Published online Jun 28, 2014. doi: 10.4329/wjr.v6.i6.344
Revised: April 10, 2014
Accepted: May 14, 2014
Published online: June 28, 2014
Bladder-sparing strategy for muscle-invasive bladder cancer (MIBC) is increasingly demanded instead of radical cystectomy plus urinary diversion. Multimodal therapeutic approaches consisting of transurethral resection, chemotherapy, radiotherapy and/or partial cystectomy improve patients’ quality of life by preserving their native bladder and sexual function without compromising oncological outcomes. Because a favorable response to chemoradiotherapy (CRT) is a prerequisite for successful bladder preservation, predicting and monitoring therapeutic response is an essential part of this approach. Diffusion-weighted magnetic resonance imaging (DW-MRI) is a functional imaging technique increasingly applied to various types of cancers. Contrast in this imaging technique derives from differences in the motion of water molecules among tissues and this information is useful in assessing the biological behavior of cancers. Promising results in predicting and monitoring the response to CRT have been reported in several types of cancers. Recently, growing evidence has emerged showing that DW-MRI can serve as an imaging biomarker in the management of bladder cancer. The qualitative analysis of DW-MRI can be applied to detecting cancerous lesion and monitoring the response to CRT. Furthermore, the potential role of quantitative analysis by evaluating apparent diffusion coefficient values has been shown in characterizing bladder cancer for biological aggressiveness and sensitivity to CRT. DW-MRI is a potentially useful tool for the management of bladder cancer, particularly in multimodal bladder-sparing approaches for MIBC.
Core tip: Diffusion-weighted magnetic resonance imaging (DW-MRI) is a functional imaging increasingly applied in the management of bladder cancer. This imaging offers unique information reflecting physiological character of the tissues by quantifying the diffusion of water molecules. DW-MRI provides accurate information for the diagnosis of bladder cancer in a noninvasive manner. Furthermore, growing evidence has emerged showing that DW-MRI can serve as an imaging biomarker of bladder cancer for assessing biologic aggressiveness and therapeutic sensitivity and for monitoring the therapeutic response. This review focuses on the potential role of DW-MRI in multimodal organ-preservation strategies for bladder cancer.
- Citation: Yoshida S, Koga F, Kobayashi S, Tanaka H, Satoh S, Fujii Y, Kihara K. Diffusion-weighted magnetic resonance imaging in management of bladder cancer, particularly with multimodal bladder-sparing strategy. World J Radiol 2014; 6(6): 344-354
- URL: https://www.wjgnet.com/1949-8470/full/v6/i6/344.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i6.344
Bladder cancer is the second most common genitourinary cancer in the United States and some 55600 new cases and 15100 deaths from bladder cancer are estimated to have occurred in 2012[1]. At the initial diagnosis, a third of all cases are diagnosed as muscle-invasive bladder cancer (MIBC)[2], and radical cystectomy has long been the treatment of choice for the treatment of localized MIBC. However, concern for patients’ quality of life has strengthened the trend toward bladder-sparing approaches with various treatment modalities[3]. In this treatment approach, meticulous evaluation of the bladder cancer is essential. Diffusion-weighted magnetic resonance imaging (DW-MRI) is a functional imaging technique increasingly applied to various types of cancer. Recently, growing evidence has emerged showing that DW-MRI can serve as an imaging technique that is useful for characterizing the pathophysiology of cancer. The biological behavior assessed with this imaging technique will play an important role in multimodal organ-preserving strategies for MIBC. Thus, this review focuses on the potential role of DW-MRI in multimodal organ-preservation strategies for MIBC.
Favorable oncological and functional outcomes using bladder-sparing trimodality therapy combined with transurethral resection of bladder tumor (TURBT), chemotherapy and radiotherapy have been reported by several groups including Harvard University, the University of Paris and the University of Erlangen in Germany[4-6]. In most trimodality bladder-sparing approaches, patients who achieve complete response (CR) after the trimodal treatment are selectively subjected to consolidative therapies for bladder preservation, whereas those who do not achieve CR are advised to undergo early radical cystectomy. The 5-year survival rates after trimodality bladder-preserving trials were reported to be 50%-60%, which is comparable to those of radical cystectomy series[7,8].
In the trimodality bladder preservation strategies, clinically tumor-free status after TURBT followed by chemoradiotherapy (CRT), as well as lower T stage and completeness of the TURBT, are important prognostic factors[6,9-11]. However, even the patients who clinically achieved CR after TURBT followed by CRT still may develop local tumor recurrence and lymph node metastases. Zietman et al[12] reported that two-thirds of non-MIBC (NMIBC) recurrences developed in the original MIBC sites. Tunio et al[13] also showed that 21% of the MIBC patients who achieved CR after trimodality therapy developed MIBC recurrence, and 69% of the recurrences arose from the original MIBC site. This problem could be due, in part, to subclinical viable bladder cancer cells remaining in the original MIBC site, which were missed by conventional imaging studies and biopsy-based evaluation[14].
Contrast-enhanced CT and conventional MRI are the standard techniques that have been used for the radiological evaluation of urinary system tumors. While CT is generally used to screen for metastasis, MRI plays a pivotal role in the staging of bladder cancer because of its superior soft tissue delineation, especially in the context of muscle-invasion. The diagnostic accuracy of MRI in differentiating MIBC from NMIBC is reported to be 75%-92%[15,16]. However, these anatomical imaging techniques are not ideal for tissue characterization and assessing tumor aggressiveness. Furthermore, these anatomical imaging techniques often overestimate the extent of tumor after TURBT and CRT due to the post-treatment changes. In multimodal organ-preserving strategies, generally, prior to CRT, TURBT is performed for debulking of the tumor. Both TURBT and CRT can induce local fibrotic and inflammatory changes, both of which manifest as bladder wall thickening[17]. Additionally, after the combined therapy, bladder cancer may regress and present as a flat lesion. Therefore, anatomical assessment of therapeutic response based on the response evaluation criteria in solid tumors on T2WI is not appropriate for discriminating small remnants of cancerous tissue from these secondary changes. Dobson et al[18] showed the utility of dynamic contrast-enhanced (DCE) MRI for discriminating cancerous tissue from radiation-induced fibrosis in thickened bladder walls. However, inflammatory changes secondary to treatments may persist for many years[19]. These false-positive results on DCE are often problematic, and they lower its specificity for detecting residual bladder cancer[19]. Thus, the utility of T2WI and DCE is still limited in monitoring the therapeutic response after TURBT and CRT[20].
