Published online Jul 28, 2014. doi: 10.4329/wjr.v6.i7.480
Revised: March 20, 2014
Accepted: May 16, 2014
Published online: July 28, 2014
Various single or multi-modality therapeutic options are available to treat pain of bone metastasis in patients with prostate cancer. Different radionuclides that emit β-rays such as 153Samarium and 89Strontium and achieve palliation are commercially available. In contrast to β-emitters, 223Radium as a α-emitter has a short path-length. The advantage of the α-emitter is thus a highly localized biological effect that is caused by radiation induced DNA double-strand breaks and subsequent cell killing and/or limited effectiveness of cellular repair mechanisms. Due to the limited range of the α-particles the bone surface to red bone marrow dose ratio is also lower for 223Radium which is expressed in a lower myelotoxicity. The α emitter 223Radium dichloride is the first radiopharmaceutical that significantly prolongs life in castrate resistant prostate cancer patients with wide-spread bone metastatic disease. In a phase III, randomized, double-blind, placebo-controlled study 921 patients with castration-resistant prostate cancer and bone metastases were randomly assigned. The analysis confirmed the 223Radium survival benefit compared to the placebo (median, 14.9 mo vs 11.3 mo; P < 0.001). In addition, the treatment results in pain palliation and thus, improved quality of life and a delay of skeletal related events. At the same time the toxicity profile of 223Radium was favourable. Since May 2013, 223Radium dichloride (Xofigo®) is approved by the US Food and Drug Administration.
Core tip: The incidence rate of prostate cancer worldwide is high. Ninety percent of patients dying of prostate cancer have bone metastases with varying symptoms which are significantly impairing their quality of life. 223Radium is the first therapeutic that results in a survival benefit for patients with bone metastatic, castrate resistant prostate cancer. 223Radium was also associated with low myelosuppression rates and fewer adverse events.This article provides an overview of the pre-clinical and clinical trials with 223Radium.
- Citation: Wieder HA, Lassmann M, Allen-Auerbach MS, Czernin J, Herrmann K. Clinical use of bone-targeting radiopharmaceuticals with focus on alpha-emitters. World J Radiol 2014; 6(7): 480-485
- URL: https://www.wjgnet.com/1949-8470/full/v6/i7/480.htm
- DOI: https://dx.doi.org/10.4329/wjr.v6.i7.480
According to estimates from the International Agency for Research on Cancer (IARC, GLOBOCAN 2008), the incidence rate of prostate cancer worldwide is 13.6 per 100000 inhabitants per year, with a mortality of 6.1 per 100000 per year[1]. The incidence rate differs quite significantly among the various regions of the world. It is lowest in Central Asia with 4.1 per 100000 and highest in Australia/New Zealand with 104 per 100000[1]. Ninety% of patients dying of prostate cancer have bone metastases[2]. Patients with bone metastases have varying symptoms such as pain, pathological fractures, neurological disorders, spinal cord compression, and bone marrow failure, which are significantly impairing their quality of life[3,4].
An optimal therapy leads to pain reduction, improved quality of life, and prolonged survival. Various single or multi-modality therapeutic options are available to treat bone pain. These include analgesics, hormone therapy, chemotherapy, external beam radiation, biphosphonates, or β-emitting radionuclides.
Combined pain medication and external radiotherapy result in pain relief in up to 70% of patients with localized pain[5]. However, external radiotherapy is only possible to a limited extent in patients with multiple bone metastases and diffuse bone pain.
