Published online Apr 28, 2013. doi: 10.4329/wjr.v5.i4.178
Revised: January 22, 2013
Accepted: February 2, 2013
Published online: April 28, 2013
AIM: To evaluate the role of thyroid blood flow assessment by color-flow Doppler ultrasonography in the differential diagnosis of thyrotoxicosis and compare it to technetium pertechnetate thyroid scanning.
METHODS: Twenty-six patients with thyrotoxicosis were included in the study. Clinical history was taken and physical examination and thyroid function tests were performed for all patients. Thyroid autoantibodies were measured. The thyroid glands of all patients were evaluated by gray scale ultrasonography for size, shape and echotexture. Color-flow Doppler ultrasonography of the thyroid tissue was performed and spectral flow analysis of both inferior thyroid arteries was assessed. Technetium99 pertechnetate scanning of the thyroid gland was done for all patients. According to thyroid scintigraphy, the patients were divided into two groups: 18 cases with Graves’ disease and 8 cases with Hashimoto’s thyroiditis. All patients had suppressed thyrotropin. The diagnosis of Graves’ disease and Hashimoto’s thyroiditis was supported by the clinical picture and follow up of patients.
RESULTS: Peak systolic velocities of the inferior thyroid arteries were significantly higher in patients with Graves’ disease than in patients with thyroiditis (P = 0.004 in the right inferior thyroid artery and P = 0.001 in left inferior thyroid artery). Color-flow Doppler ultrasonography parameters demonstrated a sensitivity of 88.9% and a specificity of 87.5% in the differential diagnosis of thyrotoxicosis.
CONCLUSION: Color Doppler flow of the inferior thyroid artery can be used in the differential diagnosis of thyrotoxicosis, especially when there is a contraindication of thyroid scintigraphy by radioactive material in some patients.
- Citation: Donkol RH, Nada AM, Boughattas S. Role of color Doppler in differentiation of Graves' disease and thyroiditis in thyrotoxicosis. World J Radiol 2013; 5(4): 178-183
- URL: https://www.wjgnet.com/1949-8470/full/v5/i4/178.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i4.178
Thyrotoxicosis refers to the hypercatabolic state resulting from elevated serum levels of thyroid hormone, mainly free tetraiodothyronine (FT) 4 and/or triiodothyronine FT3. Thyrotoxicosis is not synonymous with hyperthyroidism[1]. It may be caused either by hyperthyroidism or by inflammation of the thyroid with release of stored thyroid hormone but is not accelerated synthesis. It may also be caused by ingestion of exogenous thyroid hormone. Graves’ disease causes hyperthyroidism with diffuse thyroid disease while thyrotoxicosis due to destructive thyroiditis includes various subsets like lymphocytic thyroiditis, subacute thyroiditis and postpartum thyroiditis[2-5].
Differentiation between causes of thyrotoxicosis at time of diagnosis, either hyperthyroidism due to Graves’ disease or destructive thyrotoxicosis due to thyroiditis, is very important as management of each case is completely different. The absence of specific signs of Graves’ disease like ophthalmopathy, skin and nail changes may make it difficult to distinguish it from thyroiditis, especially when the disease is mild or subclinical. Thyroid scintigraphy by technetium99 (Tcm99) pertechnetate or iodine 123 radioisotopes is used for this purpose. Measuring thyrotropin (TSH) receptor antibody levels can be also used[6]. However, these methods are not usually available. Nuclear imaging is expensive and contraindicated during pregnancy and lactation.
Thyroid hypoechogenicity at ultrasound is a characteristic of autoimmune thyroid diseases, with an overlap of this echographic pattern in patients affected by Graves’ disease or Hashimoto’s thyroiditis. However, a diffusely increased thyroid blood flow is pathognomonic of untreated Graves’ disease and an abnormal color flow Doppler (CFD) pattern identifies the majority of Graves’ patients with a normal thyroid ultrasound pattern. Thus, CFD sonography may be useful in distinguishing patients with Graves’ disease and Hashimoto’s thyroiditis with a similar thyroid echographic pattern.