The DW-MRI technique was initially devised by Stejskal and Tanner in 1965. Since 1985, DW-MRI has been mainly used for neuroimaging, especially for diagnosis of acute cerebral infarction and intracranial tumors[21]. With the recent advent of echo planar imaging, high gradient amplitudes, multichannel coils, and parallel imaging, DW-MRI of the abdomen and pelvis has become possible, and a growing number of studies have demonstrated the usefulness of this imaging technique in the diagnosis of malignant tumors of the abdomen[22,23]. Because the signal of DW-MRI is derived from the inherent tissue contrast, this imaging technique requires no contrast agent and is applicable to patients with allergies to contrast agents or those with renal insufficiency. Furthermore, the addition of DW-MRI to a routine MRI examination requires only a few additional minutes and can be adopted for most current clinical MRI scanners.
DW-MRI is a functional imaging technique, the contrast of which results from quantifying the microscopic mobility of water molecules in tissue[22,23]. In biological tissues, the diffusion of water molecule is inversely correlated to the tissue cellularity and the integrity of cell membranes. In the area of tumor tissues, which have a high cellular density with intact cell membranes, water molecule diffusion is restricted, while the diffusion of water molecule is less restricted in areas of low cellular density. Areas where the diffusion is restricted generally show high signal intensity on DW-MRI, and malignant lesions typically show high signal intensity because of their higher cellularity, tissue disorganization, and decreased extracellular space, all of which restrict water diffusion. In recent years, an increasing number of studies have shown the usefulness of visual assessment of DW-MRI for detecting malignant tumors, and DW-MRI has quickly become a useful adjunct for assessing various types of tumors including bladder cancer[24-27].
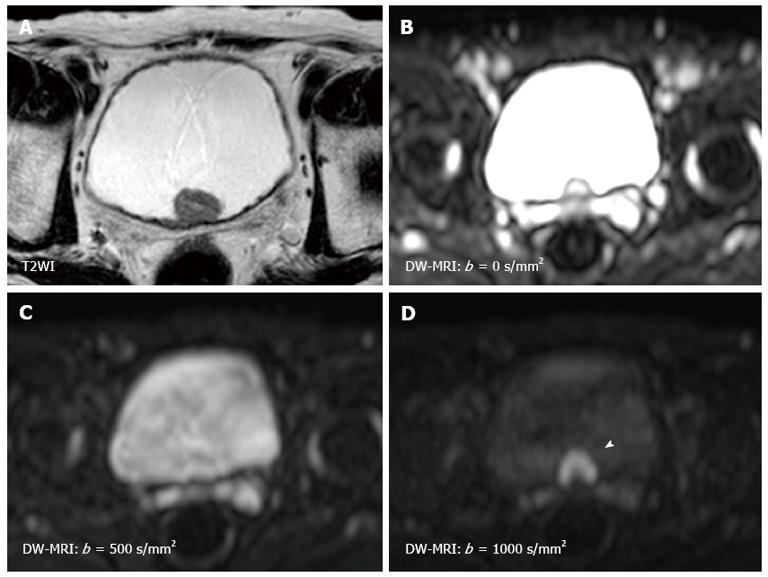
The sensitivity of the diffusion is varied by changing the “b-value” which is proportional to the gradient amplitude, the duration of the applied gradient, and the time interval between the paired gradients[22,23]. Small b-values attenuate the signals of water molecules with a large degree of motion or a great diffusion distance. By using higher b-values, the perfusion in the intra-vascular space is restricted and slow-moving water molecules or small diffusion distances can be distinguished (Figure 1). Therefore, DW-MRI should be performed using three or more b-values including b = 0 s/mm2, b≥ 100 s/mm2, and b≥ 500 s/mm2. Comparing the images obtained at different b-values is useful for characterizing the lesion. The apparent diffusion coefficient (ADC) value is assessed for quantitative evaluation of DW-MRI by evaluating the signal attenuation of tissue on DW-MRI with increasing b-values. Generally, the software automatically calculates the ADC values, and the calculated ADC values for each pixel of the image are displayed as a parametric map. By drawing regions of interests (ROI) on this ADC map, the ADC value of the delineated region can be easily obtained. However, because of their poor anatomical details, DW-MRI and ADC maps should be evaluated in combination with T1WI and T2WI, and the correlation with anatomical images is important to accurately set the ROI for the target lesion. Quantitative evaluation of DW-MRI by assessing the ADC value is potentially useful for tissue characterization based on the differences in water diffusion. The correlation of tumor ADC values with their biological aggressiveness has been reported for various types of malignancies[28-30]. However, the reproducibility of the ADC value is an intrinsic limitation in ADC measurement because the ADC value depends on the MRI system and imaging protocol used. To standardize the ADC assessment, some trials using ADC ratio, calculated with respect to surrounding normal tissues, have been performed recently.
The important clinical implication of DW-MRI in multimodal organ preservation strategies for MIBC is the ability to predict therapeutic response prior to treatment. In a number of prospective studies in various types of cancers including brain tumors and cervical and rectal cancers[31-35], the potential of DW-MRI to predict the sensitivity to radiotherapy has been shown. The tumors with higher ADC values are less likely to respond to the treatment. The hypothesized mechanism underlying this relationship is the presence of necrosis reflected in a higher ADC value, which predicts a poor outcome related to hypoxia-mediated radioresistance. Meanwhile, soon after the initiation of chemotherapy and/or radiotherapy, immediate cell death can be observed after the commencement of the treatment, which is reflected as an early increase in the ADC value. In cervical cancer and rectal cancer, this early increase in ADC value is observed in patients who show good response to CRT, and can be a potential early biomarker for treatment outcomes[35-38]. Following this early ADC increase, edema and fibrosis cause a subsequent ADC decrease[35-37].
Importantly, the DW-MRI can be an imaging biomarker in monitoring treatment effect. In response to successful treatment, cell necrosis and loss of cell membrane integrity are induced, leading to increased water diffusion. Furthermore, tumor apoptosis induced by treatment results in cell shrinkage. These changes are reflected by increases in ADC value[22]. Clinical studies in many types of malignancies, including liver cancer, cerebral gliomas, and soft-tissue sarcoma, have demonstrated the correlation between therapeutic effect and changes in water diffusion in tumors[39-41].
Since the first report by Matsuki et al[26] showing the utility of DW-MRI for detecting bladder cancer, a number of studies have shown the usefulness of DW-MRI for the diagnosis of bladder cancer[24-27]. On DW-MRI with a high b-value, bladder cancers generally show a hyperintense signal, while the signals of the surrounding tissues, including urine, are much less intense[26,42] (Figure 1). This good signal contrast is obtained between bladder cancer and the surrounding tissue. The sensitivity, specificity and accuracy for detecting bladder cancer were reported to be 90%-98%, 92%-93% and 91%-97%, respectively[24,25,27]. In several studies, quantitative analysis consistently showed restricted diffusion and lower ADC values in bladder cancer compared with the surrounding structures[26,42].
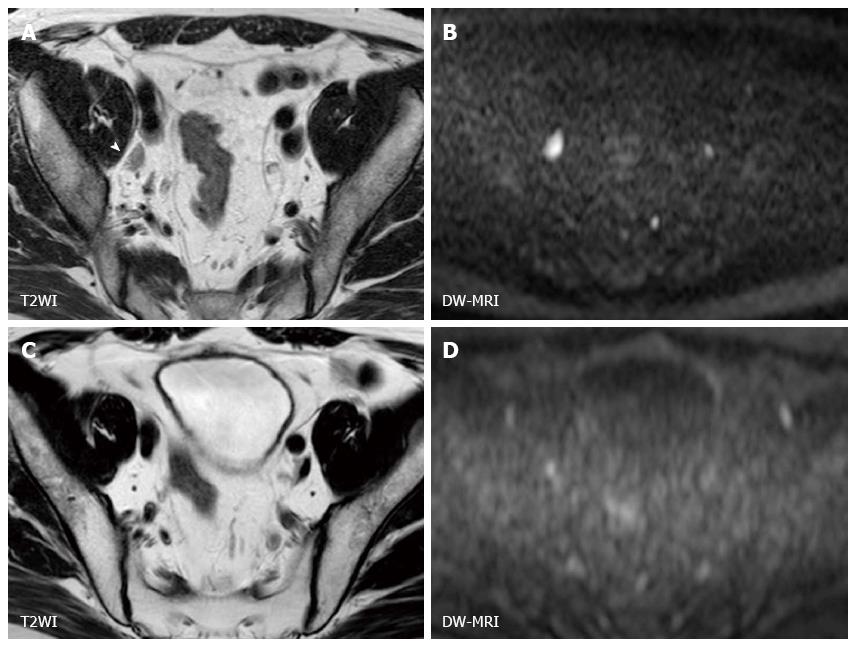
MIBC has the potential to metastasize to lymph nodes and distant organs, and detecting metastatic lesion is another problem in managing MIBC. At the time of surgery, 25% of the patients who undergo radical cystectomy have a lymph node metastasis. Lymph node staging has been generally performed by CT or conventional MRI based on size criteria and morphological appearance, and the accuracy for staging nodal disease ranges from 73% to 90%[43]. On DW-MRI, benign lymph nodes show high signal intensity due to their highly cellular structures composed of lymphoid elements (Figure 2). The utility of DW-MRI has been shown in lymph node staging in various cancers[44-48]. Papalia et al[49] showed that malignant lymph nodes have a significantly lower ADC value than benign lymph nodes with sensitivity of 76.4% and specificity of 89.4% in a study that included 36 patients with bladder cancer undergoing radical cystectomy. However, there is a substantial overlap in ADC values between malignant and benign lymph nodes, and discriminating malignant nodes from benign nodes on DW-MRI is still challenging[50]. Recently, Thoeny et al[51] reported an excellent diagnostic accuracy of 90% in detecting pelvic lymph nodal involvement by the combined use of ultra-small super paramagnetic iron oxide (USPIO) and DW-MRI. This agent is taken up by macrophages resulting signal loss in normal lymph nodes, while the signal of metastatic lymph nodes is not influenced[51-55]. Further studies are needed to confirm this encouraging result.
DW-MRI for evaluating primary bladder cancer occasionally shows abnormal signals of pelvic bones or femur heads. Bone metastasis typically shows clear high signal intensity on DW-MRI[56,57]. However, as well as benign bone tumors, hematopoietic bone marrow also appears as a hyperintense lesion on DW-MRI because of rich hematopoietic cells[58,59]. These false-positive findings in detecting metastasis should be kept in mind for staging bladder cancer[60]. Furthermore, identifying microscopic metastases or developing metastases remains a challenge, and a third of MIBC patients have undetected metastases at the initial diagnosis[61].
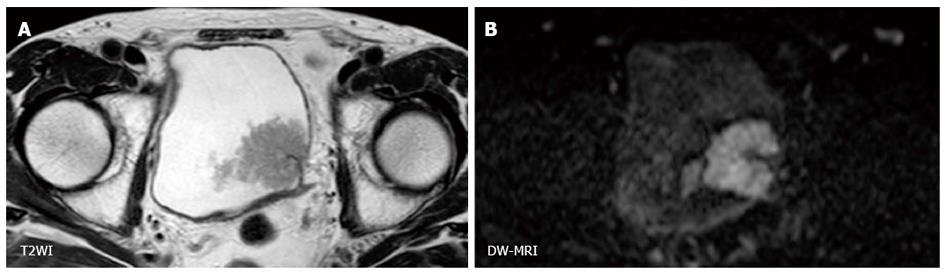
Because the contrast of DW-MRI is based on difference in the degree of water diffusion between tissues, the spatial resolution of DW-MRI is generally low. However, using the clear contrast between bladder cancer and the surrounding tissues, the utility of DW-MRI for staging of bladder cancer based on the signal shape and contrast has been shown (Figure 3). On DW-MRI, bladder cancers generally show a hyperintense signal in distinct contrast to the hypointense signal of the submucosal layer and the intermediate signal of the intact bladder wall. On the basis of these findings, El-Assmy et al[62] reported the ability to discriminate MIBC from NMIBC with an accuracy of 63.6% in a study that included 106 patients. Takeuchi et al[63] reported that bladder cancer staging accuracy improved from 67 to 88% when DW-MRI was added to T2WI.
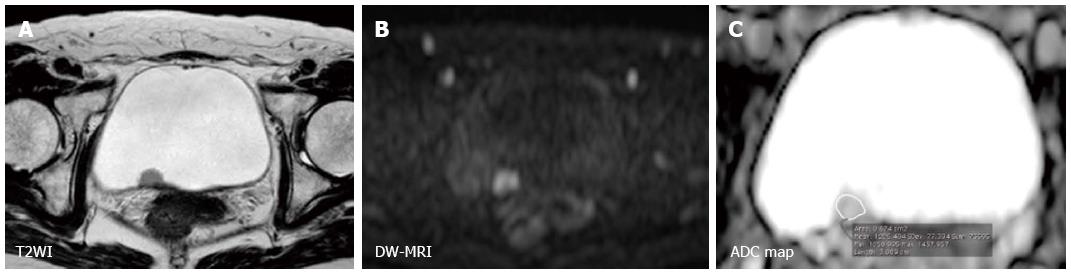
Furthermore, the utility of DW-MRI in characterizing bladder cancer has been consistently shown in multiple studies using quantitative analysis (Figures 4 and 5). Takeuchi et al[63] reported that the ADC value of grade 3 tumors was significantly lower than that of grade 1 and 2 tumors in a prospective study that included 40 patients. Avcu et al[64] also reported similar results showing an inverse correlation between the ADC value and the histological grade. The existence of a substantial overlap between the histological grades or stages poses a limit to qualitative analysis and the clinical application of this technique. However, these studies indicated that advanced and aggressive bladder cancers tend to have a low ADC values. Actually, Kobayashi et al[27] found that clinically aggressive tumors, including MIBC and high-grade T1 tumors, had a significantly lower ADC value than the other less aggressive tumors. A threshold ADC value differentiated these two entities with 87% accuracy in a series of 121 patients. The underlying mechanisms whereby the ADC value reflects these tumor characters are thought to be the tumor cell morphological characters such as dense cellularity and large cellular size[22,23]. Recent studies have shown an inverse correlation between ADC value and the Ki-67 labeling index, a marker of cell proliferation, in bladder cancer[65-67]. These data suggest the potential of ADC value to serve as a quantitative biomarker characterizing the biological features of bladder cancer.
The potential role of ADC values in predicting the metastatic potential of localized high-grade bladder cancers was shown in a small study that included 17 patients. This study showed that invasive high-grade bladder cancers with metastasis had lower ADC values than those without metastasis[68]. ADC value can be a supplemental parameter for predicting the presence of metastasis, which has a great impact on treatment decisions.
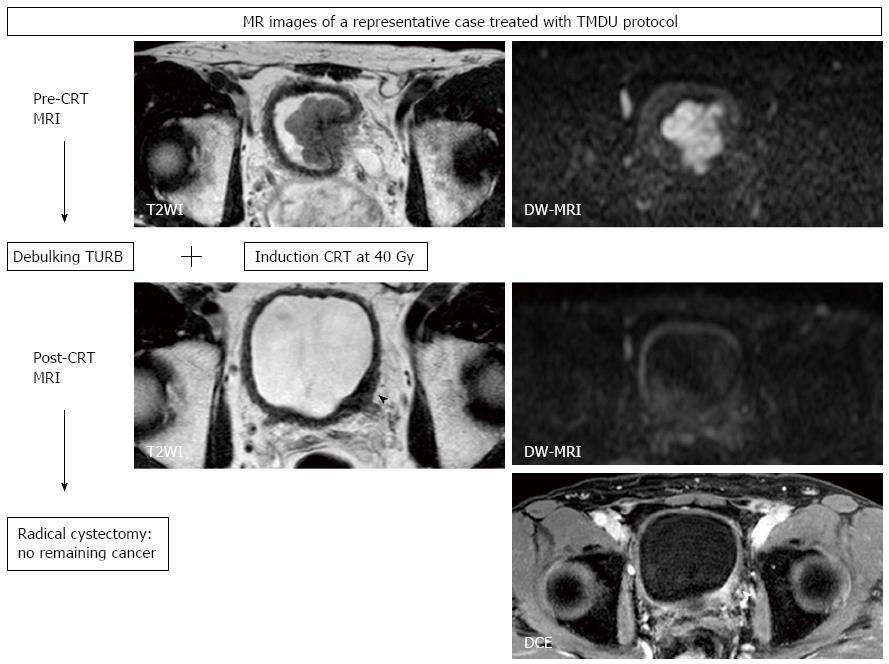
We started a pilot study of a selective bladder-sparing protocol incorporating consolidative partial cystectomy with pelvic lymph node dissection after induction low-dose chemoradiotherapy (LCRT) in 1997 at Tokyo Medical and Dental University (TMDU)[10,11,14,69-71]. Consolidative partial cystectomy with pelvic lymph node dissection is intended to eradicate possible remaining subclinical residual tumor tissue in the original MIBC sites and micrometastases in the pelvic lymph nodes. Candidates for bladder preservation are selected based on the extent, location, and post-LCRT status of the tumor. More than one-third of MIBC patients without any metastasis meet our criteria for partial cystectomy. Partial cystectomy with pelvic lymph node dissection was performed in 70 patients following LCRT. A functional native bladder was preserved in 91% of patients, and none has developed MIBC or lymph node recurrence[10,14].