Different radionuclides that emit β-rays such as 153Samarium and 89Strontium and achieve palliation are commercially available. 89Strontium is a pure β-emitter with a relatively long half-life of 50.5 d. 153Samarium has a shorter half-life of 1.9 d and emits β-rays in addition to γ-rays (Table 1). Thus, imaging of the skeletal samarium distribution post therapy is feasible. In more than half of the cases, administration of these radiopharmaceuticals results in a decrease of pain[6-8]. Yet, an effect of this therapy on patient survival is not investigated in randomised phase III studies.
| Radionuclide | Half-life | Maximum energy (MeV) | Mean energy (MeV) | Maximum range | y-Emission (keV) |
| 89Sr | 50.5 d | 1.4 (β) | 0.583 (β) | 7 mm | None |
| 153Sm | 1.9 d | 0.81 (β) | 0.229 (β) | 4 mm | 103 |
| 223Ra | 11.4 d | 5.78 (α) average | - | < 10 μm | 154 |
β-emitters with a low linear energy transfer (LET) and a long β-range can lead to a high radiation burden of the bone marrow and thus carry the risk of a significant myelosuppression. This bone marrow suppression may be dose limiting.
In contrast to β-emitters, α-emitters have a short path-length of less than 0.1 mm which increases the local anti-tumour effect without affecting the bone marrow. The α-emitter 223Radium dichloride is the first radiopharmaceutical that significantly prolongs life in castrate resistant prostate cancer patients with wide-spread bone metastatic disease. Radium-223 has been developed by the Norwegian company Algeta ASA, in a partnership with Bayer, under the trade name Xofigo®. 223Radium dichloride is approved by the US Food and Drug Administration (FDA) and by the European Commission (EC).
This article provides an overview of the pre-clinical and clinical trials with 223Radium.
Lassmann et al[9] provided a comprehensive dosimetry calculation of absorbed organ doses after intravenous administration of 223Radium chloride for 25 organs or tissues. Bone surface and red bone marrow show the highest dose coefficients followed by liver, colon, and intestines. Six cycles of 223Radium at 0.05 MBq/kg[10] (corresponding to 21 MBq for a 70 kg patient), the absorbed a dose to the bone surface was calculated at around 16 Gy with a dose of approximately 1.5 Gy to the bone marrow. Patient-specific dosimetry data have not been published yet.
Radium was discovered in December 1898 by the physicist Marie Curie and her husband Pierre Curie. 223Radium decays originates from uranium and has a natural decay balance with uranium. The α-emitter 223Radium is water soluble as 223Radium chloride. 223Radium can be relatively easily gained from 227Actinium through a cation exchange system. Due to the long half-life of 227Actinium (21.7 years), it could potentially be used as a long-term generator.
223Radium has a half-life of 11.4 d (Table 1) and decays via seven daughter nuclides into stable 207Lead-207. The half-life of the daughter nuclides ranges from seconds to minutes. During the decay of 223Radium, approximately four α particles and two β particles (electrons) are released. The combined energy of the particles emitted during the decay chain of 223Radium and its daughter nuclides is 27.5 MeV, with α-particles emitting 95.3% of the energy and β-particles emitting 3.6%. One point one percent are emitted as gamma rays.
Because of the electric charge and the relatively high mass of 4u, α-particles have a very low penetration depth in organic matter which ranges from 40 to 100 μm which approximately equals the size of micro metastases. α-particles produce high-linear energy-transfer (LET) radiation. The advantage of the α-emitter is thus a highly localized biological effect that is caused by radiation induced DNA double-strand breaks and subsequent cell killing and/or limited effectiveness of cellular repair mechanisms.
The penetration depth into the surrounding tissue of the β-particles is higher than with 223Radium (Table 1). Due to the limited range of the α-particles the bone surface to red bone marrow dose ratio is also lower for 223Radium which is expressed in a lower myelotoxicity of 223Radium as compared to the “traditional” radiopharmaceuticals.
As a calcium analogue, 223Radium dichloride is absorbed by the bone after intravenous injection without the necessity of a carrier. Initially, approximately 25% of the injected 223Radium dichloride is bound to the bone surface and from there quickly absorbed into the bone volume or returned into blood[9]. Eighty percent of the activity is transferred from the exchangeable bone volume back to the bone surface at a biological half-life of 30 d. The amount of 223Radium dichloride that the bone absorbs depends on the regional bone metabolism. The target of 223Radium dichloride in the bone is calcium hydroxylapatite. The radiopharmaceutical accumulates in regions of osteoblast activity, therefore allowing the simultaneous treatment of multiple bone metastases. In addition to the bones 223Radium dichloride is mainly absorbed from blood into soft tissue, including the liver. Its excretion is predominantly via the intestines, i.e., the feces. Renal excretion is minimal. In contrast, 153Samarium and 89Strontium undergo predominantly renal excretion thereby increasing the probability of renal toxicity.
Because of its very limited tissue penetration the environmental risk of 223Radium application is minimal if existent at all. Thus, 223Radium dichloride can be administered safely in an outpatient setting.
In animal experimental studies, 223Radium had the same bone distribution as 89Strontium which suggested that a therapeutically relevant dose of 223Radium could be applied to bone metastases[11]. In addition, 223Radium had a lower myelotoxicity than β-emitters[11]. Rats which received chemotherapy and had biphosphonate resistant bone metastases, showed a longer survival rate when they were treated with 223Radium[12]. This suggested that 223Radium may not only be used for palliation but may in fact prolong life.
The pharmacokinetics, pharmacodynamics, and biodistribution were investigated in a phase I study in 10 patients with bone metastatic prostate cancer[13]. Three patients received injections of 50 kBq/kg radium, another three received 100 kBq/kg radium, and 4 patients received 250 kBq/kg. After 6 wk, 6 of the 10 patients had another injection of 50 kBq/kg. A rapid clearance of 223Radium from the blood was observed whereby only 0.5% of 223Radium remained in the blood after 24 h. On average, 52% of the 223Radium was cleared via the intestines, which was the main route of excretion of 223Radium. The excretion via the kidneys was relatively low with a mean of 4%. No dose limiting toxicity could be verified.
In another phase I study, a total of 25 patients with bone metastatic prostate cancer (n = 15) and breast cancer (n = 10) received a single dose of 250 kBq/kg 223Radium (46, 93, 163, 213, or 250 kBq/kg)[14]. The goal of this dose escalation study was to investigate the safety profile and the pain response to 223Radium. The pain scale was documented before the first injection and at 1, 4, and 8 wk after the injection. In addition, in 6 patients the distribution of the daughter nuclide 219Radium was imaged with a gamma camera and compared to the pre-therapeutic bone scintigraphy.
The patients exhibited mild and reversible myelosuppression. In one patient, a grade 1 thrombocytopenia was observed; 2 patients had a grade 3 neutropenia, and 3 patients showed a grade 3 leukopenia. Four weeks after the injection of 223Radium a pain reduction was observed in most patients (60%). 24 h after the injection, the activity of 223Radium rapidly decreased to below 1% (redundant). Thus, 223Radium was well tolerated at therapeutically relevant doses. The pre- and post-therapeutic gamma camera images showed a good correlation with the 223Radium accumulation in bone metastases.
In a randomized, double-blind multicentre phase II study the effect of the repeated administration of 223Radium was investigated in patients with symptomatic hormone refractory metastatic prostate cancer. Inclusion criteria were multiple bone metastases or a painful osseous lesion with two consecutive rising PSA values[15]. The endpoints of the study were the efficacy of 223Radium with respect to the decrease of bone-specific alkaline phosphatase (ALK) concentration and the time to the occurrence of a skeletal event. All patients also underwent external beam radiation. Sixty-four patients were recruited of whom 33 received external beam radiation and 223Radium while 31 received external beam radiation and placebo (saline). Patients received up to 4 injections of 50 kBq/kg 223Radium or placebo at intervals of 4 wk. Eight patients in the 223Radium group and 21 patients in the placebo group completed the protocol. The study demonstrated an excellent safety profile for 223Radium. There were no differences in haemotoxicity between the groups and no patient in the 223Radium group terminated the study due to treatment related toxic effects. In the 223Radium group, 3 patients had a grade 2/3 neutropenia, which, however, was reversible.
In addition, there was evidence of biologic effects and efficacy with 223Radium. Patients in the 223Radium arm had a significantly greater reduction in bone-ALP (-65.6%, P < 0.0001) than those in the placebo group (-9.3%). The median time to skeletal related events was 124 wk in the 223Radium group vs 11 wk in the placebo arm. The median time until PSA progression was 126 wk in the 223Radium group vs 8 wk in the placebo group (P = 0.048). Four weeks after the last injection, the median relative change of the PSA was -23.8% in the 223Radium arm, vs +44.9% in the placebo group (P = 0.003). Importantly, there was a trend toward improved survival in the 223Radium group (65.3 wk) vs the placebo group (46.4 wk; P = 0.066). Thus, 223Radium was tolerated, reduced serum ALP levels and tended to improve survival. In an additional study, the same authors published the 24 mo follow-up of the patients and confirmed the results of the previous study[16]. They confirmed the excellent safety profile and demonstrated no increased risk for a secondary malignancy. A trend towards improved survival was again demonstrated (P = 0.056).
The effect of different dosages of 223Radium on the pain reduction was investigated in another randomized and double-blinded study, involving 100 patients with castration-resistant, bone metastatic prostate cancer[17]. More than 50% of the patients had 20 or more metastases, or a superscan. The patients received single doses of 5, 25, 50, or 100 kBq/kg 223Radium. Patients were classified as responders or non-responders using a bone pain index. Already after 2 wk, a significant pain reduction was evident (P = 0.35). After 8 wk, 40%, 63%, 56%, and 71% of the patients were classified as responders in the 5, 25, 50, and 100 kBq/kg groups, respectively. In the group of responders the pain was reduced by a mean of -30, -31, -27, and -28 mm [according to the visual analogue scale (VAS)]. Furthermore, the favourable safety profile of 223Radium was confirmed.
A recently published phase III trial reported the results about Xofigo® from the Symptomatic Prostate Cancer Patients (ALSYMPCA) study[10]. This multi-national, randomized, double-blind study started in 2008 and by February 2011 921 patients were enrolled. The goal of the study was to compare the efficacy and safety of 223Radium vs placebo in patients with castration-resistant prostate cancer and bone metastases.
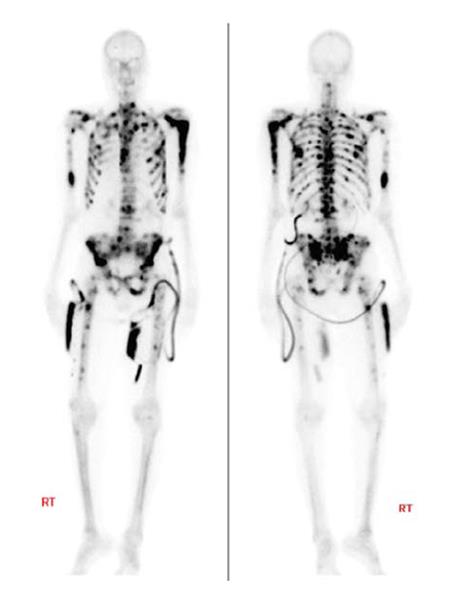
Patients were included in the study when they showed two or more bone metastases on skeletal scintigraphy, had no visceral metastases, and had been treated with docetaxel or if they were unable to receive docetaxel (Figure 1).
The patients were randomized in a ratio of 2:1 and received intravenous injections of 223Radium (at a dose of 50 kBq per kilogram of body weight) or saline injections as placebo. Patient received 6 injections in 4-wk intervals. Each patient was provided with the best standard of care including local external-beam radiation therapy or treatment with glucocorticoids, antiandrogens, ketoconazole, or estrogens. Chemotherapy, hemibody external radiotherapy, and other systemic radionuclides were not permitted.