CFD ultrasonography is a useful, inexpensive, non-invasive and widely available method for measuring tissue vascularization and blood flow. The evaluation can be both qualitative (visual assessment of thyroid vascularity) and quantitative (measuring peak systolic, end diastolic and mean velocities in the inferior thyroid arteries). CFD ultrasonography of the thyroid gland can provide valuable information about underlying thyroid functional status and is useful in the differential diagnosis of thyrotoxicosis[7-10].
The aim of the study is to evaluate the efficiency of CFD in differentiation of causes of thyrotoxicosis at time of diagnosis, either hyperthyroidism due to Graves’ disease or destructive thyrotoxicosis due to thyroiditis, and compare its sensitivity and specificity to technetium thyroid scintigraphy to know if both investigations can be used as alternatives in cases of thyrotoxicosis.
The Research and Ethics committee of our hospital approved the study and written informed consent was acquired from all patients. The study population consisted of 26 patients presenting to the endocrine clinic with thyrotoxicosis during the period from January to July 2011. Exclusion criteria included toxic nodule, history of thyroid surgery, radioiodine therapy or radiation exposure to neck. Patients whose goiter was multinodular or diffuse were included in the study. Clinical history, including sex, age, symptoms and signs, was performed. Serum levels of TSH, free T3, free T4, antithyroid peroxidase and antithyroglobulin antibodies were measured in all patients.
Graves’ disease was diagnosed on the basis of clinical parameters (marked weight loss, adrenergic symptoms, goiter, skin and nail changes, eye signs) and high uptake on Tcm99 thyroid scanning. Thyroiditis was diagnosed on the basis of low Tcm99 uptake scan, the presence of insignificant symptoms (no or minimal weight loss, occasional palpitations, absent eye signs with or without goiter) or later development of hypothyroidism.
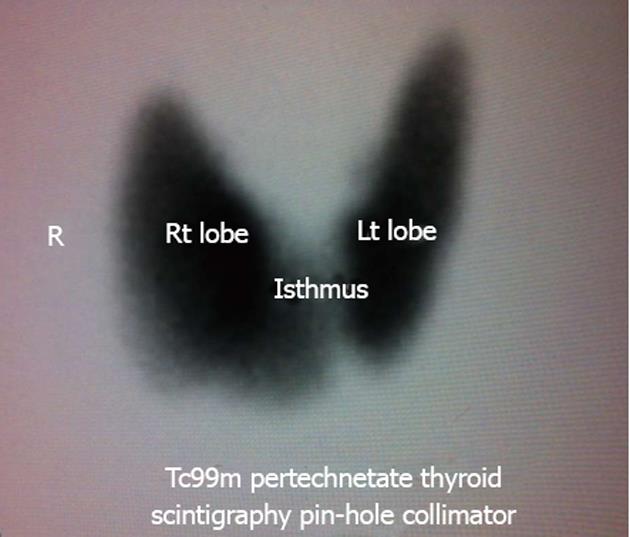
A radiologist with twenty years of experience in sonography, who was blinded to the full clinical status, performed all thyroid ultrasound examinations. A color Doppler ultrasound scanner (iU22, Philips Ultrasound, Bothell, WA, United States) equipped with a 3-9 MHz broadband linear array transducer was used. The grey scale ultrasound examinations of the thyroid gland were performed regarding the size, shape and echotexture of the gland, as well as the presence or absence of nodules (Figure 1A). The color Doppler pattern of the glands were studied (Figure 1B). Parameters for color Doppler are F. 6.6 MHz, G.76%, pulse-repetition frequency (PRF) 2.1 KHz and wall filter (WF) was M. The Doppler spectral analysis was of the right and left inferior thyroid arteries in the transverse scanning, in which the vessels crossed the common carotid arteries posteriorly, or in the longitudinal scanning of the ascending parts of the arteries, in which the vessels lie parallel to the common carotid arteries (Figure 1C). Parameters for color Doppler are F. 6.6 MHz, G.64%, PRF 5.6 KHz and WF 50 Hz. The angle correction cursor was parallel to the direction of flow and the Doppler angle was kept at or below 60°. The peak systolic velocity, end diastolic velocity and mean velocity were obtained. Peak systolic velocity of the inferior thyroid artery of 40 cm/s is considered significantly high and suggestive for Graves’ disease[11,12]. Another radiologist who was blinded to the clinical picture and ultrasound examination performed all isotopic thyroid scans (Figure 2). A technetium pertechnetate scan was done in all patients as the gold standard test for differentiation between both causes of thyrotoxicosis.
Frequency, arithmetic mean and standard deviation were used to present the data. Student’s t test was used as a test of significance at 5% level. Screening test evaluation was carried out with positive/negative outcomes, sensitivity, specificity, positive and negative predictive values and likelihood ratios for positive and negative tests were calculated with the concomitant 95%CIs.
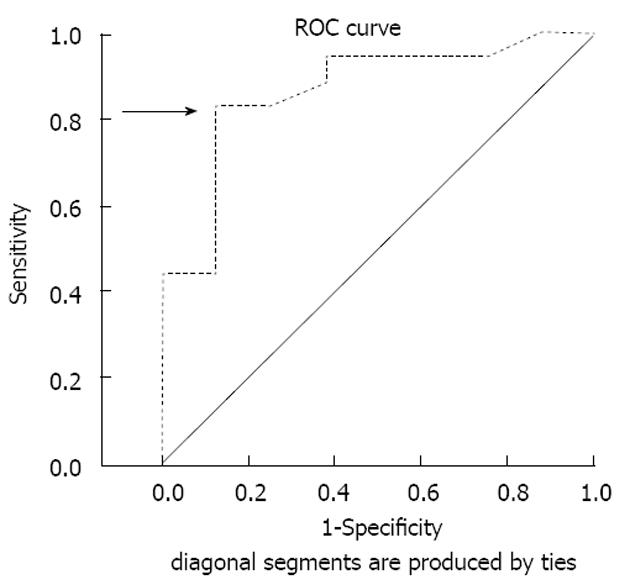
The McNemar test was applied to compare diagnostic performance of pulsed Doppler with the technetium scan for discriminating between Grave’s disease and thyroiditis. The power analysis and sample size calculation were performed based on the equation for sample size for two compared proportions with normal approximation to the binomial distribution to determine the minimal required sample size. Applying the “Shapiro-Wilk test” assessed the normal distribution of study variables. Receiver operating characteristic (ROC) analysis of the results was done to determine the appropriate cut-off value of peak systolic velocity to differentiate Graves’ disease from thyroiditis.
All patients who participated in this study have suppressed TSH level (0.08-0.005 IU/L) with normal or high free T4 and T3 levels. Thyroid scanning by Tcm99 was done for all patients as the gold standard test for differentiation between Graves’ disease and thyroiditis. Supported by the clinical picture of patients, eighteen patients had Graves’ disease and eight patients had destructive thyrotoxicosis. No significant difference in age between both groups (P = 0.565) was found.
Thyroid blood flow, as assessed by color flow imaging and Doppler spectral analysis of the inferior thyroid arteries, was significantly higher in patients with Graves’ disease than in patients with destructive thyroiditis (P = 0.004 in the right inferior thyroid artery and P = 0.001, in the left inferior thyroid artery). End diastolic velocity was significantly higher in Graves’ patients than in patients with thyroiditis (P = 0.007, in the right inferior thyroid artery and P = 0.001 in the left inferior thyroid artery). Consequently, mean velocity in the inferior thyroid artery was significantly higher in patients with Graves’ than in patients with thyroiditis (Table 1).
| Parameter | Graves | Thyroiditis | P value |
| Age, yr (mean ± SD) | 31.1 ± 8.4 | 33.1 ± 7.5 | 0.565 |
| Thyroid volume (cm3) | 24.2 ± 10.1 | 14.8 ± 7.54 | 0.028 |
| RPSV (cm/s) | 50.4 ± 23.4 | 21.7 ± 14.8 | 0.004 |
| REDV (cm/s) | 31.3 ± 16.6 | 12.8 ± 9.4 | 0.007 |
| RMV (cm/s) | 68.9 ± 31.6 | 30.6 ± 20.3 | 0.004 |
| LPSV (cm/s) | 49 ± 25.7 | 17.8 ± 6 | 0.001 |
| LEDV (cm/s) | 29.6 ± 17.5 | 10 ± 4.5 | 0.001 |
| LMV (cm/s) | 68.4 ± 35.2 | 25.5 ± 8.3 | 0.001 |
Sixteen out of 18 patients diagnosed as Graves’ disease by Tcm99 scan had an inferior thyroid artery flow velocity greater than 40 cm/s. Diagnosis of Graves’ disease in the remaining two patients was established by increased uptake on the thyroid scan and clinical findings that favor Graves’ disease. Seven out of 8 patients with destructive thyroiditis had an inferior thyroid artery flow less than 40 cm/s. The last patient was diagnosed as thyroiditis due to low Tcm99 uptake and by its clinical picture and follow up of patients.
Comparing volume of the thyroid gland between both groups revealed significantly larger volume in Graves’ patients than in patients with thyroiditis (P = 0.028) (Table 1).
CFD showed a sensitivity of 88.9% and a specificity of 87.5%, positive predictive value of 94.1%, negative predictive value of 77.8% and a diagnostic accuracy of 88.5% in the differential diagnosis of thyrotoxicosis compared to thyroid scanning by Tcm99 pertechnetate (Table 2). Also, the McNemar test result was significant (P = 0.453) and indicates that the two diagnostic tests (technetium scan and pulsed Doppler) are not significantly different with respect to sensitivity.
| Parameter | Estimate | Lower-upper 95%CI |
| Sensitivity | 88.9% | 67.2-96.9 |
| Specificity | 87.5% | 52.9-97.8 |
| Positive predictive value | 94.1% | 73.0-99 |
| Negative predictive value | 77.8% | 45.3-93.7 |
| Diagnostic accuracy | 88.5% | 71-96 |
| Likelihood ratio of a positive test | 7.1 | 0.99-51.3 |
| Likelihood ratio of a negative test | 0.1 | 0.05-0.4 |
| Diagnostic odds | 56 | 4.3-724.1 |
| Cohen's kappa (unweighted) | 0.7 | 0.4-1.1 |
The power analysis and sample size calculation were performed based on the equation for sample size for two compared proportions with normal approximation to the binomial distribution and b = 0.2, i.e., power = 0.8. Applying the “Shapiro-Wilk test” assessed the normal distribution of study variables and showed that most variables proved to be normally distributed, (e.g., volume of the thyroid gland P = 0.128, T3 serum level P = 0.076). ROC analysis of the results showed that cut-off value of 40-cm/s peak systolic velocity was considered significant to differentiate between Graves’ disease and thyroiditis (Figure 3).
Clinical manifestations of thyrotoxicosis in cases of thyroiditis and early or mild Graves’ disease may be difficult to differentiate. Although persistence of symptoms and signs in Graves’ disease can distinguish it from thyroiditis, it is very important to diagnose the disease early for the proper management. Isotope uptake scan of the thyroid is one of the definitive diagnostic tools, especially when there is clinical confusion between the two conditions. However, limited availability, high cost and contraindications to a radioisotope scan during pregnancy and lactation may restrict its application.
Although radioactive iodine is often useful in the diagnosis and treatment of thyrotoxicosis, such tests cannot be performed in many patients because of recent use of iodinated contrast for other diagnostic studies, such as computed tomography (CT) scanning which interfere with the accuracy of radioactive iodine tests. In their study, Phillips et al[13] found that 45% of patients with newly diagnosed thyrotoxicosis had received iodinated contrast within 2 wk before endocrinology evaluation; 43% had received iodine for CT and the other 2% for angiography.
In this study, Tcm99 pertechnetate was used as the definitive radiological investigation to differentiate the two types of thyrotoxicosis and thyroid blood flow was evaluated by color Doppler as a parameter to differentiate the types of thyrotoxicosis and compare its sensitivity and specificity to Tcm99 thyroid uptake.
Peak systolic, end diastolic and mean velocities of inferior thyroid artery in patients with Graves’ disease were significantly higher than patients with thyroiditis[14]. CFD ultrasonography in our study showed a sensitivity of 88.9% with specificity of 87.5%. These results are comparable to the results of a study carried out by Kurita et al[15] on 75 patients with thyrotoxicosis, which demonstrated that CFD ultrasonography had a sensitivity of 84% and specificity of 90% in the differential diagnosis of thyrotoxicosis.
On the other hand, Hari Kumar et al[16] in 2009 studied 65 patients with thyrotoxicosis. He found significantly higher blood flow in inferior thyroid arteries in Graves’ disease than in destructive thyrotoxicosis. He also demonstrated that CFD ultrasonography had a sensitivity of 96% and a specificity of 95% in the differential diagnosis of thyrotoxicosis.
Other forms of estimation of thyroid blood flow assessment, like thyroid blood flow area, vascularization index and high-resolution power Doppler, have been used by investigators to provide better differentiation[17-19]. A cut-off value of 40 cm/s was considered in differentiation between Graves’ diseases from thyroiditis based on a review of relevant literature[16,20-23]. This cut-off was also in agreement with obtained results from our own data, as ROC curve of different values of sensitivity and specificity justifies this decision.
Referring to this study and similar various studies[24-30], color Doppler ultrasonography of the thyroid gland is considered to be one of the initial investigations that can give great help in the differentiation of Graves’ disease and Hashimoto’s thyroiditis. Color Doppler is a cheap simple technique with no ionizing radiation exposure and is cost effective in the diagnosis of thyrotoxicosis[31-35].
Inferior thyroid artery blood flow is a useful parameter in the differential diagnosis of thyrotoxicosis. It has a sensitivity of 88.9% with a specificity of 87.5% in the differentiation between Graves’ disease and other forms of autoimmune thyroiditis. It is an acceptable alternative to radioisotope scans, especially when there is a contraindication to nuclear imaging of the thyroid. We recommend measurement of thyroid blood flow by Doppler as an essential part of initial investigations of thyrotoxicosis.
The authors acknowledge the great help of the laboratory team, radiology technicians, nursing staff and everybody who participated in the collaboration of this research.
Thyrotoxicosis may be caused either by hyperthyroidism of Graves’ disease or be due to destructive thyroiditis. Differentiation between causes of thyrotoxicosis at time of diagnosis is very important as management of each one is completely different. Thyroid scintigraphy is routinely used for this purpose but it is expensive and uses ionizing radiation.
Color flow Doppler (CFD) ultrasonography is a safe, inexpensive, non-invasive and widely available method for measuring thyroid vascularization and blood flow. It can provide valuable information to differentiate causes of thyrotoxicosis. In this study, the clinical, laboratory, thyroid scan and Doppler results of 26 patients with thyrotoxicosis are described and compared with the aim of evaluating the efficiency of CFD in the differentiation of causes of thyrotoxicosis and comparing its sensitivity and specificity to thyroid scintigraphy.
This study highlighted the usefulness of color Doppler in differentiating between the two common causes of thyrotoxicosis with a sensitivity of 88.9% and a specificity of 87.5%. The authors emphasized that color Doppler is an acceptable alternative to radioisotope scans in the diagnosis of thyrotoxicosis.
Color Doppler flow of the inferior thyroid artery can be used in the differential diagnosis of thyrotoxicosis, especially when there is contraindication of thyroid scintigraphy by radioactive material in some patients.
Thyrotoxicosis is a hypercatabolic state resulting from elevated serum levels of thyroid hormone. Graves’ disease is a diffuse thyroid disease with hyperthyroidism due to accelerated synthesis of thyroid hormones. Hashimoto’s disease is autoimmune inflammation of the thyroid with release of stored thyroid hormones.
This is an interesting paper addressing the role of color/pulsed Doppler for the differential diagnosis of thyrotoxicosis. This is a very careful and well thought out study of an important clinical problem. The study design, methods and data analysis are appropriate although the sample size is rather small and not well described. The authors’ conclusions are supported by the data and the manuscript is very well written.
P- Reviewers Juan A, Russell LD S- Editor Jiang L L- Editor Roemmele A E- Editor Xiong L
| 1. | Kittisupamongkol W. Hyperthyroidism or thyrotoxicosis? (NOVEMBER 2008). Cleve Clin J Med. 2009;76:152. [PubMed] [Cited in This Article: ] |
| 2. | De Waele S, Van den Bruel A, Selleslag D, Van Den Berghe I, Decallonne B. Acute thyrotoxicosis after SCT. Bone Marrow Transplant. 2009;43:663-664. [PubMed] [Cited in This Article: ] |
| 3. | Kasagi K, Hattori H. A case of destructive thyrotoxicosis induced by neck trauma. Thyroid. 2008;18:1333-1335. [PubMed] [Cited in This Article: ] |
| 4. | Motohashi K, Sakai R, Hagihara M, Enaka M, Kanamori H, Maruta A, Ishigatsubo Y. [Thyrotoxicosis after cord blood transplantation for acute myelogenous leukemia]. Rinsho Ketsueki. 2008;49:1631-1633. [PubMed] [Cited in This Article: ] |
| 5. | Romero-Rodríguez N, Cabeza Letrán ML, Villa Gil Ortega M, Ballesteros Pradas S. Thyrotoxicosis-induced vasospastic angina. Rev Esp Cardiol. 2008;61:1355-1356. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Amino N, Yabu Y, Miyai K. Differentiation of thyrotoxicosis induced by thyroid destruction from Graves’disease. Lancet. 1978;2:344-346. [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Vitti P, Rago T, Mazzeo S, Brogioni S, Lampis M, De Liperi A, Bartolozzi C, Pinchera A, Martino E. Thyroid blood flow evaluation by color-flow Doppler sonography distinguishes Graves’ disease from Hashimoto’s thyroiditis. J Endocrinol Invest. 1995;18:857-861. [PubMed] [Cited in This Article: ] |
| 8. | Ota H, Amino N, Morita S, Kobayashi K, Kubota S, Fukata S, Kamiyama N, Miyauchi A. Quantitative measurement of thyroid blood flow for differentiation of painless thyroiditis from Graves’ disease. Clin Endocrinol (Oxf). 2007;67:41-45. [PubMed] [Cited in This Article: ] |
| 9. | Erdoğan MF, Anil C, Cesur M, Başkal N, Erdoğan G. Color flow Doppler sonography for the etiologic diagnosis of hyperthyroidism. Thyroid. 2007;17:223-228. [PubMed] [Cited in This Article: ] |
| 10. | Vitti P, Rago T, Mazzeo S, Brogioni S, Lampis M, De Liperi A, Bartolozzi C, Pinchera A, Martino E. Thyroid blood flow evaluation by color-flow Doppler sonography distinguishes Graves' disease from Hashimoto's thyroiditis. J Endocrinol Invest. 1995;18:857-861. [PubMed] [Cited in This Article: ] |
| 11. | Macedo TA, Chammas MC, Jorge PT, Pereira de Souza L, Farage L, Pegoraro BL, Pessa SU, Cerri GG. Reference values for Doppler ultrasound parameters of the thyroid in a healthy iodine-non-deficient population. Br J Radiol. 2007;80:625-630. [PubMed] [Cited in This Article: ] |
| 12. | Sponza M, Fabris B, Bertolotto M, Ricci C, Armini L. [Role of Doppler color ultrasonography and of flowmetric analysis in the diagnosis and follow-up of Grave’s disease]. Radiol Med. 1997;93:405-409. [PubMed] [Cited in This Article: ] |
| 13. | Phillips BD, Hennessey JV. Iodinated contrast prior to evaluation for thyrotoxicosis. J Hosp Med. 2009;4:285-288. [PubMed] [Cited in This Article: ] |
| 14. | Bogazzi F, Bartalena L, Brogioni S, Burelli A, Manetti L, Tanda ML, Gasperi M, Martino E. Thyroid vascularity and blood flow are not dependent on serum thyroid hormone levels: studies in vivo by color flow doppler sonography. Eur J Endocrinol. 1999;140:452-456. [PubMed] [Cited in This Article: ] |
| 15. | Kurita S, Sakurai M, Kita Y, Ota T, Ando H, Kaneko S, Takamura T. Measurement of thyroid blood flow area is useful for diagnosing the cause of thyrotoxicosis. Thyroid. 2005;15:1249-1252. [PubMed] [Cited in This Article: ] |
| 16. | Hari Kumar KV, Pasupuleti V, Jayaraman M, Abhyuday V, Rayudu B R, Modi KD. Role of thyroid Doppler in differential diagnosis of thyrotoxicosis. Endocr Pract. 2009;15:6-9. [PubMed] [Cited in This Article: ] |
| 17. | Arslan H, Unal O, Algün E, Harman M, Sakarya ME. Power Doppler sonography in the diagnosis of Graves’ disease. Eur J Ultrasound. 2000;11:117-122. [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ralls PW, Mayekawa DS, Lee KP, Colletti PM, Radin DR, Boswell WD, Halls JM. Color-flow Doppler sonography in Graves disease: “thyroid inferno”. AJR Am J Roentgenol. 1988;150:781-784. [PubMed] [Cited in This Article: ] |
| 19. | Cappelli C, Pirola I, De Martino E, Agosti B, Delbarba A, Castellano M, Rosei EA. The role of imaging in Graves’ disease: a cost-effectiveness analysis. Eur J Radiol. 2008;65:99-103. [PubMed] [Cited in This Article: ] |
| 20. | Levine RA. Doppler ultrasound. Thyroid ultrasound and ultrasound-guided FNA. 2nd ed. New York: Springer; 2008; 27-43. [DOI] [Cited in This Article: ] |
| 21. | Tan GH, Gharib H, Reading CC. Solitary thyroid nodule. Comparison between palpation and ultrasonography. Arch Intern Med. 1995;155:2418-2423. [PubMed] [Cited in This Article: ] |
| 22. | Wiest PW, Hartshorne MF, Inskip PD, Crooks LA, Vela BS, Telepak RJ, Williamson MR, Blumhardt R, Bauman JM, Tekkel M. Thyroid palpation versus high-resolution thyroid ultrasonography in the detection of nodules. J Ultrasound Med. 1998;17:487-496. [PubMed] [Cited in This Article: ] |
| 23. | Gharib H, Papini E, Valcavi R, Baskin HJ, Crescenzi A, Dottorini ME, Duick DS, Guglielmi R, Hamilton CR, Zeiger MA. American Association of Clinical Endocrinologists and Associazione Medici Endocrinologi medical guidelines for clinical practice for the diagnosis and management of thyroid nodules. Endocr Pract. 2006;12:63-102. [PubMed] [Cited in This Article: ] |
| 24. | Bogazzi F, Vitti P. Could improved ultrasound and power Doppler replace thyroidal radioiodine uptake to assess thyroid disease? Nat Clin Pract Endocrinol Metab. 2008;4:70-71. [PubMed] [Cited in This Article: ] |
| 25. | Vlachopapadopoulou E, Thomas D, Karachaliou F, Chatzimarkou F, Memalai L, Vakaki M, Kaldrymides P, Michalacos S. Evolution of sonographic appearance of the thyroid gland in children with Hashimoto’s thyroiditis. J Pediatr Endocrinol Metab. 2009;22:339-344. [PubMed] [Cited in This Article: ] |
| 26. | Eaton SE, Euinton HA, Newman CM, Weetman AP, Bennet WM. Clinical experience of amiodarone-induced thyrotoxicosis over a 3-year period: role of colour-flow Doppler sonography. Clin Endocrinol (Oxf). 2002;56:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 103] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Macedo TA, Chammas MC, Jorge PT, Souza LP, Farage L, Watanabe T, Santos VA, Cerri GG. Differentiation between the two types of amiodarone-associated thyrotoxicosis using duplex and amplitude Doppler sonography. Acta Radiol. 2007;48:412-421. [PubMed] [Cited in This Article: ] |
| 28. | Saleh A, Furst G, Feldkamp J, Godehardt E, Grust A, Modder U. Estimation of antithyroid drug dose in Graves ‘ disease: value of quantification of thyroid blood flow with color duplex sonography. Ultrasound Med Biol. 2001;27:1137-1141. [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Wang CY, Chang TC. Thyroid Doppler ultrasonography and resistive index in the evaluation of the need for ablative or antithyroid drug therapy in Graves’ hyperthyroidism. J Formos Med Assoc. 2001;100:753-757. [PubMed] [Cited in This Article: ] |
| 30. | Nagasaki T, Inaba M, Kumeda Y, Fujiwara-Ueda M, Hiura Y, Nishizawa Y. Significance of thyroid blood flow as a predictor of methimazole sensitivity in untreated hyperthyroid patients with Graves’ disease. Biomed Pharmacother. 2007;61:472-476. [PubMed] [Cited in This Article: ] |
| 31. | Loy M, Perra E, Melis A, Cianchetti ME, Piga M, Serra A, Pinna G, Mariotti S. Color-flow Doppler sonography in the differential diagnosis and management of amiodarone-induced thyrotoxicosis. Acta Radiol. 2007;48:628-634. [PubMed] [Cited in This Article: ] |
| 32. | Markovic V, Eterovic D. Thyroid echogenicity predicts outcome of radioiodine therapy in patients with Graves’ disease. J Clin Endocrinol Metab. 2007;92:3547-3552. [PubMed] [Cited in This Article: ] |
| 33. | Cohen O, Pinhas-Hamiel O, Sivan E, Dolitski M, Lipitz S, Achiron R. Serial in utero ultrasonographic measurements of the fetal thyroid: a new complementary tool in the management of maternal hyperthyroidism in pregnancy. Prenat Diagn. 2003;23:740-742. [PubMed] [Cited in This Article: ] |
| 34. | Cappelli C, Castellano M, Pirola I, Cumetti D, Agosti B, Gandossi E, Agabiti Rosei E. The predictive value of ultrasound findings in the management of thyroid nodules. QJM. 2007;100:29-35. [PubMed] [Cited in This Article: ] |
| 35. | Solivetti FM, Bacaro D, Cecconi P, Baldelli R, Marandino F. Small hyperechogenic nodules in thyroiditis: usefulness of cytological characterization. J Exp Clin Cancer Res. 2004;23:433-435. [PubMed] [Cited in This Article: ] |