Published online Feb 6, 2012. doi: 10.4292/wjgpt.v3.i1.1
Revised: January 29, 2012
Accepted: February 3, 2012
Published online: February 6, 2012
AIM: To find the way to improve the eradication rate of first-line therapy in Japanese patients.
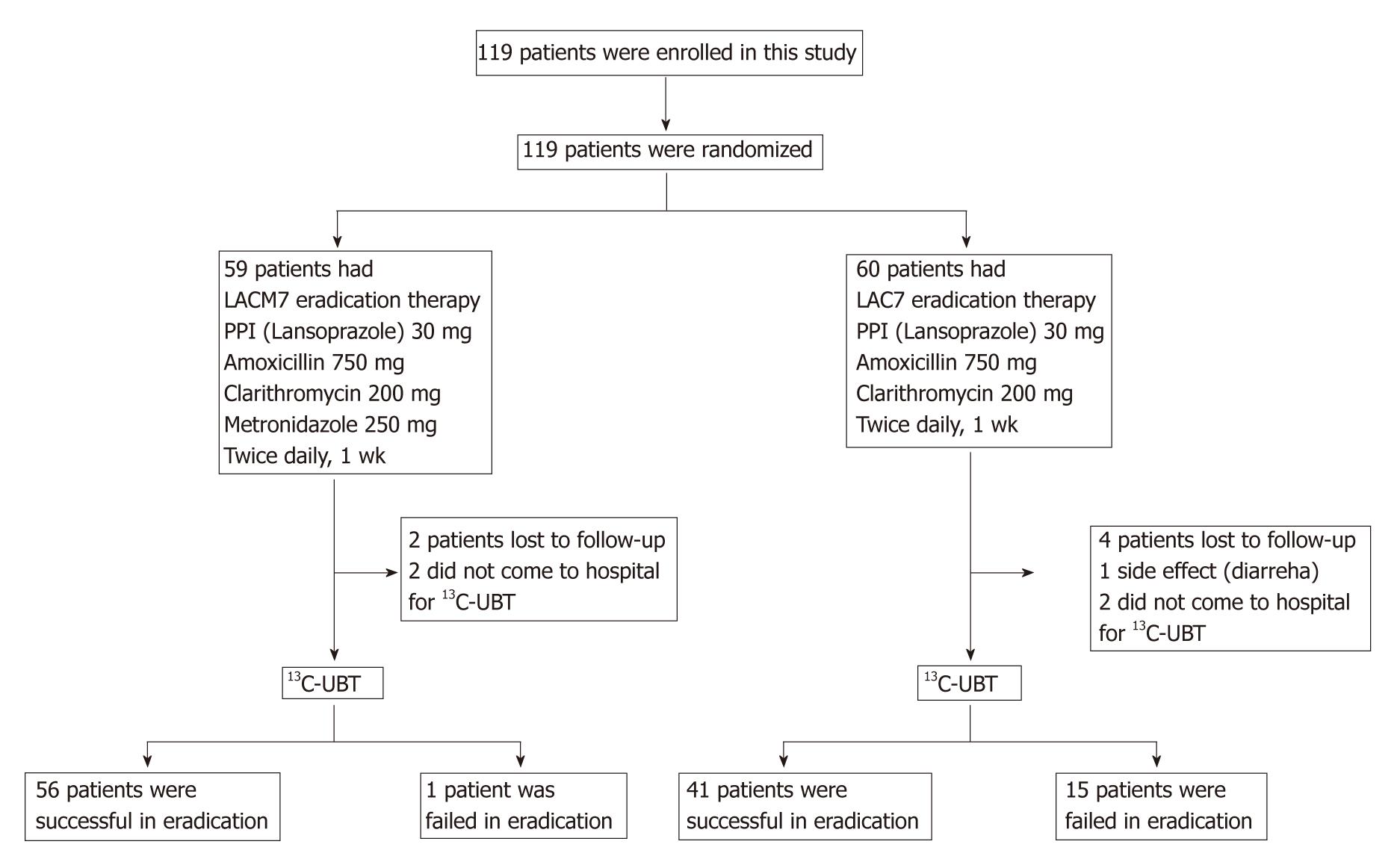
METHODS: We prospectively compared the effectiveness of 7-d quadruple therapy to standard 7 d triple therapy in Japanese patients infected with Helicobacter pylori (H. pylori). One hundred and nineteen patients were randomly assigned to receive 7-d non-bismuth quadruple therapy with lansoprazole, amoxicillin, clarithromycin and metronidazole (LACM7) or 7-d triple therapy with lansoprazole, amoxicillin and clarithromycin (LAC7). After three months, H. pylori status was analyzed by 13C-urea breath test. Incidence rates of adverse events were evaluated by use of questionnaires.
RESULTS: By intention-to-treat (ITT) analysis, the eradication rate in the LACM7 group was 94.9%, which was significantly higher than the LAC7 group (68.3%, P < 0.001). Per protocol analysis also showed a significantly higher eradication rate in the LACM7 group (98.3%) than the LAC7 group (73.2%, P < 0.001). Nevertheless, the incidence of serious adverse events did not differ between the two groups (RR: 1.10, 95% CI: 0.70-1.73, P = 0.67).
CONCLUSION: Seven day non-bismuth quadruple therapy (LACM7) was superior to standard 7-d triple therapy (LAC7) for first-line eradication.
- Citation: Yanai A, Sakamoto K, Akanuma M, Ogura K, Maeda S. Non-bismuth quadruple therapy for first-line Helicobacter pylori eradication: A randomized study in Japan. World J Gastrointest Pharmacol Ther 2012; 3(1): 1-6
- URL: https://www.wjgnet.com/2150-5349/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.4292/wjgpt.v3.i1.1
Helicobacter pylori (H. pylori) infection plays a crucial role in the pathogenesis of chronic atrophic gastritis and peptic ulcer. Moreover, it has been identified as one of the most important causes of gastric cancer and contributes to cancer initiation and progression[1]. H. pylori is associated strongly with the development of intestinal-type gastric cancer and metachronous cancer after endoscopic resection therapy[2,3] and eradication should reduce the risk of developing gastric cancer[4,5].
The recommended treatment for H. pylori is a proton pump inhibitor (PPI)-based triple therapy using PPI, amoxicillin and clarithromycin or metronidazole given twice daily and considerable data support the use of this treatment[5]. However, the eradication rate for standard triple therapy has declined in recent years despite being > 80% before 2000[6,7]. At present, patients who fail first-line therapy usually receive second-line therapy in Japan. However, a second course of eradication therapy increases the financial burden and is time-consuming[8].
Many attempts have been made recently to improve the initial eradication rates of H. pylori. Some studies have suggested prolonging the eradication period or taking drugs four or more times daily, but that might reduce compliance[9,10]. In this study, to find a more effective regimen to complete H. pylori eradication in first-line therapy, we compared the effectiveness of 7 d quadruple therapy of lansoprazole, amoxicillin, clarithromycin and metronidazole (LACM7) to 7 d standard triple therapy with lansoprazole, amoxicillin and clarithromycin (LAC7) for H. pylori eradication.
The present study was a randomized, prospective trial performed at the Institute for Adult Diseases, Asahi Life Foundation, Tokyo, Japan, between March 2007 and January 2009. Patients who were diagnosed with peptic ulcer or atrophic gastritis by endoscopic examinations were included this study. Exclusion criteria were as follows: (1) younger than 18 years; (2) allergy to antibiotics; (3) active peptic ulcer undergoing PPI treatment; (4) present malignancy or history of malignancy; (5) previous total or subtotal gastrectomy; (6) pregnancy or nursing; and (7) previous H. pylori eradication therapy.