The primary end point was overall survival, defined as the time from randomization to the date of death, regardless of cause. The main secondary end points were the time to an increase in the total ALP level, a total alkaline phosphatase response, the time to the first symptomatic skeletal event, normalization of the total alkaline phosphatase level and the time to an increase in the PSA level.
The study was designed to provide a statistical power of 90% to detect a hazard ratio of 0.76 for the risk of death in the 223Radium group vs the placebo group with a two-sided alpha significance level of 0.05. In total, 614 patients were enrolled in the 223Radium group and 307 patients in the placebo group. The patients were enrolled in 136 study centres in 19 countries. The baseline patient characteristics in both groups were largely identical.
A pre-defined interim analysis was conducted after 314 deaths had occurred to assess the effect of 223Radium on the primary end point (overall survival). On the basis of this interim analysis, which showed a survival advantage with 223Radium and an acceptable safety profile, early discontinuation of the trial and crossover from placebo to 223Radium was recommended. The authors report in this study the results of an updated descriptive analysis of the efficacy and safety data, performed when 528 deaths had occurred, before any crossover treatment with 223Radium was administered.
The median overall survival was 14.9 mo in the 223Radium group and 11.3 mo in the placebo group. The mortality risk was 30% lower in the 223Radium group than in the placebo group (P < 0.001). In total 528 patients died; 333 of the 614 patients in the 223Radium group (54%) and 195 of the 307 patients in the placebo group (64%). A secondary endpoint also confirmed the superiority of 223Radium over the best standard of care. The time to the first symptomatic skeletal event was 15.6 mo in the 223Radium group vs 9.8 mo in the placebo group (P < 0.001). The time to increases in the total ALP (P < 0.001) and PSA levels (P < 0.001) was significantly longer in the 223Radium group. Finally, a significantly larger proportion of patients in the 223Radium group showed a response of the total ALP and PSA levels (≥ 30% reduction, P < 0.001). Sixteen and 24 wk after initiation of 223Radium treatment, patients had significantly less pain compared to baseline (P < 0.001 and P = 0.001, respectively).
No clear difference in the appearance of grade 3 and 4 adverse events (according to the Common Terminology Criteria for Adverse Events) was reported between the two groups. One patient in each group showed grade 3 febrile neutropenia. In the 223Radium group one grade 5 haematologic adverse event (thrombocytopenia) occured. Serious adverse events that occurred in > 5% of the patients in the 223Radium or the placebo group were disease progression, bone pain, anaemia, and spinal cord compression. Overall the probability for the appearance of adverse events of all grades was lower in the 223Radium group than in the placebo group. The quality of life (according to the Functional Assessment of Cancer Therapy-Prostate) improved significantly in the 223Radium group.
Because of the interim analysis of the ALSYMPCA study, 223Radium was approved by the FDA as a treatment for patients with bone metastatic castrate-resistant prostate cancer. The approval was given for patients without visceral metastases.
The suggested regimen follows that of the ALSYMPCA study and includes 50 kBq/kg at an interval of 4 wk with a maximum of 6 doses. A simultaneous administration of 223Radium and chemotherapy is not permitted outside of clinical trials because of the unclear potential of additive effects on myelosuppression.
With its approval the FDA requested additional trials to determine the efficacy and safety of 223Radium when given at doses > 50 kBq/kg. The FDA also requested to investigate the long term safety, the effects of 223Radium on healthy bone marrow and the risk of the treatment for developing secondary malignancies.
223Radium is the first therapeutic that results in a survival benefit for patients with bone metastatic, castrate resistant prostate cancer. In addition, the treatment results in pain palliation and thus, improved quality of life and a delay of skeletal related events. At the same time the toxicity profile of 223Radium was favourable. Thus 223Radium may provide a new standard of care for patients with CRPC and bone metastases.
P- Reviewer: Cheng Z S- Editor: Wen LL L- Editor: A E- Editor: Lu YJ
| 1. | GLOBOCAN 2008. Cancer Fact Sheet Prostate Cancer. 2008;. [Cited in This Article: ] |
| 2. | Coleman R. Management of bone metastases. Cancer Treat Rev. 1997;23 Suppl 1:S69-S75. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s-6249s. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1481] [Cited by in F6Publishing: 1518] [Article Influence: 84.3] [Reference Citation Analysis (0)] |
| 4. | Lange PH, Vessella RL. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998;17:331-336. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 230] [Reference Citation Analysis (0)] |
| 5. | Wu JS, Wong R, Johnston M, Bezjak A, Whelan T. Meta-analysis of dose-fractionation radiotherapy trials for the palliation of painful bone metastases. Int J Radiat Oncol Biol Phys. 2003;55:594-605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 431] [Cited by in F6Publishing: 348] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 6. | Robinson RG, Preston DF, Schiefelbein M, Baxter KG. Strontium 89 therapy for the palliation of pain due to osseous metastases. JAMA. 1995;274:420-424. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 111] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Sartor O. Prostate cancer and bone: a unique relationship with multiple opportunities for targeted therapy. Clin Prostate Cancer. 2004;3:71-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Serafini AN, Houston SJ, Resche I, Quick DP, Grund FM, Ell PJ, Bertrand A, Ahmann FR, Orihuela E, Reid RH. Palliation of pain associated with metastatic bone cancer using samarium-153 lexidronam: a double-blind placebo-controlled clinical trial. J Clin Oncol. 1998;16:1574-1581. [PubMed] [Cited in This Article: ] |
| 9. | Lassmann M, Nosske D. Dosimetry of 223Ra-chloride: dose to normal organs and tissues. Eur J Nucl Med Mol Imaging. 2013;40:207-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 10. | Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fosså SD, Chodacki A, Wiechno P, Logue J, Seke M. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369:213-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2211] [Cited by in F6Publishing: 2160] [Article Influence: 196.4] [Reference Citation Analysis (0)] |
| 11. | Henriksen G, Fisher DR, Roeske JC, Bruland ØS, Larsen RH. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med. 2003;44:252-259. [PubMed] [Cited in This Article: ] |
| 12. | Henriksen G, Breistøl K, Bruland ØS, Fodstad Ø, Larsen RH. Significant antitumor effect from bone-seeking, alpha-particle-emitting (223)Ra demonstrated in an experimental skeletal metastases model. Cancer Res. 2002;62:3120-3125. [PubMed] [Cited in This Article: ] |
| 13. | Carrasquillo JA, O’Donoghue JA, Pandit-Taskar N, Humm JL, Rathkopf DE, Slovin SF, Williamson MJ, Lacuna K, Aksnes AK, Larson SM. Phase I pharmacokinetic and biodistribution study with escalating doses of ²²³Ra-dichloride in men with castration-resistant metastatic prostate cancer. Eur J Nucl Med Mol Imaging. 2013;40:1384-1393. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Nilsson S, Larsen RH, Fosså SD, Balteskard L, Borch KW, Westlin JE, Salberg G, Bruland OS. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res. 2005;11:4451-4459. [PubMed] [Cited in This Article: ] |
| 15. | Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal M. Bone-targeted radium-223 in symptomatic, hormone-refractory prostate cancer: a randomised, multicentre, placebo-controlled phase II study. Lancet Oncol. 2007;8:587-594. [PubMed] [Cited in This Article: ] |
| 16. | Nilsson S, Franzén L, Parker C, Tyrrell C, Blom R, Tennvall J, Lennernäs B, Petersson U, Johannessen DC, Sokal M. Two-year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin Genitourin Cancer. 2013;11:20-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Nilsson S, Strang P, Aksnes AK, Franzèn L, Olivier P, Pecking A, Staffurth J, Vasanthan S, Andersson C, Bruland ØS. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur J Cancer. 2012;48:678-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |