, Dhillon AP, Burroughs AK. A systematic review of the quality of liver biopsy specimens. Am J Clin Pathol. 2006;125:710-721. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 17. | Desmet VJ, Gerber M, Hoofnagle JH, Manns M, Scheuer PJ. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology. 1994;19:1513-1520. [PubMed] [Cited in This Article: ] |
| 18. | Bedossa P, Poynard T. An algorithm for the grading of activity in chronic hepatitis C. The METAVIR Cooperative Study Group. Hepatology. 1996;24:289-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2860] [Cited by in F6Publishing: 2932] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 19. | Leroy V, Sturm N, Faure P, Trocme C, Marlu A, Hilleret MN, Morel F, Zarski JP. Prospective evaluation of FibroTest®, FibroMeter®, and HepaScore® for staging liver fibrosis in chronic hepatitis B: comparison with hepatitis C. J Hepatol. 2014;61:28-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696-699. [PubMed] [Cited in This Article: ] |
| 21. | Chevallier M, Guerret S, Chossegros P, Gerard F, Grimaud JA. A histological semiquantitative scoring system for evaluation of hepatic fibrosis in needle liver biopsy specimens: comparison with morphometric studies. Hepatology. 1994;20:349-355. [PubMed] [Cited in This Article: ] |
| 22. | Venturi C, Sempoux C, Quinones JA, Bourdeaux C, Hoyos SP, Sokal E, Reding R. Dynamics of allograft fibrosis in pediatric liver transplantation. Am J Transplant. 2014;14:1648-1656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 23. | Shin NY, Kim MJ, Lee MJ, Han SJ, Koh H, Namgung R, Park YN. Transient elastography and sonography for prediction of liver fibrosis in infants with biliary atresia. J Ultrasound Med. 2014;33:853-864. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 24. | Díaz JJ, Gura KM, Roda J, Perez-Atayde AR, Duggan C, Jaksic T, Lo CW. Aspartate aminotransferase to platelet ratio index correlates with hepatic cirrhosis but not with fibrosis in pediatric patients with intestinal failure. J Pediatr Gastroenterol Nutr. 2013;57:367-371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Mutanen A, Lohi J, Heikkilä P, Koivusalo AI, Rintala RJ, Pakarinen MP. Persistent abnormal liver fibrosis after weaning off parenteral nutrition in pediatric intestinal failure. Hepatology. 2013;58:729-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 26. | Venturi C, Sempoux C, Bueno J, Ferreres Pinas JC, Bourdeaux C, Abarca-Quinones J, Rahier J, Reding R. Novel histologic scoring system for long-term allograft fibrosis after liver transplantation in children. Am J Transplant. 2012;12:2986-2996. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 81] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 27. | Schwartz MC, Sullivan L, Cohen MS, Russo P, John AS, Guo R, Guttenberg M, Rand EB. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg. 2012;143:904-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 28. | Delladetsima JK, Rassidakis G, Tassopoulos NC, Papatheodoridis GV, Smyrnoff T, Vafiadis I. Histopathology of chronic hepatitis C in relation to epidemiological factors. J Hepatol. 1996;24:27-32. [PubMed] [Cited in This Article: ] |
| 29. | Li ZX, He Y, Wu J, Liang DM, Zhang BL, Yang H, Wang LL, Ma Y, Wei KL. Noninvasive evaluation of hepatic fibrosis in children with infant hepatitis syndrome. World J Gastroenterol. 2006;12:7155-7160. [PubMed] [Cited in This Article: ] |
| 30. | ter Borg F, ten Kate FJ, Cuypers HT, Leentvaar-Kuijpers A, Oosting J, Wertheim-van Dillen PM, Honkoop P, Rasch MC, de Man RA, van Hattum J. A survey of liver pathology in needle biopsies from HBsAg and anti-HBe positive individuals. J Clin Pathol. 2000;53:541-548. [PubMed] [Cited in This Article: ] |
| 31. | Zaitoun AM, Al Mardini H, Awad S, Ukabam S, Makadisi S, Record CO. Quantitative assessment of fibrosis and steatosis in liver biopsies from patients with chronic hepatitis C. J Clin Pathol. 2001;54:461-465. [PubMed] [Cited in This Article: ] |
| 32. | Molleston JP, Schwimmer JB, Yates KP, Murray KF, Cummings OW, Lavine JE, Brunt EM, Scheimann AO, Unalp-Arida A. Histological abnormalities in children with nonalcoholic fatty liver disease and normal or mildly elevated alanine aminotransferase levels. J Pediatr. 2014;164:707-713.e3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 33. | Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1917] [Cited by in F6Publishing: 2018] [Article Influence: 118.7] [Reference Citation Analysis (1)] |
| 34. | Alkhouri N, Mansoor S, Giammaria P, Liccardo D, Lopez R, Nobili V. The development of the pediatric NAFLD fibrosis score (PNFS) to predict the presence of advanced fibrosis in children with nonalcoholic fatty liver disease. PLoS One. 2014;9:e104558. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 35. | Sandrin L, Fourquet B, Hasquenoph JM, Yon S, Fournier C, Mal F, Christidis C, Ziol M, Poulet B, Kazemi F. Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol. 2003;29:1705-1713. [PubMed] [Cited in This Article: ] |
| 36. | Frulio N, Trillaud H. Ultrasound elastography in liver. Diagn Interv Imaging. 2013;94:515-534. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 37. | Yeshua H, Oren R. Non invasive assessment of liver fibrosis. Ann Transplant. 2008;13:5-11. [PubMed] [Cited in This Article: ] |
| 38. | Castera L, Forns X, Alberti A. Non-invasive evaluation of liver fibrosis using transient elastography. J Hepatol. 2008;48:835-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 972] [Cited by in F6Publishing: 974] [Article Influence: 60.9] [Reference Citation Analysis (0)] |
| 39. | Ziol M, Handra-Luca A, Kettaneh A, Christidis C, Mal F, Kazemi F, de Lédinghen V, Marcellin P, Dhumeaux D, Trinchet JC. Noninvasive assessment of liver fibrosis by measurement of stiffness in patients with chronic hepatitis C. Hepatology. 2005;41:48-54. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1090] [Cited by in F6Publishing: 1062] [Article Influence: 55.9] [Reference Citation Analysis (0)] |
| 40. | Fung J, Lai CL, Chan SC, But D, Seto WK, Cheng C, Wong DK, Lo CM, Fan ST, Yuen MF. Correlation of liver stiffness and histological features in healthy persons and in patients with occult hepatitis B, chronic active hepatitis B, or hepatitis B cirrhosis. Am J Gastroenterol. 2010;105:1116-1122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 41. | Castéra L, Vergniol J, Foucher J, Le Bail B, Chanteloup E, Haaser M, Darriet M, Couzigou P, De Lédinghen V. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology. 2005;128:343-350. [PubMed] [Cited in This Article: ] |
| 42. | de Lédinghen V, Douvin C, Kettaneh A, Ziol M, Roulot D, Marcellin P, Dhumeaux D, Beaugrand M. Diagnosis of hepatic fibrosis and cirrhosis by transient elastography in HIV/hepatitis C virus-coinfected patients. J Acquir Immune Defic Syndr. 2006;41:175-179. [PubMed] [Cited in This Article: ] |
| 43. | Sirli R, Sporea I, Tudora A, Deleanu A, Popescu A. Transient elastographic evaluation of subjects without known hepatic pathology: does age change the liver stiffness? J Gastrointestin Liver Dis. 2009;18:57-60. [PubMed] [Cited in This Article: ] |
| 44. | Roulot D, Czernichow S, Le Clésiau H, Costes JL, Vergnaud AC, Beaugrand M. Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome. J Hepatol. 2008;48:606-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 315] [Cited by in F6Publishing: 303] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 45. | Engelmann G, Gebhardt C, Wenning D, Wühl E, Hoffmann GF, Selmi B, Grulich-Henn J, Schenk JP, Teufel U. Feasibility study and control values of transient elastography in healthy children. Eur J Pediatr. 2012;171:353-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Pavlov CS, Casazza G, Nikolova D, Tsochatzis E, Burroughs AK, Ivashkin VT, Gluud C. Transient elastography for diagnosis of stages of hepatic fibrosis and cirrhosis in people with alcoholic liver disease. Cochrane Database Syst Rev. 2015;1:CD010542. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2014;60:392-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 635] [Cited by in F6Publishing: 646] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 48. | Tomita H, Ohkuma K, Masugi Y, Hosoe N, Hoshino K, Fuchimoto Y, Fujino A, Shimizu T, Kato M, Fujimura T. Diagnosing native liver fibrosis and esophageal varices using liver and spleen stiffness measurements in biliary atresia: a pilot study. Pediatr Radiol. 2016;46:1409-1417. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Pedersen MR, Osther PJ, Rafaelsen SR. Testicular microlithiasis and preliminary experience of acoustic radiation force impulse imaging. Acta Radiol Open. 2016;5:2058460116658686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Hofauer B, Mansour N, Heiser C, Wirth M, Straßen U, Loeffelbein D, Bas M, Knopf A. Reproducibility of Acoustic Radiation Force Impulse Imaging in Thyroid and Salivary Glands with Experienced and Inexperienced Examiners. Ultrasound Med Biol. 2016;42:2545-2552. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Zhou J, Yang Z, Zhan W, Dong Y, Zhou C. Anisotropic Properties of Breast Tissue Measured by Acoustic Radiation Force Impulse Quantification. Ultrasound Med Biol. 2016;42:2372-2382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Karaman E, Arslan H, Çetin O, Şahin HG, Bora A, Yavuz A, Elasan S, Akbudak İ. Comparison of placental elasticity in normal and pre-eclamptic pregnant women by acoustic radiation force impulse elastosonography. J Obstet Gynaecol Res. 2016;42:1464-1470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Llamoza-Torres CJ, Fuentes-Pardo M, Álvarez-Higueras FJ, Alberca-de-Las-Parras F, Carballo-Álvarez F. Usefulness of percutaneous elastography by acoustic radiation force impulse for the non-invasive diagnosis of chronic pancreatitis. Rev Esp Enferm Dig. 2016;108:450-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Yang C, Jin Y, Wu S, Li L, Hu M, Xu M, Rong R, Zhu T, He W. Prediction of Renal Allograft Acute Rejection Using a Novel Non-Invasive Model Based on Acoustic Radiation Force Impulse. Ultrasound Med Biol. 2016;42:2167-2179. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 168] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 56. | Hanquinet S, Courvoisier D, Kanavaki A, Dhouib A, Anooshiravani M. Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children. Pediatr Radiol. 2013;43:539-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Haque M, Robinson C, Owen D, Yoshida EM, Harris A. Comparison of acoustic radiation force impulse imaging (ARFI) to liver biopsy histologic scores in the evaluation of chronic liver disease: A pilot study. Ann Hepatol. 2010;9:289-293. [PubMed] [Cited in This Article: ] |
| 58. | Belei O, Sporea I, Gradinaru-Tascau O, Olariu L, Popescu A, Simedrea I, Marginean O. Comparison of three ultrasound based elastographic techniques in children and adolescents with chronic diffuse liver diseases. Med Ultrason. 2016;18:145-150. [PubMed] [Cited in This Article: ] |
| 59. | Eiler J, Kleinholdermann U, Albers D, Dahms J, Hermann F, Behrens C, Luedemann M, Klingmueller V, Alzen GF. Standard value of ultrasound elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents. Ultraschall Med. 2012;33:474-479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Shima H, Igarashi G, Wakisaka M, Hamano S, Nagae H, Koyama M, Kitagawa H. Noninvasive acoustic radiation force impulse (ARFI) elastography for assessing the severity of fibrosis in the post-operative patients with biliary atresia. Pediatr Surg Int. 2012;28:869-872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 61. | Behrens CB, Langholz JH, Eiler J, Jenewein R, Naehrlich L, Fuchs K, Harth S, Krombach GA, Alzen GF. A pilot study of the characterization of hepatic tissue strain in children with cystic-fibrosis-associated liver disease (CFLD) by acoustic radiation force impulse imaging. Pediatr Radiol. 2013;43:552-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 62. | Tomita H, Hoshino K, Fuchimoto Y, Ebinuma H, Ohkuma K, Tanami Y, Du W, Masugi Y, Shimojima N, Fujino A. Acoustic radiation force impulse imaging for assessing graft fibrosis after pediatric living donor liver transplantation: a pilot study. Liver Transpl. 2013;19:1202-1213. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 63. | Hanquinet S, Courvoisier DS, Rougemont AL, Dhouib A, Rubbia-Brandt L, Wildhaber BE, Merlini L, McLin VA, Anooshiravani M. Contribution of acoustic radiation force impulse (ARFI) elastography to the ultrasound diagnosis of biliary atresia. Pediatr Radiol. 2015;45:1489-1495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 64. | Hanquinet S, Courvoisier DS, Rougemont AL, Wildhaber BE, Merlini L, McLin VA, Anooshiravani M. Acoustic radiation force impulse sonography in assessing children with biliary atresia for liver transplantation. Pediatr Radiol. 2016;46:1011-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 65. | Hanquinet S, Rougemont AL, Courvoisier D, Rubbia-Brandt L, McLin V, Tempia M, Anooshiravani M. Acoustic radiation force impulse (ARFI) elastography for the noninvasive diagnosis of liver fibrosis in children. Pediatr Radiol. 2013;43:545-551. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 66. | Sandulescu L, Rogoveanu I, Gheonea IA, Cazacu S, Saftoiu A. Real-time elastography applications in liver pathology between expectations and results. J Gastrointestin Liver Dis. 2013;22:221-227. [PubMed] [Cited in This Article: ] |
