Published online Sep 28, 2018. doi: 10.4329/wjr.v10.i9.91
Peer-review started: April 26, 2018
First decision: June 14, 2018
Revised: July 21, 2018
Accepted: August 4, 2018
Article in press: August 5, 2018
Published online: September 28, 2018
Among the many causes of forefoot pain, Morton’s neuroma (MN) is often suspected, particularly in women, due to its high incidence. However, there remain controversies about its relationship with symptomatology and which diagnostic and treatment choices to choose. This article mainly focuses on the role of the various imaging methods and their abilities to support an accurate diagnosis of MN, ruling out other causes of forefoot pain, and as a way of providing targeted imaging-guided therapy for patients with MN.
Core tip: Nowadays, ultrasound and magnetic resonance imaging provide accurate diagnosis of Morton’s neuroma (MN) and are invaluable tools for ruling out other causes of forefoot pain. This extended review is intended to show the potential of imaging methods for diagnosis as well as treatment of MN.
- Citation: Santiago FR, Muñoz PT, Pryest P, Martínez AM, Olleta NP. Role of imaging methods in diagnosis and treatment of Morton’s neuroma. World J Radiol 2018; 10(9): 91-99
- URL: https://www.wjgnet.com/1949-8470/full/v10/i9/91.htm
- DOI: https://dx.doi.org/10.4329/wjr.v10.i9.91
Morton’s neuroma (MN) is considered a nerve entrapment neuropathy, causing symptoms relating to impingement of the common plantar digital and proper plantar digital nerves, such as burning, tingling or numbness. It is a common problem but mainly affects middle-aged women, occurring more than 5 times more frequently in women than in men[1].
It is considered that MN occurs secondary to mechanical stress on the nerves, leading to proliferation of fibrosis in and around the affected nerve[2]. It has been reported to be more frequent in the 3rd web space (in 68% of cases), followed by the 2nd web space (in 32% of cases). The presence of a neuroma in the 1st and 4th web spaces is extremely rare[3]. Two main anatomical reasons have been postulated to explain this distribution; firstly, the 3rd metatarsal nerve is theoretically thicker because it is usually formed by the confluence of medial and lateral plantar nerves[4]; and, secondly, the larger shearing forces that occur at the 3rd web space due to the relatively greater mobility of the 4th metatarsal relative to the 3rd metatarsal[1].
Other authors have found a similar proportion of MN in the 2nd and 3rd web spaces[5,6] and this could be explained by the fact that the 2nd and 3rd intermetatarsal spaces are the narrowest of the foot[7]. The occurrence of multiple neuromas in the same foot has also been reported, but is considered rare by some authors[8]; although, in other case series, it has been found in between 28% and 65% of cases[5,6,9].
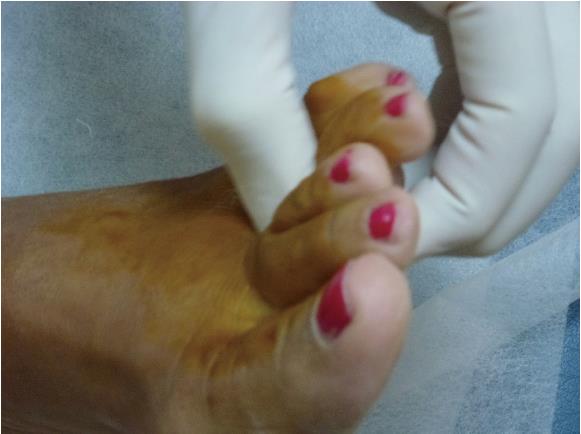
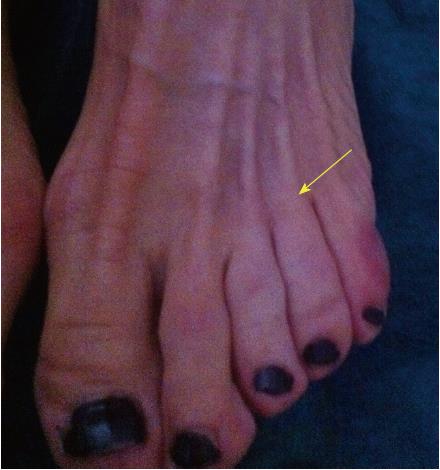
Not all patients with MN show clinical symptoms. The prevalence of asymptomatic MN has been reported as 33%-54% when using ultrasound (US) or magnetic resonance imaging (MRI) for diagnosis[9,10]. Nevertheless, when forefoot pain secondary to MN is present, it is elicited under pressure of the web space. The thumb index finger squeeze test has been reported to be the most sensitive screening method for clinical diagnosis of MN, having accuracy of around 96% (Figure 1). In this maneuver, the intermetatarsal space is squeezed between the tips of the index finger (dorsal) and thumb (plantar). The Mulder’s click is reproduced by firm medial to lateral compression of the metatarsal heads, when one hand is clasping the forefoot. A palpable and/or audible click is perceived, though it is mainly present for larger neuromas; its accuracy is around 62%[5].
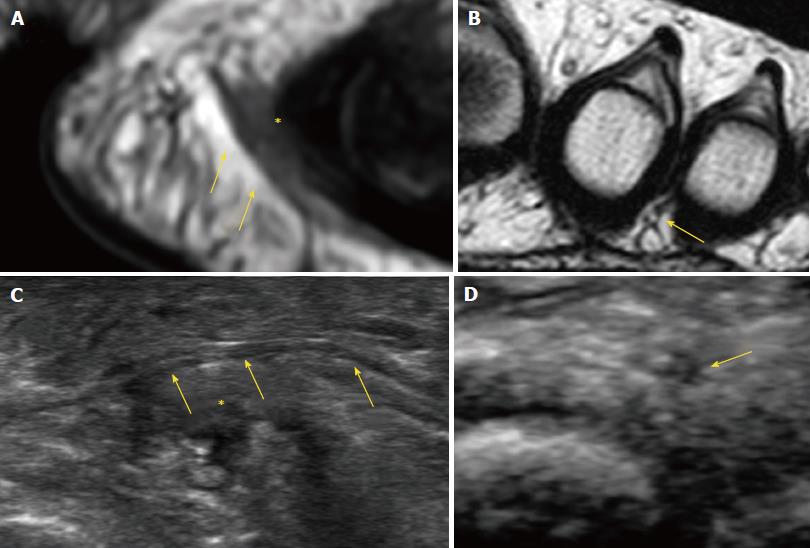
Pathognomonic diagnostic clinical tests for MN do not exist, and many of the clinical tests provide false positive results in feet with alternative pathologies[11]. Therefore, imaging may be required to confirm the diagnosis of MN and exclude other causes of forefoot pain, such as metatarsophalangeal joint arthritis or intermetatarsal bursitis. The available evidence suggests that US is more accurate than MRI for diagnosis of MN. Analysis of pooled sensitivity showed similarity between US (90%) and MRI (93%), both being relatively high; yet, MRI (68%) showed a relatively lower specificity than US (88%) in the detection of MN[12]. Diameter of the normal plantar digital nerve is around 1 mm at the level of the intermetatarsal heads[13] and can be identified on high-resolution sonography and MRI (Figure 2).
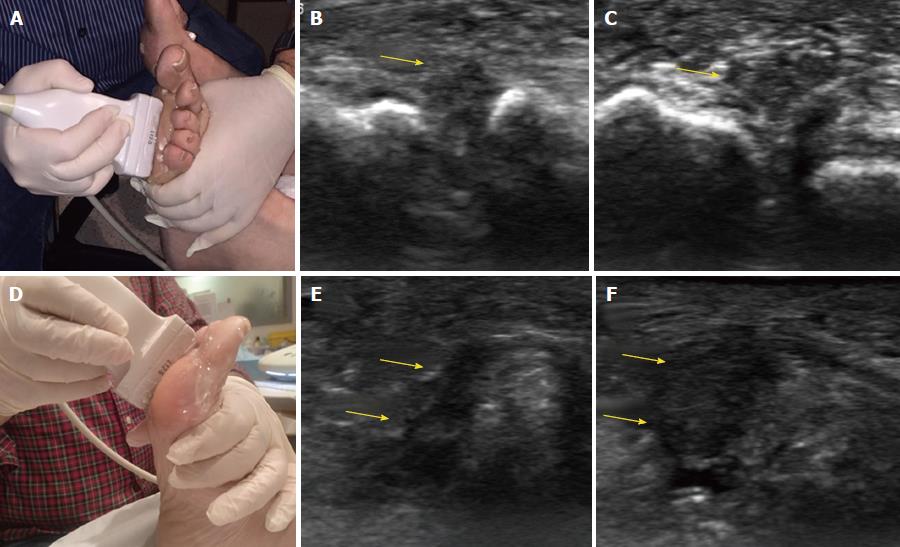
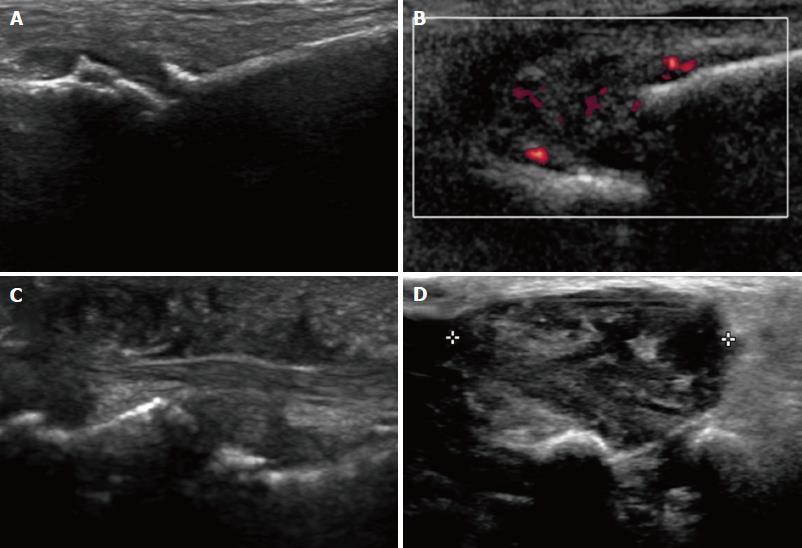
In US, MN is detected as a well-defined, round or ovoid mass in the short axis view and as an elongated/fusiform mass in the long axis view; it is hypoechoic relative to the adjacent tissues and located between the metatarsal heads along the plantar aspect of the intermetatarsal spaces[14] (Figure 3). Squeezing of the lateral aspect of the forefoot helps to extrude the intermetatarsal contents toward the plantar aspect of the forefoot, leading to a better delineation of the MN. This displacement may coincide with a palpable and/or audible click, known as the sonographic Mulder sign[15].
The extruding content may be formed by the MN and/or the bursa. It is theorized that the click will only happen when there is a MN because a thickened bursa is much more pliable than a neuroma[16]. As both entities may coexist, we can complete the US scan with another maneuver that will help in differentiating between MN and bursal tissue. The intermetatarsal space is compressed dorsally by the fingers whilst the probe is scanning along the plantar aspect of the web space in the long axis view. A MN will have a fusiform elongated shape and be displaceable, but it will not be as compressible as the intermetatarsal bursa. The bursa, located along the dorsal aspect of the intermetatarsal nerve, will show a more rounded or oval shape and will be compressible to a greater extent that the neural tissue (Figure 4).
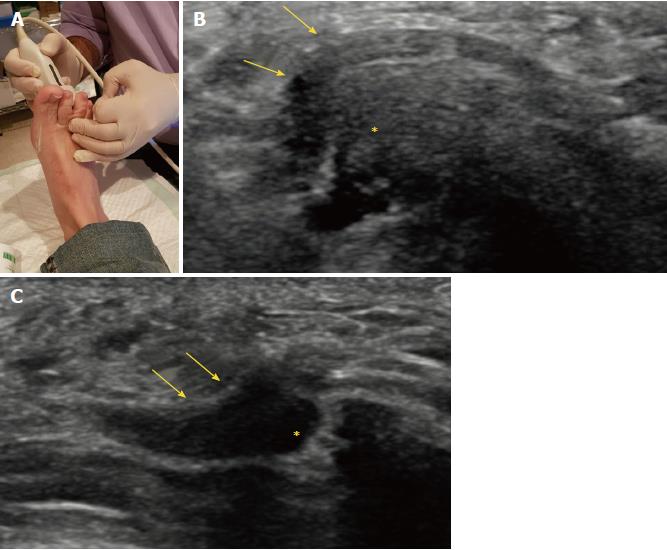
US may also detect other causes of forefoot pain, such as joint pathology (osteoarthritis or synovitis), soft tissue abnormalities (bursitis or inflammation-related change) and even bone lesions (stress fracture, bone erosion or cyst). Soft tissue masses different from MN can also be characterized, differentiating solid from cystic content, as well as the presence of neovascularity. Any intermetatarsal mass longer than 20 mm should raise suspicion that it is not a neuroma[17]. If appropriate diagnosis is not achieved with imaging features alone, biopsy under US guidance may help to obtain an accurate diagnosis (Figure 5).
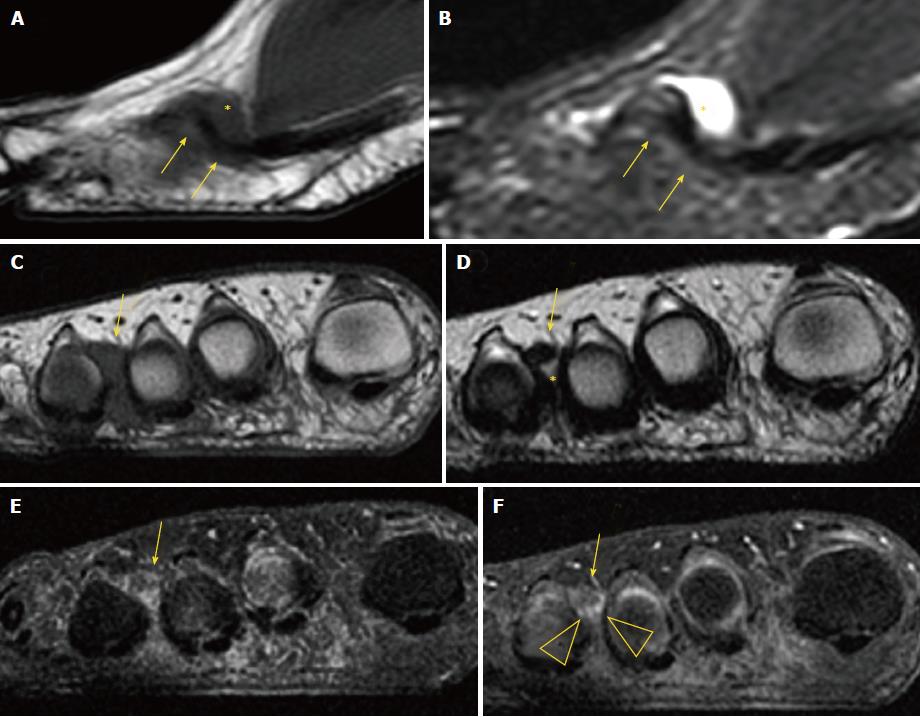
On MRI, MN is usually seen as a well-demarcated ovoid or dumbbell-shaped intermetatarsal mass. This mass shows intermediate to low signal intensity on both T1-weighted images (T1WI) and T2WI[18]. By contrast, bursal tissue appears hypo- or isointense on T1WI and hyperintense on T2WI, allowing for differentiation between bursal and neural tissues. There is no typical enhancement pattern of MN after intravenous administration of gadolinium contrast medium, with varying degrees of enhancement reported in the literature[19]; in our experience, however, the enhancement of most MN is low to zero. When present, this is usually due to the enhancement of the bursal tissue surrounding the neuroma. In the appropriate clinical setting, administration of gadolinium contrast medium is not required for a reliable diagnosis of MN (Figure 6).
MRI may also detect other causes of forefoot pain, including abnormalities of the bone (edema, fracture, erosion and cyst) and soft tissues (such as bursitis, synovitis, muscle edema and atrophy, and plantar plate tears) (Figure 7).
The treatment for MN should initially be conservative, including shoe modification, use of insoles and administration of antiinflammatory drugs. Percutaneous therapies may be the second step, and eventually surgery, if previous measures fail[2]. Success of conservative management approaches that include use of insoles and shoe modification has been reported to be around 48%[20].
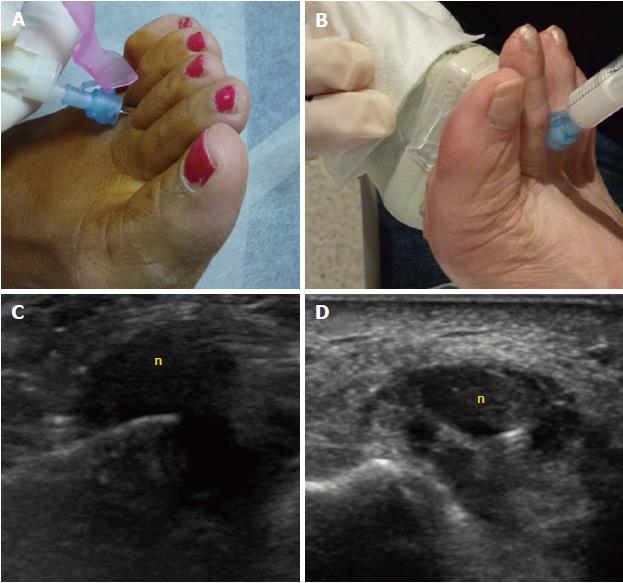
The percutaneous treatments mainly include injections of local anesthetics with or without steroids, or of alcohol. Percutaneous injection may be performed blindly or under US guidance (Figure 8). Studies of US-guided injections have shown that anesthetics with corticosteroids produce an effect (at 3 mo follow-up) that is superior to anesthetic injections alone[21]. Previous nonrandomized cohort studies have also shown a degree of improvement of about 45% for both blind and single US-guided injections[22], and of around 75% to 80% for blind multiple injections[23]. We only found one study comparing the blind technique, based on anatomical landmarks, to the echo-guided technique. In that study, no significant differences were found between the injection methods, but the sample size (only 36 cases) constitutes a substantial limitation that might have prevented the study from reaching a statistically significant difference[24].
Previous studies comparing modification of the forefoot loading area with injection of steroids have demonstrated greater pain improvement (at 6 mo follow-up) experienced by the steroid-treated group, but this difference disappeared at 1 year of follow-up[25].
The influence of neuroma size on treatment response to injections has also been debated. One study showed that neuromas less than 5 mm had a better response at 6 mo than those greater than 5 mm; at 12 mo, however, the response to injections was no longer significantly different for the two size groups[26]. A recent retrospective case series study found a cut-off value of 6.3 mm, above which larger MN s failed to respond, as well as to corticosteroid injections[27]. Overall, most of the reported data in the literature do not support any influence of neuroma size on the outcome[23,24,28].
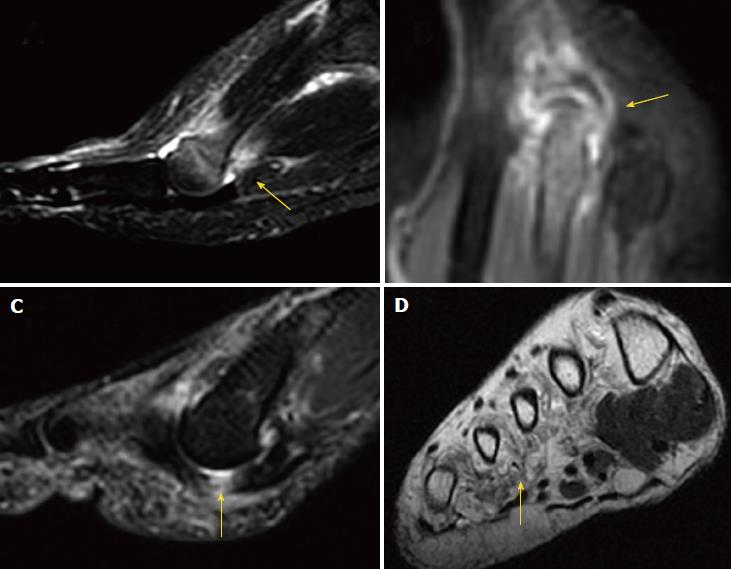
Corticosteroids may cause dermal/subcutaneous tissue atrophy, hypopigmentation and fat necrosis, with reported incidence of 1%-5%[21,29] (Figure 9). Particulate steroids have greater dermal and subcutaneous tissue atrophy. Triamcinolone, in particular, has greater risk for inducing dermal changes than methylprednisolone, due to its increased crystal size[29].
Other drugs used in MN treatment include alcohol, botulinum toxin and hyaluronic acid. Alcohol injection has a reported success range between 69%-84%, with a complication rate of 3%[30,31]. The most specific complication of this procedure has been plantar pain, presumed due to an inflammatory reaction secondary to perilesional leakage of the alcohol[30]. Case series using botulinum toxin[32] and hyaluronic acid[33] have shown clinical benefits of 70.6% and 84% respectively. Nevertheless, more studies are needed before considering these injections an alternative to the more traditional treatments.
Percutaneous radiofrequency ablation[34] has also been used in patients unresponsive to conservative therapies. The reported success with radiofrequency ranges from 68% to 100%, probably influenced by technical factors such as variability in the number of ablation cycles and in patient’s inclusion criteria[34,35]. A case series using cryoablation showed that 77.7% of patients were completely satisfied following treatment, but this result needs to be confirmed by further prospective studies in order to be considered a valid alternative to radiofrequency ablation[36]. The global rate of complications with radiofrequency ablation is about 5%, including hematoma and persistent pain, followed by temporary nerve irritation and infection, as well as pain and numbness at the injection site[31].
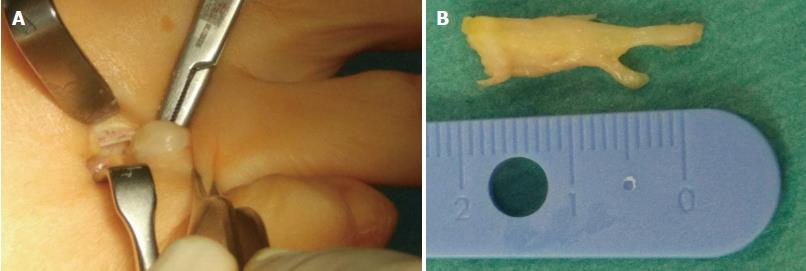
Surgical treatment is indicated after failure of conservative or percutaneous therapies. It consists of lesion excision (neurectomy) or intermetatarsal ligament resection (neurolysis) (Figure 10). Neurectomy may be performed either via a dorsal or plantar approach, according to surgeon preferences; no statistical differences in success rate have been reported. Surgical excision success ranges from 50% to 88%[31,37,38], with a complication rate of 25% for neurectomy and 7% for neurolysis, which would include infection, hematoma, hammertoe formation, hypertrophic or keloid scar formation, complex regional pain syndrome, and persistent postoperative pain, numbness and stiffness of the metatarsophalangeal joints. Recurrent painful neuromas after surgery have been described in 4% of cases and are less responsive to further surgical intervention; conservative measures would then have to be used prior to the consideration of further surgery[31,39].
A minimally invasive technique may be used for decompressing the nerve by division of the deep intermetatarsal ligament, either endoscopically or percutaneously. The reported success rate for decompression (sectioning of the deep transverse intermetatarsal ligament with/without osteotomy of the metatarsal heads) is around 94%, with a complication rate of 6%[31].
In summary, controversies still exist in the appropriate management of MN, including for the diagnosis and treatment algorithms. Although the clinical diagnosis of MN has a high accuracy, the coexistence of MN with many another causes of forefoot pain increases the importance of imaging techniques in achieving an accurate diagnosis, mainly when initial conservative measures fail to obtain any clinical improvement. Surgery should be considered when imaging findings support the clinical diagnosis of MN and when conservative measures including percutaneous treatment fail to improve the patient’s symptoms.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bazeed MF, Cerwenka H, Razek AAKA S- Editor: Ji FF L- Editor: A E- Editor: Song H
| 1. | Peters PG, Adams SB Jr, Schon LC. Interdigital neuralgia. Foot Ankle Clin. 2011;16:305-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Jain S, Mannan K. The diagnosis and management of Morton’s neuroma: a literature review. Foot Ankle Spec. 2013;6:307-317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Kasparek M, Schneider W. Surgical treatment of Morton’s neuroma: clinical results after open excision. Int Orthop. 2013;37:1857-1861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 4. | Jones JR, Klenerman L. A study of the communicating branch between the medial and lateral plantar nerves. Foot Ankle. 1984;4:313-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 5. | Mahadevan D, Venkatesan M, Bhatt R, Bhatia M. Diagnostic Accuracy of Clinical Tests for Morton’s Neuroma Compared With Ultrasonography. J Foot Ankle Surg. 2015;54:549-553. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 6. | Valero J, Gallart J, González D, Deus J, Lahoz M. Multiple interdigital neuromas: a retrospective study of 279 feet with 462 neuromas. J Foot Ankle Surg. 2015;54:320-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Levitsky KA, Alman BA, Jevsevar DS, Morehead J. Digital nerves of the foot: anatomic variations and implications regarding the pathogenesis of interdigital neuroma. Foot Ankle. 1993;14:208-214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Thompson FM, Deland JT. Occurrence of two interdigital neuromas in one foot. Foot Ankle. 1993;14:15-17. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Symeonidis PD, Iselin LD, Simmons N, Fowler S, Dracopoulos G, Stavrou P. Prevalence of interdigital nerve enlargements in an asymptomatic population. Foot Ankle Int. 2012;33:543-547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Bencardino J, Rosenberg ZS, Beltran J, Liu X, Marty-Delfaut E. Morton’s neuroma: is it always symptomatic? AJR Am J Roentgenol. 2000;175:649-653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Owens R, Gougoulias N, Guthrie H, Sakellariou A. Morton’s neuroma: clinical testing and imaging in 76 feet, compared to a control group. Foot Ankle Surg. 2011;17:197-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Xu Z, Duan X, Yu X, Wang H, Dong X, Xiang Z. The accuracy of ultrasonography and magnetic resonance imaging for the diagnosis of Morton’s neuroma: a systematic review. Clin Radiol. 2015;70:351-358. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Nissen KI. Plantar digital neuritis; Morton’s metatarsalgia. J Bone Joint Surg Br. 1948;30B:84-94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Bignotti B, Signori A, Sormani MP, Molfetta L, Martinoli C, Tagliafico A. Ultrasound versus magnetic resonance imaging for Morton neuroma: systematic review and meta-analysis. Eur Radiol. 2015;25:2254-2262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Torriani M, Kattapuram SV. Technical innovation. Dynamic sonography of the forefoot: The sonographic Mulder sign. AJR Am J Roentgenol. 2003;180:1121-1123. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Simmons DN. Imaging of the Painful Forefoot. Techniques in Foot Ankle Surgery. 2008;7:238-249. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Quinn TJ, Jacobson JA, Craig JG, van Holsbeeck MT. Sonography of Morton’s neuromas. AJR Am J Roentgenol. 2000;174:1723-1728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Weishaupt D, Treiber K, Kundert HP, Zollinger H, Vienne P, Hodler J, Willmann JK, Marincek B, Zanetti M. Morton neuroma: MR imaging in prone, supine, and upright weight-bearing body positions. Radiology. 2003;226:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Van Hul E, Vanhoenacker F, Van Dyck P, De Schepper A, Parizel PM. Pseudotumoural soft tissue lesions of the foot and ankle: a pictorial review. Insights Imaging. 2011;2:439-452. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kilmartin TE, Wallace WA. Effect of pronation and supination orthosis on Morton’s neuroma and lower extremity function. Foot Ankle Int. 1994;15:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Thomson CE, Beggs I, Martin DJ, McMillan D, Edwards RT, Russell D, Yeo ST, Russell IT, Gibson JN. Methylprednisolone injections for the treatment of Morton neuroma: a patient-blinded randomized trial. J Bone Joint Surg Am. 2013;95:790-798, S1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 22. | Hassouna H, Singh D, Taylor H, Johnson S. Ultrasound guided steroid injection in the treatment of interdigital neuralgia. Acta Orthop Belg. 2007;73:224-229. [PubMed] [Cited in This Article: ] |
| 23. | Greenfield J, Rea J Jr, Ilfeld FW. Morton’s interdigital neuroma. Indications for treatment by local injections versus surgery. Clin Orthop Relat Res. 1984;142-144. [PubMed] [Cited in This Article: ] |
| 24. | Mahadevan D, Attwal M, Bhatt R, Bhatia M. Corticosteroid injection for Morton’s neuroma with or without ultrasound guidance: a randomised controlled trial. Bone Joint J. 2016;98-B:498-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 25. | Saygi B, Yildirim Y, Saygi EK, Kara H, Esemenli T. Morton neuroma: comparative results of two conservative methods. Foot Ankle Int. 2005;26:556-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Makki D, Haddad BZ, Mahmood Z, Shahid MS, Pathak S, Garnham I. Efficacy of corticosteroid injection versus size of plantar interdigital neuroma. Foot Ankle Int. 2012;33:722-726. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Park YH, Lee JW, Choi GW, Kim HJ. Risk factors and the associated cutoff values for failure of corticosteroid injection in treatment of Morton’s neuroma. Int Orthop. 2018;42:323-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Markovic M, Crichton K, Read JW, Lam P, Slater HK. Effectiveness of ultrasound-guided corticosteroid injection in the treatment of Morton’s neuroma. Foot Ankle Int. 2008;29:483-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 29. | van Vendeloo SN, Ettema HB. Skin depigmentation along lymph vessels of the lower leg following local corticosteroid injection for interdigital neuroma. Foot Ankle Surg. 2016;22:139-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Hughes RJ, Ali K, Jones H, Kendall S, Connell DA. Treatment of Morton’s neuroma with alcohol injection under sonographic guidance: follow-up of 101 cases. AJR Am J Roentgenol. 2007;188:1535-1539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 31. | Valisena S, Petri GJ, Ferrero A. Treatment of Morton’s neuroma: A systematic review. Foot Ankle Surg. 2017;pii:S1268-7731(17)30065-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 32. | Climent JM, Mondéjar-Gómez F, Rodríguez-Ruiz C, Díaz-Llopis I, Gómez-Gallego D, Martín-Medina P. Treatment of Morton neuroma with botulinum toxin A: a pilot study. Clin Drug Investig. 2013;33:497-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 33. | Lee K, Hwang IY, Ryu CH, Lee JW, Kang SW. Ultrasound-Guided Hyaluronic Acid Injection for the Management of Morton’s Neuroma. Foot Ankle Int. 2018;39:201-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 34. | Masala S, Cuzzolino A, Morini M, Raguso M, Fiori R. Ultrasound-Guided Percutaneous Radiofrequency for the Treatment of Morton’s Neuroma. Cardiovasc Intervent Radiol. 2018;41:137-144. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Finney W, Wiener SN, Catanzariti F. Treatment of Morton’s neuroma using percutaneous electrocoagulation. J Am Podiatr Med Assoc. 1989;79:615-618. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 36. | Cazzato RL, Garnon J, Ramamurthy N, Tsoumakidou G, Caudrelier J, Thenint MA, Rao P, Koch G, Gangi A. Percutaneous MR-Guided Cryoablation of Morton’s Neuroma: Rationale and Technical Details After the First 20 Patients. Cardiovasc Intervent Radiol. 2016;39:1491-1498. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Mann RA, Reynolds JC. Interdigital neuroma--a critical clinical analysis. Foot Ankle. 1983;3:238-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 170] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Richardson DR, Dean EM. The recurrent Morton neuroma: what now? Foot Ankle Clin. 2014;19:437-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Johnson JE, Johnson KA, Unni KK. Persistent pain after excision of an interdigital neuroma. Results of reoperation. J Bone Joint Surg Am. 1988;70:651-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 136] [Article Influence: 3.8] [Reference Citation Analysis (0)] |