In the majority of CRT-based bladder-sparing protocols for localized MIBC, patients who achieve a clinical CR are subjected to consolidative treatment with CRT for bladder preservation. In these protocols, treatment effect cannot be histologically evaluated. In the above-mentioned bladder-sparing protocol incorporating partial cystectomy, histopathological therapeutic effects of LCRT can be assessed, which is one of advantages of the TMDU protocol. By comparing DW-MRIs taken before and after LCRT with this therapeutic effect, the utility of DW-MRI for predicting treatment sensitivity and in monitoring therapeutic response can be evaluated[20,67].
We found a significant inverse correlation between LCRT sensitivity and ADC value of the tumor[67]. LCRT-sensitive MIBCs had significantly lower ADC values than LCRT-resistant MIBCs. With a defined cut-off ADC value, the sensitivity, specificity and accuracy in predicting LCRT sensitivity were 92%, 90%, and 91%, respectively. These findings are consistent with previous reports on other tumors including brain, cervix and rectum[31-35]. However, the presence of necrosis is not common in MIBC, which is understood to be the background of the correlation between lower ADC values and favorable CRT response. One possible explanation of this correlation found in MIBC is that the relationship between the proliferative activity and the ADC value of MIBC; highly proliferating MIBCs show low ADC values[65,67]. Because favorable CRT response in highly proliferating MIBC has been reported[72,73], a low ADC value would be predictive of a better CRT sensitivity of MIBC.
We also showed the utility of DW-MRI in monitoring the therapeutic response of MIBC treated with LCRT, as has been reported for other cancers. The sensitivity/specificity/accuracy of T2WI, DCE, and DW-MRI in predicting pathologic CR were 43%/45%/44%, 57%/18%/33%, and 57%/92%/80%, respectively[20]. DW-MRI improved the accuracy for detecting the remaining cancer after LCRT, primarily due to its increased specificity (Figure 6). However, the low sensitivity in detecting small lesions is a notable limitation, which makes it difficult to detect microscopic residual cancers, as is the case with the other imaging techniques. Further studies are necessary to evaluate the potential of DW-MRI as an imaging technique in the context of bladder-sparing approaches. Multiple approaches, including DW-MRI and biopsies to monitor the therapeutic response, may improve the accuracy of these techniques. However, the limits discussed here in detecting remaining cancers justify partial cystectomies to eliminate the possibly of remaining microscopic tumors in the original invasive cancer site, even in the patients who achieve clinical CR after CRT.
Recent studies have shown that the DW-MRI is a unique imaging technique that provides qualitative and quantitative information on biological features of bladder cancer, and is potentially useful as an imaging technique in the management of bladder cancer, particularly in multimodal bladder-sparing strategies for MIBC. Further large prospective studies are needed to clarify the practical roles of DW-MRI in the management of bladder cancer.
P- Reviewers: Msaouel P, Plataniotis G S- Editor: Ji FF L- Editor: A E- Editor: Zhang DN
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8406] [Cited by in F6Publishing: 8901] [Article Influence: 741.8] [Reference Citation Analysis (0)] |
| 2. | Tsukamoto T, Kitamura H, Takahashi A, Masumori N. Treatment of invasive bladder cancer: lessons from the past and perspective for the future. Jpn J Clin Oncol. 2004;34:295-306. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 3. | Gilbert SM, Wood DP, Dunn RL, Weizer AZ, Lee CT, Montie JE, Wei JT. Measuring health-related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI). Cancer. 2007;109:1756-1762. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Housset M, Maulard C, Chretien Y, Dufour B, Delanian S, Huart J, Colardelle F, Brunel P, Baillet F. Combined radiation and chemotherapy for invasive transitional-cell carcinoma of the bladder: a prospective study. J Clin Oncol. 1993;11:2150-2157. [PubMed] [Cited in This Article: ] |
| 5. | Kachnic LA, Kaufman DS, Heney NM, Althausen AF, Griffin PP, Zietman AL, Shipley WU. Bladder preservation by combined modality therapy for invasive bladder cancer. J Clin Oncol. 1997;15:1022-1029. [PubMed] [Cited in This Article: ] |
| 6. | Rödel C, Grabenbauer GG, Kühn R, Papadopoulos T, Dunst J, Meyer M, Schrott KM, Sauer R. Combined-modality treatment and selective organ preservation in invasive bladder cancer: long-term results. J Clin Oncol. 2002;20:3061-3071. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 471] [Cited by in F6Publishing: 439] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 7. | Eisenberg MS, Dorin RP, Bartsch G, Cai J, Miranda G, Skinner EC. Early complications of cystectomy after high dose pelvic radiation. J Urol. 2010;184:2264-2269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Stein JP, Lieskovsky G, Cote R, Groshen S, Feng AC, Boyd S, Skinner E, Bochner B, Thangathurai D, Mikhail M. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666-675. [PubMed] [Cited in This Article: ] |
| 9. | Perdonà S, Autorino R, Damiano R, De Sio M, Morrica B, Gallo L, Silvestro G, Farella A, De Placido S, Di Lorenzo G. Bladder-sparing, combined-modality approach for muscle-invasive bladder cancer: a multi-institutional, long-term experience. Cancer. 2008;112:75-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Koga F, Kihara K, Yoshida S, Yokoyama M, Saito K, Masuda H, Fujii Y, Kawakami S. Selective bladder-sparing protocol consisting of induction low-dose chemoradiotherapy plus partial cystectomy with pelvic lymph node dissection against muscle-invasive bladder cancer: oncological outcomes of the initial 46 patients. BJU Int. 2012;109:860-866. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Koga F, Yoshida S, Kawakami S, Kageyama Y, Yokoyama M, Saito K, Fujii Y, Kobayashi T, Kihara K. Low-dose chemoradiotherapy followed by partial or radical cystectomy against muscle-invasive bladder cancer: an intent-to-treat survival analysis. Urology. 2008;72:384-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Zietman AL, Grocela J, Zehr E, Kaufman DS, Young RH, Althausen AF, Heney NM, Shipley WU. Selective bladder conservation using transurethral resection, chemotherapy, and radiation: management and consequences of Ta, T1, and Tis recurrence within the retained bladder. Urology. 2001;58:380-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 13. | Tunio MA, Hashmi A, Qayyum A, Mohsin R, Zaeem A. Whole-pelvis or bladder-only chemoradiation for lymph node-negative invasive bladder cancer: single-institution experience. Int J Radiat Oncol Biol Phys. 2012;82:e457-e462. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 98] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 14. | Koga F, Kihara K. Selective bladder preservation with curative intent for muscle-invasive bladder cancer: a contemporary review. Int J Urol. 2012;19:388-401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 15. | Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, Thompson R, Bluemke D. Dynamic MRI of bladder cancer: evaluation of staging accuracy. AJR Am J Roentgenol. 2005;184:121-127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 16. | Hayashi N, Tochigi H, Shiraishi T, Takeda K, Kawamura J. A new staging criterion for bladder carcinoma using gadolinium-enhanced magnetic resonance imaging with an endorectal surface coil: a comparison with ultrasonography. BJU Int. 2000;85:32-36. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Raza SA, Jhaveri KS. MR imaging of urinary bladder carcinoma and beyond. Radiol Clin North Am. 2012;50:1085-1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 18. | Dobson MJ, Carrington BM, Collins CD, Ryder WD, Read G, Hutchinson CE, Hawnaur JM. The assessment of irradiated bladder carcinoma using dynamic contrast-enhanced MR imaging. Clin Radiol. 2001;56:94-98. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 19. | Johnson RJ, Carrington BM, Jenkins JP, Barnard RJ, Read G, Isherwood I. Accuracy in staging carcinoma of the bladder by magnetic resonance imaging. Clin Radiol. 1990;41:258-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Yoshida S, Koga F, Kawakami S, Ishii C, Tanaka H, Numao N, Sakai Y, Saito K, Masuda H, Fujii Y. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology. 2010;75:387-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M. MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology. 1986;161:401-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2776] [Cited by in F6Publishing: 2523] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 22. | Koh DM, Collins DJ. Diffusion-weighted MRI in the body: applications and challenges in oncology. AJR Am J Roentgenol. 2007;188:1622-1635. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1418] [Cited by in F6Publishing: 1392] [Article Influence: 81.9] [Reference Citation Analysis (0)] |
| 23. | Padhani AR, Liu G, Koh DM, Chenevert TL, Thoeny HC, Takahara T, Dzik-Jurasz A, Ross BD, Van Cauteren M, Collins D. Diffusion-weighted magnetic resonance imaging as a cancer biomarker: consensus and recommendations. Neoplasia. 2009;11:102-125. [PubMed] [Cited in This Article: ] |
| 24. | Abou-El-Ghar ME, El-Assmy A, Refaie HF, El-Diasty T. Bladder cancer: diagnosis with diffusion-weighted MR imaging in patients with gross hematuria. Radiology. 2009;251:415-421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Ceylan K, Taken K, Gecit I, Pirincci N, Gunes M, Tanik S, Karaman I. Comparison of cystoscopy with diffusion-weighted magnetic resonance images used in the diagnosis and follow-up of patients with bladder tumors. Asian Pac J Cancer Prev. 2010;11:1001-1004. [PubMed] [Cited in This Article: ] |
| 26. | Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17:201-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Kobayashi S, Koga F, Yoshida S, Masuda H, Ishii C, Tanaka H, Komai Y, Yokoyama M, Saito K, Fujii Y. Diagnostic performance of diffusion-weighted magnetic resonance imaging in bladder cancer: potential utility of apparent diffusion coefficient values as a biomarker to predict clinical aggressiveness. Eur Radiol. 2011;21:2178-2186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Costantini M, Belli P, Rinaldi P, Bufi E, Giardina G, Franceschini G, Petrone G, Bonomo L. Diffusion-weighted imaging in breast cancer: relationship between apparent diffusion coefficient and tumour aggressiveness. Clin Radiol. 2010;65:1005-1012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Yoshida S, Masuda H, Ishii C, Tanaka H, Fujii Y, Kawakami S, Kihara K. Usefulness of diffusion-weighted MRI in diagnosis of upper urinary tract cancer. AJR Am J Roentgenol. 2011;196:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Kitajima K, Takahashi S, Ueno Y, Miyake H, Fujisawa M, Kawakami F, Sugimura K. Do apparent diffusion coefficient (ADC) values obtained using high b-values with a 3-T MRI correlate better than a transrectal ultrasound (TRUS)-guided biopsy with true Gleason scores obtained from radical prostatectomy specimens for patients with prostate cancer? Eur J Radiol. 2013;82:1219-1226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958-965. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 185] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Bai R, Sun H, Liu H, Zhao X, Li Y. Diffusion-weighted imaging in predicting and monitoring the response of uterine cervical cancer to combined chemoradiation. Clin Radiol. 2009;64:1067-1074. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 110] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 33. | Mardor Y, Roth Y, Ochershvilli A, Spiegelmann R, Tichler T, Daniels D, Maier SE, Nissim O, Ram Z, Baram J. Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia. 2004;6:136-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 129] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 34. | Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307-308. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 380] [Cited by in F6Publishing: 403] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 35. | Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, Zhang XY. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 36. | Hein PA, Kremser C, Judmaier W, Griebel J, Pfeiffer KP, Kreczy A, Hug EB, Lukas P, DeVries AF. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol. 2003;45:214-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 123] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 37. | Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, de Vries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179:641-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 71] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 38. | Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111:213-220. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 191] [Cited by in F6Publishing: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 39. | Chen CY, Li CW, Kuo YT, Jaw TS, Wu DK, Jao JC, Hsu JS, Liu GC. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants--initial experience. Radiology. 2006;239:448-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 143] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 40. | Chenevert TL, McKeever PE, Ross BD. Monitoring early response of experimental brain tumors to therapy using diffusion magnetic resonance imaging. Clin Cancer Res. 1997;3:1457-1466. [PubMed] [Cited in This Article: ] |
| 41. | Einarsdóttir H, Karlsson M, Wejde J, Bauer HC. Diffusion-weighted MRI of soft tissue tumours. Eur Radiol. 2004;14:959-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 126] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | El-Assmy A, Abou-El-Ghar ME, Refaie HF, El-Diasty T. Diffusion-weighted MR imaging in diagnosis of superficial and invasive urinary bladder carcinoma: a preliminary prospective study. ScientificWorldJournal. 2008;8:364-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 43. | Barentsz JO, Jager GJ, van Vierzen PB, Witjes JA, Strijk SP, Peters H, Karssemeijer N, Ruijs SH. Staging urinary bladder cancer after transurethral biopsy: value of fast dynamic contrast-enhanced MR imaging. Radiology. 1996;201:185-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 144] [Cited by in F6Publishing: 151] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 44. | Beer AJ, Eiber M, Souvatzoglou M, Holzapfel K, Ganter C, Weirich G, Maurer T, Kübler H, Wester HJ, Gaa J. Restricted water diffusibility as measured by diffusion-weighted MR imaging and choline uptake in (11)C-choline PET/CT are correlated in pelvic lymph nodes in patients with prostate cancer. Mol Imaging Biol. 2011;13:352-361. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 45. | Budiharto T, Joniau S, Lerut E, Van den Bergh L, Mottaghy F, Deroose CM, Oyen R, Ameye F, Bogaerts K, Haustermans K. Prospective evaluation of 11C-choline positron emission tomography/computed tomography and diffusion-weighted magnetic resonance imaging for the nodal staging of prostate cancer with a high risk of lymph node metastases. Eur Urol. 2011;60:125-130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 46. | Eiber M, Beer AJ, Holzapfel K, Tauber R, Ganter C, Weirich G, Krause BJ, Rummeny EJ, Gaa J. Preliminary results for characterization of pelvic lymph nodes in patients with prostate cancer by diffusion-weighted MR-imaging. Invest Radiol. 2010;45:15-23. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 47. | Nakamatsu S, Matsusue E, Miyoshi H, Kakite S, Kaminou T, Ogawa T. Correlation of apparent diffusion coefficients measured by diffusion-weighted MR imaging and standardized uptake values from FDG PET/CT in metastatic neck lymph nodes of head and neck squamous cell carcinomas. Clin Imaging. 2012;36:90-97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Rechichi G, Galimberti S, Oriani M, Perego P, Valsecchi MG, Sironi S. ADC maps in the prediction of pelvic lymph nodal metastatic regions in endometrial cancer. Eur Radiol. 2013;23:65-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 49. | Papalia R, Simone G, Grasso R, Augelli R, Faiella E, Guaglianone S, Cazzato R, Del Vescovo R, Ferriero M, Zobel B. Diffusion-weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int. 2012;109:1031-1036. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 50. | Mir N, Sohaib SA, Collins D, Koh DM. Fusion of high b-value diffusion-weighted and T2-weighted MR images improves identification of lymph nodes in the pelvis. J Med Imaging Radiat Oncol. 2010;54:358-364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 51. | Thoeny HC, Triantafyllou M, Birkhaeuser FD, Froehlich JM, Tshering DW, Binser T, Fleischmann A, Vermathen P, Studer UE. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging reliably detect pelvic lymph node metastases in normal-sized nodes of bladder and prostate cancer patients. Eur Urol. 2009;55:761-769. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 195] [Cited by in F6Publishing: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 52. | Birkhäuser FD, Studer UE, Froehlich JM, Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A, Thoeny HC. Combined ultrasmall superparamagnetic particles of iron oxide-enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. 2013;64:953-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 53. | Fortuin AS, Meijer H, Thompson LC, Witjes JA, Barentsz JO. Ferumoxtran-10 ultrasmall superparamagnetic iron oxide-enhanced diffusion-weighted imaging magnetic resonance imaging for detection of metastases in normal-sized lymph nodes in patients with bladder and prostate cancer: do we enter the era after extended pelvic lymph node dissection? Eur Urol. 2013;64:961-93; discussion 963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Froehlich JM, Triantafyllou M, Fleischmann A, Vermathen P, Thalmann GN, Thoeny HC. Does quantification of USPIO uptake-related signal loss allow differentiation of benign and malignant normal-sized pelvic lymph nodes? Contrast Media Mol Imaging. 2012;7:346-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 55. | Triantafyllou M, Studer UE, Birkhäuser FD, Fleischmann A, Bains LJ, Petralia G, Christe A, Froehlich JM, Thoeny HC. Ultrasmall superparamagnetic particles of iron oxide allow for the detection of metastases in normal sized pelvic lymph nodes of patients with bladder and/or prostate cancer. Eur J Cancer. 2013;49:616-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 86] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 56. | Lecouvet FE, El Mouedden J, Collette L, Coche E, Danse E, Jamar F, Machiels JP, Vande Berg B, Omoumi P, Tombal B. Can whole-body magnetic resonance imaging with diffusion-weighted imaging replace Tc 99m bone scanning and computed tomography for single-step detection of metastases in patients with high-risk prostate cancer? Eur Urol. 2012;62:68-75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 57. | Mosavi F, Johansson S, Sandberg DT, Turesson I, Sörensen J, Ahlström H. Whole-body diffusion-weighted MRI compared with (18)F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am J Roentgenol. 2012;199:1114-1120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 86] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Ording Müller LS, Avenarius D, Olsen OE. High signal in bone marrow at diffusion-weighted imaging with body background suppression (DWIBS) in healthy children. Pediatr Radiol. 2011;41:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 59. | Steiner RM, Mitchell DG, Rao VM, Schweitzer ME. Magnetic resonance imaging of diffuse bone marrow disease. Radiol Clin North Am. 1993;31:383-409. [PubMed] [Cited in This Article: ] |
| 60. | Takeuchi M, Suzuki T, Sasaki S, Ito M, Hamamoto S, Kawai N, Kohri K, Hara M, Shibamoto Y. Clinicopathologic significance of high signal intensity on diffusion-weighted MR imaging in the ureter, urethra, prostate and bone of patients with bladder cancer. Acad Radiol. 2012;19:827-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | Prout GR, Griffin PP, Shipley WU. Bladder carcinoma as a systemic disease. Cancer. 1979;43:2532-2539. [PubMed] [Cited in This Article: ] |
| 62. | El-Assmy A, Abou-El-Ghar ME, Mosbah A, El-Nahas AR, Refaie HF, Hekal IA, El-Diasty T, Ibrahiem el H. Bladder tumour staging: comparison of diffusion- and T2-weighted MR imaging. Eur Radiol. 2009;19:1575-1581. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 120] [Cited by in F6Publishing: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 63. | Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, Suzuki K, Oshima H, Hara M, Shibamoto Y. Urinary bladder cancer: diffusion-weighted MR imaging--accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251:112-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 243] [Cited by in F6Publishing: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 64. | Avcu S, Koseoglu MN, Ceylan K, Bulut MD, Unal O. The value of diffusion-weighted MRI in the diagnosis of malignant and benign urinary bladder lesions. Br J Radiol. 2011;84:875-882. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 65. | Kobayashi S, Koga F, Kajino K, Yoshita S, Ishii C, Tanaka H, Saito K, Masuda H, Fujii Y, Yamada T. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging. 2014;39:172-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 66. | Yoshida S, Kobayashi S, Koga F, Ishioka J, Ishii C, Tanaka H, Nakanishi Y, Matsuoka Y, Numao N, Saito K. Apparent diffusion coefficient as a prognostic biomarker of upper urinary tract cancer: a preliminary report. Eur Radiol. 2013;23:2206-2214. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 67. | Yoshida S, Koga F, Kobayashi S, Ishii C, Tanaka H, Tanaka H, Komai Y, Saito K, Masuda H, Fujii Y. Role of diffusion-weighted magnetic resonance imaging in predicting sensitivity to chemoradiotherapy in muscle-invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2012;83:e21-e27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 68. | Rosenkrantz AB, Mussi TC, Spieler B, Melamed J, Taneja SS, Huang WC. High-grade bladder cancer: association of the apparent diffusion coefficient with metastatic disease: preliminary results. J Magn Reson Imaging. 2012;35:1478-1483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 69. | Yoshida S, Saito K, Koga F, Yokoyama M, Kageyama Y, Masuda H, Kobayashi T, Kawakami S, Kihara K. C-reactive protein level predicts prognosis in patients with muscle-invasive bladder cancer treated with chemoradiotherapy. BJU Int. 2008;101:978-981. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 70. | Kageyama Y, Okada Y, Arai G, Hyochi N, Suzuki M, Masuda H, Hayashi T, Kawakami S, Okuno T, Ishizaka K. Preoperative concurrent chemoradiotherapy against muscle-invasive bladder cancer: results of partial cystectomy in elderly or high-risk patients. Jpn J Clin Oncol. 2000;30:553-556. [PubMed] [Cited in This Article: ] |
| 71. | Kageyama Y, Yokoyama M, Sakai Y, Saito K, Koga F, Yano M, Arai G, Hyochi N, Masuda H, Fujii Y. Favorable outcome of preoperative low dose chemoradiotherapy against muscle-invasive bladder cancer. Am J Clin Oncol. 2003;26:504-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 72. | Matsumoto H, Wada T, Fukunaga K, Yoshihiro S, Matsuyama H, Naito K. Bax to Bcl-2 ratio and Ki-67 index are useful predictors of neoadjuvant chemoradiation therapy in bladder cancer. Jpn J Clin Oncol. 2004;34:124-130. [PubMed] [Cited in This Article: ] |
| 73. | Rödel C, Grabenbauer GG, Rödel F, Birkenhake S, Kühn R, Martus P, Zörcher T, Fürsich D, Papadopoulos T, Dunst J. Apoptosis, p53, bcl-2, and Ki-67 in invasive bladder carcinoma: possible predictors for response to radiochemotherapy and successful bladder preservation. Int J Radiat Oncol Biol Phys. 2000;46:1213-1221. [PubMed] [Cited in This Article: ] |