One hundred and nineteen patients infected with H. pylori were enrolled in this study. Of those, 93 were men (78%) and 26 were women (22%), ranging in age from 26 to 79 years (mean age 61.4 years). Clinical characteristics are shown in Table 1.
| Treatment groups | |||
| Characteristics | LACM7 (n = 59) | LAC7 (n = 60) | P value |
| Age (mean) (yr) | 63 ± 8.7 | 60 ± 11.5 | 0.05 |
| Sex (male/female) | 48/11 | 45/15 | 0.40 |
| Endoscopic diagnosis | 0.37 | ||
| Atrophic gastritis | 31 | 23 | |
| Gastric ulcer | 15 | 16 | |
| Duodenal ulcer | 10 | 17 | |
This study was approved by the ethics committee of the Institute for Adult Diseases before the start of patient enrollment and was performed in accordance with the principles set forth in the Declaration of Helsinki. Informed consent was obtained from all patients.
Diagnosis of peptic ulcer or atrophic gastritis was determined through endoscopic examination. At endoscopy, biopsy samples were taken from the greater curvature of the antrum for rapid urease test (Helicocheck; Otsuka Pharmaceuticals, Tokyo, Japan), culture or histological examination. The rapid urease test was inspected for color change up to 2 h at room temperature after addition of the gastric samples. If the color changed from yellow to red, the test was diagnosed as positive. Culture was performed using Columbia agar plates supplemented with 5% horse blood at 37ºC under microaerobic conditions. Biopsy samples were fixed in formalin and slides were prepared with hematoxylin and eosin and/or Giemsa staining for H. pylori diagnosis. Serum anti-H. pylori antibody was measured using a commercial EIA kit (E-plate Eiken H. pylori antibody; Eiken Chemical Co., Ltd., Tokyo, Japan). Seropositivity for H. pylori antibody was defined by optical density values according to the manufacturer’s protocol. Positive result on at least one test was considered to be evidence of H. pylori infection.
Patients were assigned randomly by the envelope method to receive either quadruple LACM7 therapy or triple LAC7 therapy. Patients in the LACM7 group received 30 mg lansoprazole twice daily, 750 mg amoxicillin twice daily, 200 mg clarithromycin twice daily and 250 mg metronidazole twice daily for a week. Patients in the LAC7 group received lansoprazole 30 mg twice daily, 750 mg amoxicillin twice daily and 200 mg clarithromycin twice daily for a week (Figure 1).
The outcome of the eradication treatment was evaluated by 13C-urea breath test (13C-UBT) three months after treatment. After overnight fasting, a baseline breath sample was collected and 100 mg of 13C-urea (UBiT; Otsuka Pharmaceuticals, Tokyo, Japan) was administered with 100 mL of water. Twenty minutes after taking the 13C-urea, a further breath sample was obtained. Breath samples were analyzed by isotope-selective infrared spectrometry using the UBiT IR 300 (Otsuka Pharmaceuticals). A value of delta-over-baseline higher than 2.5% was considered positive. Compliance was checked by pill count. Incidence of adverse events was evaluated by means of a questionnaire at the time they received 13C-UBT. This questionnaire addressed nine symptoms; diarrhea, taste disturbance, loss of appetite, heart burn, nausea, abdominal pain/ distention, belching, rash and general fatigue. Degree of symptoms were scored as 0 (absent), 1 (mild), 2 (moderate) or 3 (severe).
Characteristics (sex, age, endoscopic diagnoses) between the two groups were compared with χ2 test. Eradication rates between the two arms were compared by intention-to-treat (ITT) and per-protocol analyses. ITT analysis included all patients who were enrolled and randomized to the LACM7 or LAC7 group. For per protocol analysis, patients who were lost to follow-up evaluation or deviated from the protocol were excluded. Eradication rates of these analyses were compared with Fisher’s exact test.
Frequencies and degrees of adverse events were evaluated by using χ2 test, Fisher’s exact test and Wilcoxon rank sum test. P < 0.05 was considered statistically significant.
One hundred and nineteen patients were enrolled in this study: 59 in the LACM7 group and 60 in the LAC7 group. There were no significant differences between age, sex and endoscopic diagnosis (Table 1). However, as serological tests do not always demonstrate a current infection of H. pylori[5], we also analyzed eradication rates in a subgroup excluding patients whose H. pylori status was checked only by a serological test. There were also no significant differences between the groups in terms of characteristics (Table 2).
| Treatment groups | |||
| Characteristics | LACM7 (n = 52) | LAC7 (n = 44) | P value |
| Age (mean) (yr) | 63 ± 8.9 | 63 ± 8.6 | 0.95 |
| Sex (male/female) | 41/11 | 33/1 | 0.66 |
| Endoscopic diagnosis | 0.34 | ||
| Atrophic gastritis | 24 | 13 | |
| Gastric ulcer | 15 | 14 | |
| Duodenal ulcer | 10 | 12 | |
After treatment therapy, two patients in the LACM7 group and two patients in the LAC7 group did not appear to have a urea breath test for unknown reasons. One patient in the LAC7 group interrupted eradication therapy because of severe diarrhea. By ITT analysis, H. pylori was successfully eradicated from 56 (94.9%) patients in the LACM7 group and 41 (68.3%) patients in the LAC7 group. By per protocol analysis, the eradication rate was 98.3% in the LACM7 group and 73.2% in the LAC7 group (Table 3). The eradication rate for quadruple LACM7 therapy was significantly higher than that for triple LAC7 therapy by ITT (P = 0.0002) and per protocol (P = 0.0001) analyses (Table 3). The subgroup analyses which excluded patients whose H. pylori status was checked only by serological test also showed a significantly higher eradication rate in the LACM7 group (94.2%) than in the LAC7 group (68.2%) (P = 0.0009, Table 4).
| Treatment groups | ||||||
| LACM7 | LAC7 | P value | ||||
| Eradication | n | % | n | % | ||
| ITT analysis | Success | 56 | 94.9 | 41 | 68.3 | 0.0002 |
| Failure | 3 | 5.1 | 19 | 31.7 | ||
| Per protocol analysis | Success | 56 | 98.3 | 41 | 73.2 | 0.0001 |
| Failure | 1 | 1.7 | 15 | 26.8 | ||
| Treatment groups | ||||||
| LACM7 | LAC7 | P value | ||||
| Eradication | n | % | n | % | ||
| ITT analysis | Success | 49 | 94.2 | 30 | 68.2 | 0.0009 |
| Failure | 3 | 5.8 | 14 | 31.8 | ||
| Per protocol analysis | Success | 49 | 98.0 | 30 | 75.0 | 0.0009 |
| Failure | 1 | 2.0 | 10 | 25.0 | ||
A total of 110 of 119 patients completed the questionnaire. The incidence of side effects in patients receiving LACM7 quadruple therapy was 42.9%, whereas the incidence in patients receiving LAC7 therapy was 38.9%. No statistically significant difference exists between two groups (RR: 1.10, 95% CI: 0.70-1.73, P = 0.67, Table 5). The most frequent adverse event was diarrhea in both groups. It was reported in 30.1% in the LACM7 group compared with 24.1% in the LAC group (P = 0.53). Other adverse effects such as taste disturbance, loss of appetite, heart burn, nausea, abdominal pain or distention, belching and general fatigue occurred more frequently in the LACM7 group than in the LAC7 group but none was statistically significant. One patient in the LAC7 group complained of a skin rash but it was too mild to quit the eradication therapy (Table 6).
| Treatment groups | |||||
| LACM7 | LAC7 | P value | |||
| Adverse events | n | % | n | % | |
| None | 32 | 57.1 | 33 | 61.1 | 0.67 |
| Present | 24 | 42.9 | 21 | 38.9 | |
| Adverse events | LACM7n = 56 (%) | LAC7n = 54 (%) | P value |
| Diarrhea | 17 (30.1) | 13 (24.1) | 0.53 |
| Taste disturbance | 7 (12.5) | 2 (3.7) | 0.16 |
| Loss of appetite | 5 (8.9) | 0 (0.0) | 0.06 |
| Heart burn | 5 (8.9) | 4 (7.4) | 1.00 |
| Nausea | 2 (3.6) | 0 (0.0) | 0.50 |
| Abdominal pain/distention | 7 (12.5) | 3 (5.6) | 0.32 |
| Belching | 3 (5.4) | 2 (3.7) | 1.00 |
| Rash | 0 (0.0) | 1 (1.9) | 0.49 |
| General fatigue | 2 (3.6) | 1 (1.9) | 1.00 |
This study was designed as a randomized prospective study to find the effectiveness of LACM7 quadruple eradication therapy and clearly showed that first-line 7-d quadruple therapy using lansoprazole, amoxicillin, clarithromycin and metronidazole significantly improved the eradication rate of H. pylori compared to standard 7-d triple therapy. Furthermore, LACM7 quadruple therapy showed no significant increases in the risk of adverse events.
In the Asia-Pacific consensus guidelines for H. pylori infection, the currently recommended first-line eradication therapy is PPI-based triple therapy with clarithromycin and either amoxicillin or metronidazole for 7 d[11]. PPI-based triple therapy is also recommended in the Maastricht III consensus report, whereas bismuth-containing quadruple therapy is presented as option[5].
Recently, some non-bismuth quadruple therapies were reported to obtain higher eradication rates[12]. Neville reported that the eradication rate of quadruple therapy (lansoprazole 30 mg, amoxicillin 1 g, clarithromycin 250 mg and metronidazole 400 mg, twice daily for 5 d) was 88%[13]. Garcia reported that seven day quadruple therapy of PPI, amoxicillin, trinidazole and bismuth showed a high eradication rate of 84%[14]. In randomized control studies, Kateralis compared quadruple therapy (PPI, bismuth, tetracycline and metronidazole for 7 d) with triple therapy (PPI, amoxicillin, clarithromycin) and eradication rates were 82% and 78%, respectively[15]. There were some reports of higher eradication rates in quadruple therapies than in triple therapies[16-18]. On the other hand, some reports showed better eradication rates in triple therapies than in quadruple therapies. Mantzaris showed that the eradication rate of quadruple therapy (64.8%) was significantly lower than that of triple therapy (78.2%)[19]. Some other studies also showed higher eradication rate in triple therapies than in quadruple therapies[20,21]. Thus, the effectiveness of first-line quadruple therapy is controversial. In this study, we have demonstrated that the eradication rates were 94.9%/98.3% by ITT/per protocol analyses using LACM7 therapy, which showed a higher eradication rate compared to previous treatment regimens. It seems that LACM7 therapy is superior to other first-line eradication therapies.
However, quadruple therapies have not been prevalent in Japan. The concern is that quadruple therapies may induce more severe adverse events than standard triple therapies. In this study, the overall incidence rate of adverse effects in patients receiving LACM7 therapy was 42.9%, which is higher than that in patients receiving LAC7 therapy (38.9%, Table 5). Diarrhea was the most frequent adverse event which was reported in 30.1% of LACM7 compared with 24.1% of LAC7. Other adverse events that occurred with a frequency of more than 10% were taste disturbance (12.5%) and abdominal pain/distention (12.5%) in the LACM7 group. Nevertheless, the rate of adverse events did not differ significantly between two groups (P = 0.06-1.00, Table 6). Whereas some previous reports reported that metronidazole induces acute pancreatitis or brain tumors, these effects are extremely rare and are related to high doses or long-term administration[22,23]. Metronidazole use in the short-term may be favorable to achieve eradication therapy efficiently.
The serology test, which is non-invasive, is widely available to diagnose H. pylori infection[5]. However, as the serology test does not always indicate current infection of H. pylori, patients whose H. pylori status was checked only by serological test were excluded in subgroup analysis (Tables 2 and 4). In this subgroup analysis, eradication rates of LACM7/LAC7 were 94.2%/68.2%, which were similar to results in Table 3. These results confirmed the efficacy of LACM7 therapy.
Recently, the eradication rates in Japan have been decreasing. In other Asian countries, eradication rates have also been decreasing because of drug resistance. The clarithromycin resistance rate is increasing in many countries. In Japan, clarithromycin resistance currently accounts for approximately 25% compared to around 15% in the 1990s[7]. Hence, it is reasonable to use metronidazole for first-line therapy. In addition, clarithromycin is metabolized by the CYP3A4 family of P-450, which is abundant in the liver, and metronidazole is also metabolized in the liver by oxidation. Several drug interactions may occur due to inhibition of oxidative drug metabolism. It was also reported that the combination of metronidazole and clarithromycin induced long-term and concentration-dependent effects[24]. On account of these pharmacological effects, combination use of clarithromycin and metronidazole is more effective than using either drug alone.
There is concern that using LACM7 could cause metronidazole resistance. Actually, metronidazole resistance has also increasing and the resistance rate in other Asian countries is 50%-70%, which is higher than in Japan (4.5%)[25]. In these countries, metronidazole is frequently used for gynecological infections such as Trichomonas vaginalis, which has a high resistance rate. In contrast, metronidazole resistance rate in Japan is lower than in other Asian countries because of the restriction on its use. For these reasons, the influence of short-term use of metronidazole on its resistance might be slight.
Previously, we evaluated 7 d first-line triple therapy with metronidazole (lansoprazole 30 mg, amoxicillin 750 mg, metronidazole 200 mg twice daily for 7 d: LAM) in expectation of a higher eradication rate than other eradication therapies. Eradication of H. pylori was achieved in 91.0%, 91.2% and 86.5% of atrophic gastritis, gastric ulcer and duodenal ulcer patients by ITT analyses (data not shown), which were lower than LACM7 (94.9%). These results suggest that metronidazole-containing quadruple therapy is superior to metronidazole-containing triple therapy as first-line eradication.
LACM7 therapy may be useful when confident H. pylori eradication is needed; for example, gastric mucosa-associated lymphoid tissue (MALT) lymphoma and idiopathic thrombocytopenic purpura (ITP) that is linked closely to H. pylori infection[26,27]. As for MALT lymphoma, immediate H. pylori eradication is recognized as an effective primary treatment[5]. Rapid induction of eradication therapy may reduce tumor size and is expected to prevent undergoing surgical therapy or tumor metastasis if no API2-MALT1 gene translocations exist. As for ITP, when the platelet count is < 3000-5000/μL, immediate H. pylori eradication is strongly recommended to prevent cerebral hemorrhage. Immediate H. pylori eradication improves quality of life and decreases the mortality rate. In these cases, to achieve eradication in the first treatment is so important that the LACM7 regimen should be considered for first-line therapy.
We sincerely thank Dr. Yoshihiro Hirata and Dr. Yutaka Yamaji from University of Tokyo for some valuable suggestions to the study. We sincerely thank Mr. Tachibana and Ms. Iigaya for the management of the clinical database.
The most widely recommended Helicobacter pylori (H. pylori) first-line eradication regimen is PPI-based triple therapy (PPI, clarithromycin plus amoxicillin or metronidazole) for at least 7 d. However, the eradication rate of H. pylori first-line treatment is decreasing.
Various four-drug combination regimens have been reported. Most of them contain bismuth citrate. The research hotspot is how to modify the H. pylori eradication therapy to improve its effectiveness.
Bismuth is not available currently in Japan. Metronidazole is approved only for second-line eradication therapy. Hence, the authors used non-bismuth quadruple therapy (PPI, amoxicillin, clarithromycin and metronidazole) to improve the eradication rate of first-line therapy. The eradication rate in non-bismuth quadruple therapy was significantly higher than standard triple therapy. Nevertheless, the incidence of serious adverse events did not differ between two groups.
Non-bismuth quadruple therapy would be helpful to increase the first-line eradication rate in Japan.
Quadruple therapy: therapy with bismuth, two antibiotics and PPI or H2RA.
Non-bismuth quadruple therapy: therapy with PPI, clarithromycin, amoxicillin and nitroimidazole.
In this study, the authors prospectively compared the effectiveness of 7-d quadruple therapy to standard 7-d triple therapy in Japanese patients infected with H. pylori. Although many studies have been performed regarding the treatment of H. pylori infection, this current study still has its significance, in particular for patients in Japan or in Asia.
Peer reviewer: Ming-Xian Yan, MD, PhD, Associate Professor, Department of Gastroenterology, Shandong Qianfoshan Hospital, 16766 Jingshi Road, Jinan 250014, Shangdong Province, China
S- Editor Wang JL L- Editor Roemmele A E- Editor Zhang DN
| 1. | Boussioutas A, Li H, Liu J, Waring P, Lade S, Holloway AJ, Taupin D, Gorringe K, Haviv I, Desmond PV. Distinctive patterns of gene expression in premalignant gastric mucosa and gastric cancer. Cancer Res. 2003;63:2569-2577. [PubMed] [Cited in This Article: ] |
| 2. | Uemura N, Mukai T, Okamoto S, Yamaguchi S, Mashiba H, Taniyama K, Sasaki N, Haruma K, Sumii K, Kajiyama G. Effect of Helicobacter pylori eradication on subsequent development of cancer after endoscopic resection of early gastric cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:639-642. [PubMed] [Cited in This Article: ] |
| 3. | Fukase K, Kato M, Kikuchi S, Inoue K, Uemura N, Okamoto S, Terao S, Amagai K, Hayashi S, Asaka M. Effect of eradication of Helicobacter pylori on incidence of metachronous gastric carcinoma after endoscopic resection of early gastric cancer: an open-label, randomised controlled trial. Lancet. 2008;372:392-397. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 876] [Cited by in F6Publishing: 850] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 4. | Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, Taniyama K, Sasaki N, Schlemper RJ. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784-789. [PubMed] [Cited in This Article: ] |
| 5. | Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D, Hunt R, Rokkas T, Vakil N, Kuipers EJ. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772-781. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1295] [Article Influence: 76.2] [Reference Citation Analysis (0)] |
| 6. | Asaka M, Sugiyama T, Kato M, Satoh K, Kuwayama H, Fukuda Y, Fujioka T, Takemoto T, Kimura K, Shimoyama T. A multicenter, double-blind study on triple therapy with lansoprazole, amoxicillin and clarithromycin for eradication of Helicobacter pylori in Japanese peptic ulcer patients. Helicobacter. 2001;6:254-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Kobayashi I, Murakami K, Kato M, Kato S, Azuma T, Takahashi S, Uemura N, Katsuyama T, Fukuda Y, Haruma K. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45:4006-4010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Fujioka T, Yoshiiwa A, Okimoto T, Kodama M, Murakami K. Guidelines for the management of Helicobacter pylori infection in Japan: current status and future prospects. J Gastroenterol. 2007;42 Suppl 17:3-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Shirai N, Sugimoto M, Kodaira C, Nishino M, Ikuma M, Kajimura M, Ohashi K, Ishizaki T, Hishida A, Furuta T. Dual therapy with high doses of rabeprazole and amoxicillin versus triple therapy with rabeprazole, amoxicillin, and metronidazole as a rescue regimen for Helicobacter pylori infection after the standard triple therapy. Eur J Clin Pharmacol. 2007;63:743-749. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch Intern Med. 1999;159:1562-1566. [PubMed] [Cited in This Article: ] |
| 11. | Fock KM, Katelaris P, Sugano K, Ang TL, Hunt R, Talley NJ, Lam SK, Xiao SD, Tan HJ, Wu CY. Second Asia-Pacific Consensus Guidelines for Helicobacter pylori infection. J Gastroenterol Hepatol. 2009;24:1587-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 384] [Cited by in F6Publishing: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 12. | Gisbert JP, Calvet X. Review article: non-bismuth quadruple (concomitant) therapy for eradication of Helicobater pylori. Aliment Pharmacol Ther. 2011;34:604-617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 121] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Neville PM, Everett S, Langworthy H, Tompkins D, Mapstone NP, Axon AT, Moayyedi P. The optimal antibiotic combination in a 5-day Helicobacter pylori eradication regimen. Aliment Pharmacol Ther. 1999;13:497-501. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Garcia N, Calvet X, Gené E, Campo R, Brullet E. Limited usefulness of a seven-day twice-a-day quadruple therapy. Eur J Gastroenterol Hepatol. 2000;12:1315-1318. [PubMed] [Cited in This Article: ] |
| 15. | Katelaris PH, Forbes GM, Talley NJ, Crotty B. A randomized comparison of quadruple and triple therapies for Helicobacter pylori eradication: The QUADRATE Study. Gastroenterology. 2002;123:1763-1769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Calvet X, Ducons J, Guardiola J, Tito L, Andreu V, Bory F, Guirao R. One-week triple vs. quadruple therapy for Helicobacter pylori infection - a randomized trial. Aliment Pharmacol Ther. 2002;16:1261-1267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Laine L, Hunt R, El-Zimaity H, Nguyen B, Osato M, Spénard J. Bismuth-based quadruple therapy using a single capsule of bismuth biskalcitrate, metronidazole, and tetracycline given with omeprazole versus omeprazole, amoxicillin, and clarithromycin for eradication of Helicobacter pylori in duodenal ulcer patients: a prospective, randomized, multicenter, North American trial. Am J Gastroenterol. 2003;98:562-567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 18. | Uygun A, Kadayifci A, Safali M, Ilgan S, Bagci S. The efficacy of bismuth containing quadruple therapy as a first-line treatment option for Helicobacter pylori. J Dig Dis. 2007;8:211-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Mantzaris GJ, Petraki K, Archavlis E, Amberiadis P, Christoforidis P, Kourtessas D, Chiotakakou E, Triantafyllou G. Omeprazole triple therapy versus omeprazole quadruple therapy for healing duodenal ulcer and eradication of Helicobacter pylori infection: a 24-month follow-up study. Eur J Gastroenterol Hepatol. 2002;14:1237-1243. [PubMed] [Cited in This Article: ] |
| 20. | Pai CG, Thomas CP, Biswas A, Rao S, Ramnarayan K. Quadruple therapy for initial eradication of Helicobacter pylori in peptic ulcer: comparison with triple therapy. Indian J Gastroenterol. 2003;22:85-87. [PubMed] [Cited in This Article: ] |
| 21. | Jang HJ, Choi MH, Kim YS, Seo YA, Baik KH, Baik IH, Eun CS, Kim JB, Kae SH, Kim DJ. [Effectiveness of triple therapy and quadruple therapy for Helicobacter pylori eradication]. Korean J Gastroenterol. 2005;46:368-372. [PubMed] [Cited in This Article: ] |
| 22. | Celifarco A, Warschauer C, Burakoff R. Metronidazole-induced pancreatitis. Am J Gastroenterol. 1989;84:958-960. [PubMed] [Cited in This Article: ] |
| 23. | Beard CM, Noller KL, O'Fallon WM, Kurland LT, Dahlin DC. Cancer after exposure to metronidazole. Mayo Clin Proc. 1988;63:147-153. [PubMed] [Cited in This Article: ] |
| 24. | Svensson M, Nilsson LE, Ström M, Nilsson M, Sörberg M. Pharmacodynamic effects of nitroimidazoles alone and in combination with clarithromycin on Helicobacter pylori. Antimicrob Agents Chemother. 2002;46:2244-2248. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 25. | Koletzko S, Richy F, Bontems P, Crone J, Kalach N, Monteiro ML, Gottrand F, Celinska-Cedro D, Roma-Giannikou E, Orderda G. Prospective multicentre study on antibiotic resistance of Helicobacter pylori strains obtained from children living in Europe. Gut. 2006;55:1711-1716. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 159] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 26. | Fischbach W, Goebeler-Kolve ME, Dragosics B, Greiner A, Stolte M. Long term outcome of patients with gastric marginal zone B cell lymphoma of mucosa associated lymphoid tissue (MALT) following exclusive Helicobacter pylori eradication therapy: experience from a large prospective series. Gut. 2004;53:34-37. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 256] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 27. | Stasi R, Sarpatwari A, Segal JB, Osborn J, Evangelista ML, Cooper N, Provan D, Newland A, Amadori S, Bussel JB. Effects of eradication of Helicobacter pylori infection in patients with immune thrombocytopenic purpura: a systematic review. Blood. 2009;113:1231-1240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 223] [Article Influence: 14.9] [Reference Citation Analysis (0)] |