| 67. | Morikawa H, Fukuda K, Kobayashi S, Fujii H, Iwai S, Enomoto M, Tamori A, Sakaguchi H, Kawada N. Real-time tissue elastography as a tool for the noninvasive assessment of liver stiffness in patients with chronic hepatitis C. J Gastroenterol. 2011;46:350-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 68. | Schenk JP, Selmi B, Flechtenmacher C, Sakka SE, Teufel U, Engelmann G. Real-time tissue elastography (RTE) for noninvasive evaluation of fibrosis in liver diseases in children in comparison to liver biopsy. J Med Ultrason (2001). 2014;41:455-462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Ishikawa E, Yada N, Hagiwara S, Inoue T, Minami Y, Chung H. Non-invasive evaluation of hepatic fibrosis for type C chronic hepatitis. Intervirology. 2010;53:76-81. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 70. | Kobayashi K, Nakao H, Nishiyama T, Lin Y, Kikuchi S, Kobayashi Y, Yamamoto T, Ishii N, Ohashi T, Satoh K. Diagnostic accuracy of real-time tissue elastography for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2015;25:230-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Binkovitz LA, El-Youssef M, Glaser KJ, Yin M, Binkovitz AK, Ehman RL. Pediatric MR elastography of hepatic fibrosis: principles, technique and early clinical experience. Pediatr Radiol. 2012;42:402-409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | Takamura T, Motosugi U, Ichikawa S, Sano K, Morisaka H, Ichikawa T, Enomoto N, Onishi H. Usefulness of MR elastography for detecting clinical progression of cirrhosis from child-pugh class A to B in patients with type C viral hepatitis. J Magn Reson Imaging. 2016;44:715-722. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Lemaitre C, Dominique S, Billoud E, Eliezer M, Montialoux H, Quillard M, Riachi G, Koning E, Morisse-Pradier H, Savoye G. Relevance of 3D Cholangiography and Transient Elastography to Assess Cystic Fibrosis-Associated Liver Disease? Can Respir J. 2016;2016:4592702. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 74. | Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J Hepatol. 2016;65:1006-1016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 253] [Cited by in F6Publishing: 236] [Article Influence: 29.5] [Reference Citation Analysis (0)] |
| 75. | de Alwis NM, Anstee QM, Day CP. How to Diagnose Nonalcoholic Fatty Liver Disease. Dig Dis. 2016;34 Suppl 1:19-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 76. | Mansoor S, Collyer E, Alkhouri N. A comprehensive review of noninvasive liver fibrosis tests in pediatric nonalcoholic Fatty liver disease. Curr Gastroenterol Rep. 2015;17:23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 77. | Selmi B, Engelmann G, Teufel U, El Sakka S, Dadrich M, Schenk JP. Normal values of liver elasticity measured by real-time tissue elastography (RTE) in healthy infants and children. J Med Ultrason (2001). 2014;41:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 78. | Wang J, Guo L, Shi X, Pan W, Bai Y, Ai H. Real-time elastography with a novel quantitative technology for assessment of liver fibrosis in chronic hepatitis B. Eur J Radiol. 2012;81:e31-e36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 79. | Xanthakos SA, Podberesky DJ, Serai SD, Miles L, King EC, Balistreri WF, Kohli R. Use of magnetic resonance elastography to assess hepatic fibrosis in children with chronic liver disease. J Pediatr. 2014;164:186-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 80. | Singh S, Venkatesh SK, Wang Z, Miller FH, Motosugi U, Low RN, Hassanein T, Asbach P, Godfrey EM, Yin M. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clin Gastroenterol Hepatol. 2015;13:440-451.e6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 359] [Cited by in F6Publishing: 367] [Article Influence: 40.8] [Reference Citation Analysis (0)] |
| 81. | Matos H, Trindade A, Noruegas MJ. Acoustic radiation force impulse imaging in paediatric patients: normal liver values. J Pediatr Gastroenterol Nutr. 2014;59:684-688. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |