Published online Dec 28, 2015. doi: 10.4329/wjr.v7.i12.509
Peer-review started: January 26, 2015
First decision: March 6, 2015
Revised: July 1, 2015
Accepted: November 3, 2015
Article in press: November 4, 2015
Published online: December 28, 2015
AIM: To assess the clinical diagnostic value of functional imaging, combining quantitative parameters of apparent diffusion coefficient (ADC) and standardized uptake value (SUV)max, before and after chemo-radiation therapy, in prediction of tumor response of patients with rectal cancer, related to tumor regression grade at histology.
METHODS: A total of 31 patients with biopsy proven diagnosis of rectal carcinoma were enrolled in our study. All patients underwent a whole body 18FDG positron emission tomography (PET)/computed tomography (CT) scan and a pelvic magnetic resonance (MR) examination including diffusion weighted (DW) imaging for staging (PET1, RM1) and after completion (6.6 wk) of neoadjuvant treatment (PET2, RM2). Subsequently all patients underwent total mesorectal excision and the histological results were compared with imaging findings. The MR scanning, performed on 1.5 T magnet (Philips, Achieva), included T2-weighted multiplanar imaging and in addition DW images with b-value of 0 and 1000 mm²/s. On PET/CT the SUVmax of the rectal lesion were calculated in PET1 and PET2. The percentage decrease of SUVmax (ΔSUV) and ADC (ΔADC) values from baseline to presurgical scan were assessed and correlated with pathologic response classified as tumor regression grade (Mandard’s criteria; TRG1 = complete regression, TRG5 = no regression).
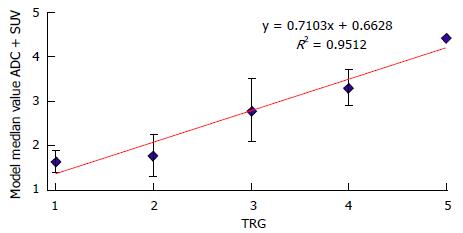
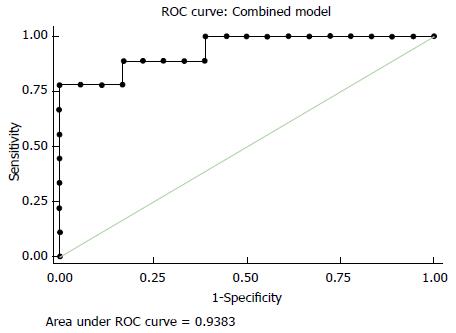
RESULTS: After completion of therapy, all the patients were submitted to surgery. According to the Mandard’s criteria, 22 tumors showed complete (TRG1) or subtotal regression (TRG2) and were classified as responders; 9 tumors were classified as non responders (TRG3, 4 and 5). Considering all patients the mean values of SUVmax in PET 1 was higher than the mean value of SUVmax in PET 2 (P < 0.001), whereas the mean ADC values was lower in RM1 than RM2 (P < 0.001), with a ΔSUV and ΔADC respectively of 60.2% and 66.8%. The best predictors for TRG response were SUV2 (threshold of 4.4) and ADC2 (1.29 × 10-3 mm2/s) with high sensitivity and specificity. Combining in a single analysis both the obtained median value, the positive predictive value, in predicting the different group category response in related to TRG system, presented R2 of 0.95.
CONCLUSION: The functional imaging combining ADC and SUVmax in a single analysis permits to detect changes in cellular tissue structures useful for the assessment of tumour response after the neoadjuvant therapy in rectal cancer, increasing the sensitivity in correct depiction of treatment response than either method alone.
Core tip: In our study we evaluated the combination of changes of glucose metabolism values expressed as SUVmax and the changes of apparent diffusion coefficient (ADC map) values, before and after neoadjuvant therapy, in patients with advanced rectal cancer in order to predict, in vivo, the therapy response. The importance of this work consist of the possibility to offer, in the era of positron emission tomography (PET)/magnetic resonance imaging scanner, a new advanced tool that allows the non-invasive evaluation of response to neoadjuvant chemotherapy treatment in patients with rectal cancer, by adding quantitative value information on diffusion weighted images and on PET/computed tomography imaging.
- Citation: Ippolito D, Fior D, Trattenero C, Ponti ED, Drago S, Guerra L, Franzesi CT, Sironi S. Combined value of apparent diffusion coefficient-standardized uptake value max in evaluation of post-treated locally advanced rectal cancer. World J Radiol 2015; 7(12): 509-520
- URL: https://www.wjgnet.com/1949-8470/full/v7/i12/509.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i12.509
The use of pre-operative chemoradiation treatment (CRT) induces downsizing and downstaging of primary rectal tumors, yielding a pathologic complete response (pCR) in up to 24% of patients[1]. A pCR is known to be associated with a favourable oncologic outcome, in regard to both recurrence and patients survival[2].
The trend in treatment of rectal cancer, although is still controversial, to date is toward a more conservative approach in patients identified as complete responders after CRT. Generally, a pCR is determined with histopathologic examination after surgery, but, if the determination of CR before surgery may influence the subsequent treatment decision, an accurate clinical assessment of response becomes essential[3-5].
Recently, diffusion weighted magnetic resonance imaging (DW-MR) after CRT has demonstrated to be more valuable than standard morphologic MR study in differentiation between a pCR and the presence of residual disease. On DW images, the viable neoplastic remnants are more easily defined, since they appear hyperintense in comparison to the low signal intensity (SI) of the surroundings not neoplastic tissues[6,7]. Promising results have been shown with quantitative DW imaging analysis by quantifying the apparent diffusion coefficient (ADC) in the evaluation of treatment response to CRT in patients having rectal cancer[8-14].
Even positron emission tomography (PET)/computed tomography (CT) has been suggested to be an accurate imaging modality in the staging of newly diagnosed or in detection of recurrent rectal cancer. Furthermore, qualitative and quantitative assessment of fluorodeoxyglucose-PET provide helpful information regarding treatment response and prognosis of patients with rectal cancer[15,16] .
Both DWI imaging and PET-CT imaging have been used separately in different fields of tumor evaluation, such as detection, characterization and CRT response assessment. As both ADC and standardized uptake value (SUV) have been associated with biological behaviour and treatment response in various tumors types a correlation between SUV values, which reflect metabolic activity and ADC values which reflect cellular density might be found[17].
To date, there have been few comparative studies between ADC and SUVmax to evaluate the tumour response to preoperative CRT in locally advanced rectal cancer (LARC). The aim of this study was, along with brief review of literature, to evaluate the accuracy of combined ADC and SUVmax values in prediction of tumor regression grade (TRG) complete responders in LARC patients, using histological tumor regression grade as standard reference.
Between June 2009 and April 2012, 53 consecutive patients with diagnosis of rectal cancer were considered for eligibility. Inclusion criteria were: (1) histopathologically proved rectal adenocarcinoma (0 to 15 cm from anal verge, by means of endoscopic biopsy); (2) LARC staged by baseline MR imaging examination (≥ T3 or positive lymph nodes); (3) absence of distant metastases; (4) neoadjuvant preoperative CRT. The exclusion criteria were: (1) previous CRT for primary rectal carcinoma or tumour in other organ; (2) contraindication to MR imaging study; (3) premature discontinuation of CRT; (4) delayed (more than 8 mo after CRT) or cancelled surgery; and (5) discontinued or non-diagnostic MR imaging examinations during therapy.
A total of 31 patients (22 men and 9 women, mean age of 64.5 years with a range of 42-80) met the study criteria.
This prospective study was approved by our institutional review board, and informed consent was obtained from all patients.
The baseline MR imaging examination (MR1) was performed within a mean of 4.8 wk (I-III quartile: 3.8-6.3 wk) before the treatment for tumour staging, while the second study (MR2) was performed within a mean of 6.6 wk (I-III quartile: 5.3-7.7 wk) after the completion of CRT and before surgery. MRI was performed using a 1.5 T magnet (Philips, Achieva 1.5 T, The Netherlands). The patients were positioned supine and feet first, and scan was performed by using a five-channel high resolution phased-array body coil.
The standard protocol included multiplanar T2- TSE-weighted sequences without fat suppression, applying the following parameters: Repetition time msec/echo time msec 4750/120; slice thickness: 3 mm; slices: 18; matrix: 256 × 256; number of signal acquired (NSA): 4; axial TSE T1-weighted axial sequence Turbo Spin-Echo (TSE) T1-weighted (slice thickness: 3 mm; slice: 20; gap: 3 mm; TR: 612 ms; TE: 14 ms; flip angle: 90°; Field of View (FOV): 180; RFOV: 85; matrix: 272 × 320; NSA: 4.
The images were obtained in three different planes: sagittal, coronal and transverse, with the latter two orientations angled perpendicularly to the long axis of the tumour according to sagittal images. At the end of the examination, DW images using a Multi-slice Spin Echo Eco-planar Single Shot (SE-EPI-SSh) sequence were obtained in the axial plane with the following parameters: Repetition time msec/echo time 3000/74; slice thickness: 6 mm; slices: 12; matrix: 240 × 256; NSA: 4; b values of 0 and 1000 mm2/s; time: 1.30 min; SENSE factor 1.5.
No intravenous contrast medium was injected as part of our routine acquisition protocol for rectal cancer evaluation, according to recent guidelines about clinical management of rectal cancer patients with MRI (recommendations from ESGAR, 2012).
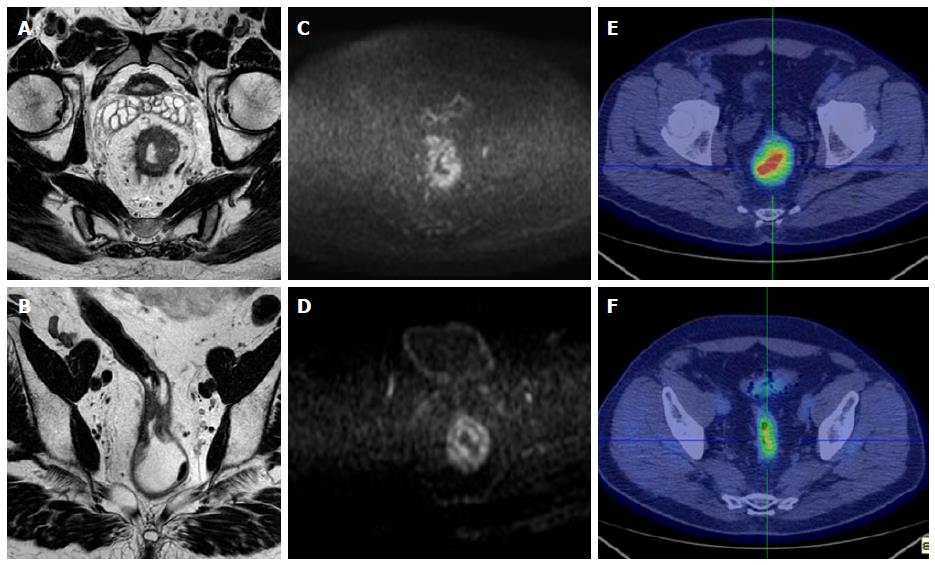
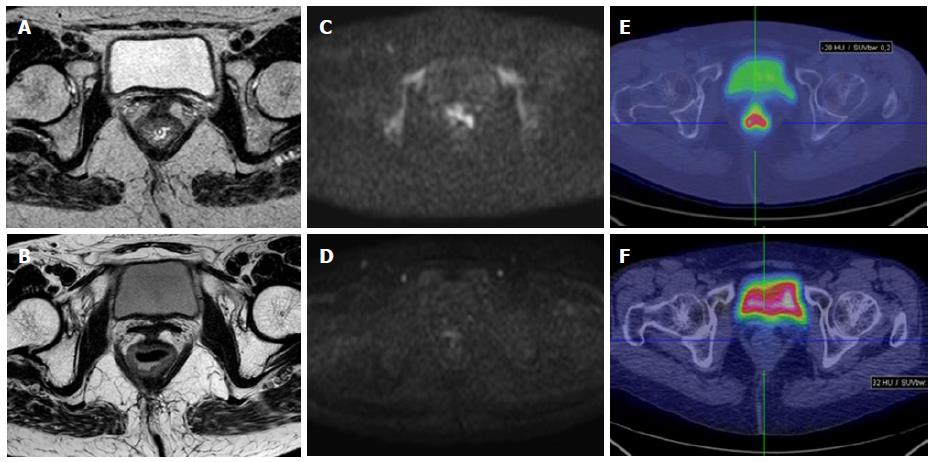
MR images were analyzed and ADC measurements were made by one radiologist experienced in abdominal radiology (DI), and who was blinded to the therapeutic response and to the histological results (Figures 1 and 2).
On post-CRT ADC maps, the region of interest (ROIs) were manually drawn on the basis of visual analysis of focal areas of residual high SI on the high-b-value images within the location of the primary tumour site, by comparing pre-CRT examination if needed. When no remaining high SI area could be depicted on the post-CRT DW images, the ROIs were drawn on the rectal wall at the former location of the primary tumour, using pre-CRT DW and T2W images as reference. The lesions were manually contoured along their edge avoiding vessels distortion areas, vessels and motion artefacts. Then, the mean and standard deviation of the ADC values were automatically calculated.
The size of ROI of one section was not less than 20 voxels. Diffusion-weighted images were of diagnostic quality in all patients, and no patients were excluded from the study.
In order to determine percentage variation of ADC before and after CRT, the ADC values in the MR1 (ADC 1) and MR2 (ADC 2) were used also to define delta ADC (ΔADC) as follows: ΔADC= [(ADC2 - ADC1)/ADC2] × 100.
All patients were investigated by FDG-PET/CT prior to the onset of CRT (PET1) and 4 wk after the completion of the pre-operative treatment (PET2). All studies were performed on a PET scanner coupled with a 8-detector rows CT scanner (Discovery ST - GE Healthcare, Milwaukee, WI, United States), thus allowing one step acquisition of co-registered PET and CT images. According to the acquisition protocol, patients fasted for at least 6 h before the intravenous administration of 3.7 MBq/kg body weight of 18F-FDG. Blood glucose levels were checked before tracer administration and patients with glucose level above 170 mg/dL were excluded from the study. All patients were orally hydrated (500 mL of water) during the FDG uptake period and were asked to empty their bladder before positioning for the scan. Sixty ± ten minutes after the trace injection, PET/CT study was performed. Unenhanced low-dose CT (LD-CT) was acquired first with the following parameters: 120 kV, 60 mA, gantry rotation time of 0.8 s, section thickness of 3.75 mm and pitch of 1.65. PET emission scanning was performed immediately after LD-CT, with the same coverage volume. All PET studies were acquired in 3D mode, with acquisition time of 3 min per FOV. Images were reconstructed with ordered subsets expectation-maximization algorithm, 128 × 128 matrix size, attenuation, random, and scatter correction. Attenuation correction was performed on the basis of CT scan data. The CT pixel values measured in hounsfield units were transformed into linear attenuation coefficients for the 511-keV energy radiation. CT and PET images were then matched and fused into transaxial, coronal, and sagittal images.
PET, CT, and fused PET/CT images were displayed on Xeleris workstation (GE Medical Systems, Milwaukee, WI). Images were interpreted by one experienced nuclear medicine physicians (LG) without knowledge of clinical and histological data, but only of the presence of primary rectal cancer. Lesion uptake was identified as an area of pathologically increased 18F-FDG uptake, excluding causes of nonspecific or physiologic accumulation of the radio- tracer (Figures 1 and 2). ROIs were drawn over the region of pathological uptake on the baseline scan (PET1) for the calculation of SUV1. At subsequent PET/CT (PET2) images were co-registered with the baseline study by means of the anatomical CT and the ROIs were drawn in the same positions of PET1 in order to calculate SUV2. SUV values were calculated using the maximum activity values within each ROI on the transaxial slices, normalized to the injected dose and patient’s body weight, as per ADC values. The SUVmax values in the PET1 (SUV1) and PET2 (SUV2) were used to define ΔSUV in percentage as follows: ΔSUV1 = [(SUV1 - SUV2)/SUV1] × 100.
Pathologic response was evaluated on resected specimens by a pathologist, with 15-year experience in gastrointestinal pathology. Each specimen was fixed in 10% buffered formalin for at least 48 h and inked. Serial transversal tissue blocks were cut at 5 mm intervals from the distal portion. Each block, consisting of full thickness of the rectal wall and the mesorectum, was embedded in paraffin. Whole-mount sections were obtained and stained with hematoxylin and eosin. The TRG definition of Mandard et al[18] was adopted for clinical response classification. Patients with TRG1-2 scores were considered as responders, while patients with TRG3-5 were classified as non-responders.
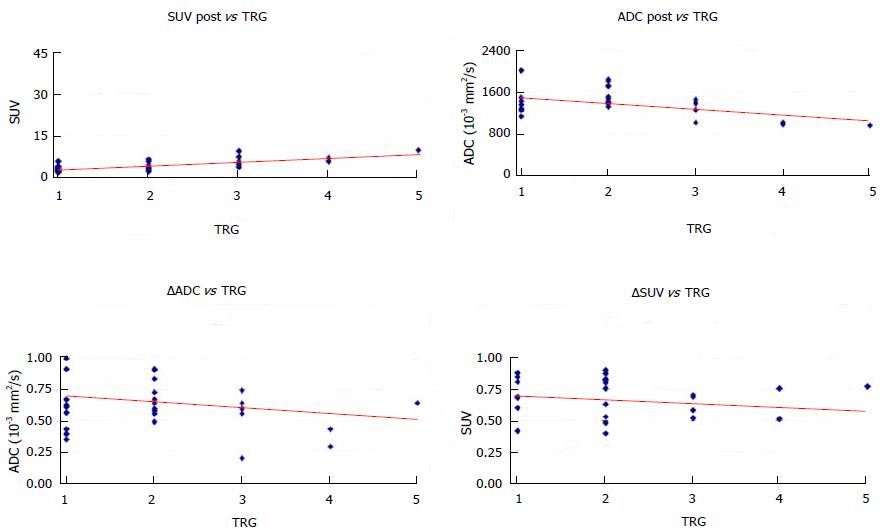
Mean and SD of the SUV1, SUV2, ADC1 and ADC2 were calculated and the comparison between SUV1 and SUV2, and between ADC1 and ADC2 was done with Wilcoxon paired test (Table 1). The comparison of the same quantitative parameter was also performed between histopathologic responders and non responders patients with the non parametric Mann-Whitney U test (Table 2). The correlation between histological TRG in the resected specimen and the ADC and SUVmax values assessed before and after surgery was analysed with the Pearson correlation test. Multivariate regression model was evaluated including those parameters with significant correlation in univariate regression analysis (Figure 3). The final model incorporated ADC and SUVmax values measured after surgery (ADCpost - SUVpost). Model predictions of histological tumour regression were also compared with true patients’ TRG and investigated with scatter diagram (Figure 4).
| Variable | Mean ± SD | P value (Wilcoxon paired) |
| SUV1 | 16.3 ± 8.6 | < 0.0001 |
| SUV2 | 4.5 ± 2.1 | |
| ΔSUV (%) | 66.8 ± 20.4 | |
| ADC1 | 0.83 ± 0.15 | < 0.0001 |
| ADC2 | 1.33 ± 0.13 | |
| ΔADC (%) | 60.2 ± 23.2 |
| Variable | Responders(Mean ± SD) | Not responders(Mean ± SD) | P value(Mann-Whitney U test) |
| SUV1 | 15.1 ± 8.0 | 19.5 ± 9.8 | 0.151 |
| SUV2 | 3.6 ± 1.4 | 6.6 ± 2.1 | 0.0009 |
| ΔSUV (%) | 68.5 ± 23.2 | 62.8 ± 10.5 | 0.151 |
| ADC1 | 0.88 ± 0.19 | 0.78 ± 0.09 | 0.076 |
| ADC2 | 1.47 ± 0.22 | 1.19 ± 0.2 | 0.009 |
| ΔADC(%) | 72.6 ± 27.1 | 55.5 ± 18.5 | 0.0078 |
Receiver operating characteristic (ROC) analysis was performed to define the best accuracy of the metabolic parameters in predicting the response to treatment.
The sensitivity, specificity and overall diagnostic accuracy for each item were calculated under the optimal cut-off value.
Stata software 9.0 (Stata Corporation, College Station, Texas, United States) was used for performing statistical analysis and a P < 0.05 was deemed as statistical significant.
All patients underwent surgical excision within 8-10 wk after CRT completion, i.e., low anterior resection (n = 24), abdominoperineal resection (n = 6) and extended resection (n = 1). The surgical approach was established considering the clinical response to CRT defined at conventional restaging.
In the whole sample of 31 patients, the mean tumor ADC before CRT in the responder group of 22 patients was 0.88 × 10-3 mm2/s; while in the non-responder group (9 patients) was 0.78 × 10-3 mm2/s. After CRT, the mean tumour ADC in the down-staged group was 1.47 × 10-3 mm2/s, while in the nondown-staged group was 1.19 × 10-3 mm2/s. ΔADC showed to be statistically relevant between responders and non responders (P = 0.0078), as shown in Table 2.
The regression analysis in comparing the ability of post-CRT ADC, ΔADC values in the identification of response to CRT demonstrates an optimal cut-off point of 1.294 for post-CRT measures [sensitivity = 86.4%, specificity = 66.7%, positive predictive value (PPV) = 86.4%, negative predictive value (NPV) = 66.7%], 0.500 for ΔADC (sensitivity = 63.4%, specificity = 66.7, PPV = 82.4%, NPV = 42.9%).
The mean SUVmax and ΔSUV values of the rectal lesion for each PET/CT study are reported (Tables 1 and 2). SUV1 was found significantly higher than SUV2 (P < 0.0001). Figure 3 shows the results of univariate and multivariate linear regression analysis comparing metabolic parameters to TRG groups. In the univariate analysis, a statistically significant correlation was found for SUV2 (P = 0.009) with TRG (Table 2).
Considering the TRG1-2 patients as responder and TRG3-5 patients as non-responder, the highest accuracy in defining the response to treatment was obtained with a SUV2 cut-off value of 4.4. With this threshold, metabolic response evaluation was true positive in 17 patients, true negative in 8 patients, false positive in 1 patients and false negative in 5 patients, obtaining sensitivity, specificity, accuracy, PPV and NPV of 77.3%, 88.9%, 80.7%, 94.4% and 61.5%, respectively.
Recently, a more conservative treatment has been advocated in patients with rectal cancer showing a good or a complete response to neoadjuvant treatments. The selection of true responders is essential and the role of imaging for restaging after CRT has been the subject of several recent studies, suggesting that neither MRI nor endorectal ultrasound or FDG-PET are enough accurate for identifying the true complete responders, with an overall PPV ranging from 17% to 50%[6,19-23].
The correlation between therapy-related changes in FDG uptake and tumour response in rectal cancer has been previously reported by several groups. Despite the differences in study set-up, scan type, pathological and metabolic evaluation, the final metabolic response to CRT in rectal cancer with FDG-PET/CT have been demonstrated to correlate with the histopathological response and therefore to be a useful method for early assessment of treatment efficacy in rectal cancer. The different studies provided similar cut-off values, however the above mentioned differences make a direct comparison of the results not possible (Table 3).
| Ref. | No. of patients | Mean SUV 1 | Mean SUV 2 | Mean SUV 3 | Sn (%) | Sp (%) | Late cut-off (%) | Sn (%) | Sp (%) | Delta SUV 1 R (%) | Delta SUV 1 NR (%) | Delta SUV 2 R (%) | Delta SUV 2 NR (%) |
| Bampo et al[31] | 30 | 17.5 | 7.1 | 73.1 | 50.2 | ||||||||
| Cascini et al[24] | 33 | 11.2 | 6 | 2.7 | 100 | 87 | 62 | 28 | |||||
| Guerra et al[46] | 31 | 16.3 | 8.1 | 4.3 | 63.2 | 55.6 | 60 | 77.3 | 55.6 | 51 | 43.1 | 68.5 | 62.8 |
| Hermann et al[26] | 28 | 9.5 | 5.2 | 3.1 | 74 | 50 | 45 | 63 | 100 | ||||
| Janssen et al[25] | 46 | 16.4 | 13 | ||||||||||
| Lambrecht et al[28] | 22 | 100 | 75 | 76 | 100 | 75 | 59 | 25 | 90 | 63 | |||
| Rosenberg et al[27] | 30 | 9.5 | 5.5 | 3.5 | 74 | 70 | 57.5 | 79 | 70 | 44.3 | 29.6 | 66 | 48.3 |
| Shanmugan et al[29] | 70 | 10.8 | 3.8 | 63 | 60 | 84 | 74 | 56 | |||||
| Sun et al[30] | 35 | 14.7 | 7.9 | 57.8 |
Cascini et al[24] showed that early responder patients, evaluated with PET/CT 12 d after the beginning of therapy, had a higher decrease of SUV than non-responder patients (62% vs 28%, respectively; P < 0.0001). Conversely, the pre-surgical PET data did not demonstrate any statistically significant correlation between mean SUV late change and TRG findings (P = 0.2) obtaining a low correlation between overall changes and the TRG (P = 0.63).
Also Janssen et al[25] found an early significant decrease of the metabolic activity after the first week of CRT, both in SUVmean and SUVmax, that decreased from respectively 8.5 ± 2.8 (range: 4.0-15.1) and 16.4 ± 5.8 (range: 7.0-28.1) to 6.9 ± 2.2 (range: 4.3-12.7) (P < 0.001) and 13.0 ± 4.8 (range: 7.6-27.4) (P < 0.001) after the first week of combined treatment. More recently Herrmann et al[26] found in 28 patients a decrease of mean SUV uptake from 9.5 at baseline to 5.5 (P < 0.001) 14 d after the onset of preoperative radiochemotherapy and in the third PET scan (4 wk after completion of treatment), mean SUV decreased to 3.1 (P < 0.001).
Rosenberg et al[27] did not obtain the same result but reported that the percentage of early SUV reduction tended to be higher (44.3%) in responders than in non-responder patients (29.6%; P = 0.085). However after the completion of therapy, the reduction of FDG uptake was 66% in histopathologically responding tumors and 48.3% in non responding tumors (P = 0.040).
Similarly also Lambrecht et al[28] found, during CRT, a mean reduction in SUVmax of 59% in patients with histopathological complete response vs a mean reduction of SUVmax of 25% in patients without complete response (P = 0.0036). Additionally, 5 wk after the completion of CRT, a 90% SUVmax reduction in the first group vs 63% in second group (P = 0.013) was found.
Shanmugan et al[29] recently evaluated seventy patients that underwent pre- and post-CRT PET/CT followed by surgery and found that patients with pCR had a lower median post-CRT SUV compared with those without (2.7 vs 4.5, P = 0.01). Median SUV decrease was 63% (7.5%-95.5%) and predicted pCR (P = 0.002); the authors concluded that post-treatment SUV and %SUV decrease correlate with pCR.
Similar results were found by Sun et al[30] in a study group of 53 patients diagnosed with clinical T3- 4 and/or N+ rectal cancer and treated with CRT followed by radical surgery after 6-8 wk. A PET/CT scan was performed before (PET/CT1) beginning of treatment and a second scan (PET/CT2) was performed within 1 wk after the completion of CRT. Thirty-five out of 53 patients also underwent a third (PET/CT3) scan within 1 wk before surgery. When patients were regrouped as having a pCR and a non-pCR significant differences were found in the percentage difference between PET/CT1 and PET/CT3 in SUVmax [(Δ% SUVmax(1-3); 69.17% vs 57.77%)].
Also Bampo et al[31] evaluated the possible predictive role of late FDG-PET/CT for the assessment of pathological response in locally advanced rectal cancer following neoadjuvant chemoradiation in 30 patients; significant differences in late SUV value and response index were observed between complete and non-complete pathological responder (P = 0.0006 and 0.03). Furthermore, with receiver operating characteristic curve analysis, a SUV threshold of 5.4% had 81% sensitivity and 100% specificity, with 90% overall accuracy.
Nevertheless the optimal timing of the post-treatment PET scan, for a proper assessment of early response to CRT, is still unclear. Radiation-induced reduction in glucose intake occurs due to cell loss, which is a prolonged effect[32]. However, this can be confounded by two transient processes that occur soon after radiochemotherapy. The first is defined as ‘‘stunning” of tumor cells and can lead to a transient reduction in glucose metabolism, increasing false negative results[33]. The second one is the possible increase in FDG uptake due to radiogenic inflammatory processes after radiotherapy and can lead to false positive results[34]. Increasing time interval between neoadjuvant treatment and PET scan should theoretically lead to a more accurate evaluation. Alternatively, 18F-FLT PET has been tested to minimize the influence of radiation-induced inflammation[35]. In previous animal studies, FLT uptake has been shown to be in inflamed tissue as compared with FDG. However, it has not shown to be a valid tool for a proper CRT response assessment in rectal cancer patients[36].
In Lambrecht et al[28] study ROC curve analysis identified a threshold value for ΔSUVmax of 40%, for differentiating patients with a complete response after 2 wk, with a sensitivity of 100%, but a specificity of 75% and a PPV of 60%. Similarly using a threshold for ΔSUVmax of 76% after CRT and before surgery, is possible to identify complete responders with a sensitivity of 100%, a specificity of 75% and a PPV of 60%.
Considering our results, ROC curves analysis have shown that SUV2 has the best accuracy (80.7%) in predicting response to neoadjuvant treatment with a threshold value of 4.4. Interestingly, to note that in our study the PPV of SUV2 in predicting response was very high (94.4%) suggesting more conservative surgical approaches only in patients with evidence of lower glucose uptake at the end of neoadjuvant treatment.
While evaluating the correlation between the changes of ADC before and after CRT we found that before CRT, the mean tumour ADC in the responder group was (0.88 ± 0.19) × 10-3 mm2/s, while that in the non-responder group was (0.78 ± 0.09) × 10-3 mm2/s. At the end of combined chemoradiation therapy the mean tumor ADC value for responder patients was (1.47 ± 0.22) × 10-3 mm2/s. For non-responder patients the mean ADC value after therapy was (1.19 ± 0.20) × 10-3 mm2/s.
Our results are in line with the previously published by Jung et al[37]. Before neoadjuvant CRT, the mean ADC of responders and non-responders were (0.93 ± 0.09) × 10-3 mm2/s and (1.03 ± 0.08) × 10-3 mm2/s, respectively. After neoadjuvant CRT the mean post-CRT ADC in responders was higher than in non-responders (P = 0.009), being respectively (1.29 ± 0.13) × 10-3 mm2/s and (1.18 ± 0.08) × 10-3 mm2/s. Using a post-CRT ADC of 1.18 × 10-3 mm2/s as cut-off value for discriminate between the responders and non-responders, the highest accuracy (77.1%) was obtained, with the following diagnostic predictive values: Sensitivity 91.3%, specificity 50.0%, positive predictive value 77.8%, and negative predictive value 75.0%.
Similarly Kim et al[6] reported that the mean ADC after CRT in the responders group [(1.62 ± 0.36) × 10-3 mm2/s)] differed significantly from the one in the non-responders group [(1.04 ± 0.24) × 10-3 mm2/s)]. Moreover, when an ADC value of 1.20 × 10-3 mm2/s was used as cut-off, the authors obtained an accuracy of 85% with the following diagnostic predictive values: sensitivity 100%, specificity 79%, positive predictive value 65%, and negative predictive value 100%.
Recently, other studies[38-40] revealed similar data. Ha et al[38] comparing the mean post-CRT ADC for the RC group vs the non-CR group [(1.33 ± 0.25) × 10-3 mm2/s vs (1.13 ± 0.32) × 10-3 mm2/s] found a significant increased (P = 0.001) of ADC value. When a post-CRT ADC of 1.20 × 10-3 mm2/s was used as a cut-off value for discriminating CR, the accuracy was 67%, sensitivity was 52.1%, specificity of 80.8%, positive predictive value of 71.4%, and negative predictive value of 64.6%.
Even the best timing to perform the follow-up MR study for the assessment of response to CRT is still debated. Some Authors performed the diffusion-MR also during the first 15 d of the combined treatment. Sun et al[12] evaluated the ADC only one week after the beginning of CRT. Before CRT the mean tumour ADC value in the down-staged group was lower than that in the nondown-staged group (1.07 × 10-3 mm2/s ± 0.13 vs 1.19 × 10-3 mm2/s ± 0.15, F = 6.91, P = 0.013). At the end of the first week, the mean tumour ADC increased significantly to 1.32 × 10-3 mm2/s ± 0.16 (F = 37.63, P < 0.001) in the down-staged group, while there was no significant ADC value increase in the nondown-staged group (F = 1.18, P = 0.291).
Also Kim et al[41] reported that the mean percentage of tumour ADC change in the responder group after 2 wk of CRT was higher in comparison with that of the non-responder group, even if not statistically significant. Seierstad et al[42] found an increase in tumour ADC values on day 11 after the beginning of CRT.
Cai et al[39] obtained an increase in the mean tumour ADC during the course of neoadjuvant CRT, especially at the 2th week (P = 0.004), with a significant increase in the mean ADC at the 2th week of neoadjuvant therapy in the T down-stage and tumour regression group (P = 0.011; 0.004). They also found a strong negative correlation between the mean pretreatment tumour ADC and tumour regression after neoadjuvant CRT (P = 0.0021).
Hypotizing that disrupted membranes increase the extracellular volume since they demonstrated higher permeability, Authors concluded that the ADC values changes in this early phase of CRT are probably the result of irreparable radiation induced DNA damage. However considering the variability among the results obtained in the recent literature (Table 4), actual evidence suggests that DWI-MR performed early after the beginning of therapy could not provide accurate and reproducible results for the evaluation of early tumour response to CRT. Hence the attention should be focused on ADC values obtained after the end of the treatment.
| Ref. | n of patients | Pre ADC mean R | Pre ADC mean NR | Post ADC mean R | Post ADC mean NR | ROC curve (highest accuracy) |
| Ippolito et al[45] | 30 | 0.88 ± 0.19 | 0.78 ± 0.09 | 1.47 ± 0.22 | 1.19 ± 0.20 | 1.28 (80%) |
| Kim et al[41] | 34 | 0.90 ± 0.06 | 0.94 ± 0.03 | |||
| Jung et al[37] | 35 | 0.93 ± 0.09 | 1.03 ± 0.08 | 1.29 ± 0.13 | 1.18 ± 0.08 | 1.18 (77.1%) |
| Kim et al[6] | 40 | 1.62 ± 0.36 | 1.04 ± 0.24 | 1.20 (85%) | ||
| Curvo Semedo et al[43] | 50 | 1.07 ± 0.15 | 1.10 ± 0.19 | 1.39 ± 0.24 | 1.45 ± 0.28 | 1.41 (53%) |
| Ha et al[38] | 100 | 0.59 ± 0.29 | 0.49 ± 0.22 | 1.33 ± 0.25 | 1.13 ± 0.32 | 1.20 (67%) |
| Genovesi[44] | 28 | 1.01 ± 0.06 | 1.29 ± 0.02 | 1.79 ± 0.51 | 1.37 ± 0.43 | 29.5 (91.3%) |
| Cai et al[39] | 15 | 0.659 | 0.885 | 0.713 | 1.027 | |
| Birlik et al[40] | 43 | 0.66 ± 0.10 | 0.72 ± 0.14 | 1.22 ± 0.26 | 0.95 ± 0.20 | 1.20 (60%) |
As well as for SUV values, in order to evaluate the prognostic value of pretreatment MR, several authors assessed[6,7,38-40,43,44] the pre-CRT ADC, obtaining controversial results. Curvo-Semedo et al[43] demonstrated that lower pre-CRT ADC values were associated with a more aggressive tumour profile: in their study mean ADCs were significantly different for mesorectal fascia tumour free (MRF) vs MRF-invaded (P = 0.013), mrN0 vs mrNþ (P = 0.011), and for the different tumour differentiation grades at histology (P = 0.025). Particularly tumours with involved MRFs, nodal-positive disease and those of less differentiated showed lower ADC values. Furthermore, a significant positive correlation (r = 0.374; P = 0.019) between ADC values and the distance of the tumour from the MRF was found.
Jung et al[37] recently reported that the mean pre-CRT ADC obtained with a 3 Tesla MR of responders was lower than that of non-responders (P = 0.034). Other authors[39,40], obtained similar results with 1.5 T MR. For instance, in the study of Birlik et al[40], before CRT the mean tumour ADC in the responder group was significantly lower than that in the nonresponder group (P < 0.001).
Other authors, however, reported that pre-treatment ADC values were not statistically different for responders and non-responders and that may be limited in predicting treatment outcome[6,38,41,44,45]. For instance, Kim et al[41] reported that, predicting the treatment outcome based on TRG, there were no significant differences among responder and non-responder groups when comparing pre-CRT ADCs and early tumour ADC increase rates: As the tumour responds to treatment, ADC values will probably rise at first, due the initial disruption of cell membranes, and then decrease at the end of the treatment, for the post-irradiation ingrowth of fibrosis restricting water mobility.
Considering these results and according to recent literature, the best reproducibility was obtained by studies that evaluated a post-treatment cut-off value of ADC and SUV; being the most promising and precise way of applying functional techniques in the clinical practice.
Moreover, as evaluated in our series of patients, combining in a single analysis the mathematical model of median values of ADC and of SUV values, the power of both functional technique improves, gaining a strictly and significant relationship with TRG system staging by linear regression analysis with R2 of 0.95 (Figure 5).
In the era of PET/MRI scanner, the future approach should be represented by the combination of PET imaging with MRI not only to increase anatomical resolution but also for cell and molecular imaging, improving the accuracy in the assessment of tumour response. Moreover, the synchronous acquisition of both technique is critical in order to avoid differences in patient position, organ motion, and tumor growth.
In conclusion, the combined functional analysis of MRI and PET imaging, by the quantitative analysis of ADC map on DW-MR imaging and glucose uptake by 18F-FDG-PET, can contribute to the management of patients with locally advanced rectal cancer increasing the overall accuracy and sensitivity for treatment response evaluation in one step, since they permit to detect changes in cellular tissue structures useful in prediction the different group category response in relation to TRG system.
The treatment of locally advanced rectal cancer has shifted in recent years toward a more conservative policy for patients identified as complete responders after chemoradiation treatment (CRT). Therefore the determination of CR before surgery would influence the subsequent treatment choice, an accurate clinical assessment of response becomes essential. Conventional imaging modalities cannot distinguish fibrosis or scar from viable tumour cells in residual masses after chemoradiotherapy; therefore, these methods have a negligible impact on the prediction of pathologic findings. For these reasons, in recent years, the functional imaging studies are increasingly being conducted to add information about changes in tumour pathophysiology.
[18F]fluorodeoxyglucose positron emission tomography (18F-FDG PET) is a non-invasive tool to detect tumour metabolic activity and can be used to assess changes in tumour glucose metabolism after a CRT treatment. The semiquantitative assessment of glucose metabolism by evaluating the standardized uptake value (SUV) has been shown to have clinical relevance in several tumour types, since a strong relationship between 18F-FDG SUV changes and pathological response has been proved in different types of cancer. DW-MRI enables noninvasive characterization of biologic tissues on the basis of their water diffusion properties (Brownian motion) microcirculation. Due to the restricted motion of water molecules, the diffusion coefficients obtained by quantitative DWI differ from the free diffusion values and are called apparent diffusion coefficients (ADC), and it can be measured. ADCs tend to decrease with increased tissue cellularity or cell density. Conversely, the cell density may be indicative of tumor aggressiveness; increased metastatic capacity of tumors with high cellularity.
The importance of this work consist of the possibility to offer, in the era of PET/MRI scanner, a new advanced quantitative tool that allows the non-invasive evaluation of response to neoadjuvant chemotherapy treatment in patients with rectal cancer, by adding quantitative value information on DW images and on PET/CT imaging, combined in a single analysis. Moreover in this manuscript the authors reviewed and commented the recent literature findings on this field by using the two different techniques modalities [i.e., PET/CT and magnetic resonance imaging (MRI)].
Considering the variability among the results obtained in the recent literature, in rectal cancer post-therapy imaging assessment, the actual evidence suggests that DWI-MR and PET/CT performed early after the beginning of therapy could not provide accurate and reproducible results for the evaluation of early tumour response to CRT. Hence the attention should be focused on functional quantitative evaluation of ADC and SUVmax obtained after the end of the treatment.
DWI: Diffusion MRI (or dMRI) is a MRI method which allows the mapping of the diffusion process of molecules, mainly water, in biological tissues, in vivo and non-invasively. Molecular diffusion in tissues is not free, but reflects interactions with many obstacles, such as macromolecules, fibers, and membranes. Water molecule diffusion patterns can therefore reveal microscopic details about tissue architecture, either normal or in a diseased state. ADC is a measure of the magnitude of diffusion (of water molecules) within tissue, and is commonly clinically calculated using MRI with diffusion weighted imaging, the extent of tissue cellularity and the presence of intact cell membrane help determine the impedance of water molecule diffusion. This impedance of water molecules diffusion can be quantitatively assessed using the ADC value. This assessment can be done using different b values via changing gradient amplitude. ADC values are calculated automatically by the software and then displayed as a parametric map that reflects the degree of diffusion of water molecules through different tissues. Then, by use of a dedicated workstation, ADC measurements are recorded for a given region by drawing regions of interest (ROIs) on the ADC map. SUVmax: The SUV is often used in PET imaging for a simple semiquantitative analysis. Its use is particularly common in the analysis of 18F-FDG images of cancer patients. It can also be used with other PET agents especially when no arterial input function is available for more detailed pharmacokinetic modeling. The SUV represents the ratio of the image derived radioactivity concentration found in a selected part of the body at a certain time point, and as reference the radioactivity concentration in the hypothetical case of an even distribution of the injected radioactivity across the whole body.
This study is well designed and well described. The conclusion that MRI and PET imaging including the quantitative analysis of ADC map on DW-MR imaging and glucose uptake by 18F-FDG-PET can contribute to the management of patients with locally advanced rectal cancer is helpful in clinical practice.
P- Reviewer: Shen J, Yazdi HR S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Curvo-Semedo L, Lambregts DM, Maas M, Thywissen T, Mehsen RT, Lammering G, Beets GL, Caseiro-Alves F, Beets-Tan RG. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy--conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260:734-743. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 216] [Article Influence: 16.6] [Reference Citation Analysis (1)] |
| 2. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1189] [Cited by in F6Publishing: 1313] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 3. | Bujko K, Kepka L, Nowacki MP. Chemoradiotherapy alone for rectal cancer: a word of caution. Lancet Oncol. 2007;8:860-862; author reply 862-863. [PubMed] [Cited in This Article: ] |
| 4. | O’Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Chemoradiotherapy alone for rectal cancer: a word of caution-author’s reply [Letter]. Lancet Oncol. 2007;8:662-863. [Cited in This Article: ] |
| 5. | O’Neill BD, Brown G, Heald RJ, Cunningham D, Tait DM. Non-operative treatment after neoadjuvant chemoradiotherapy for rectal cancer. Lancet Oncol. 2007;8:625-633. [PubMed] [Cited in This Article: ] |
| 6. | Kim SH, Lee JM, Hong SH, Kim GH, Lee JY, Han JK, Choi BI. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253:116-125. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 278] [Cited by in F6Publishing: 277] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 7. | Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG. Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study. Ann Surg Oncol. 2011;18:2224-2231. [PubMed] [Cited in This Article: ] |
| 8. | Kim SH, Lee JY, Lee JM, Han JK, Choi BI. Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer. Eur Radiol. 2011;21:987-995. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 147] [Cited by in F6Publishing: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 9. | Hein PA, Kremser C, Judmaier W, Griebel J, Pfeiffer KP, Kreczy A, Hug EB, Lukas P, DeVries AF. Diffusion-weighted magnetic resonance imaging for monitoring diffusion changes in rectal carcinoma during combined, preoperative chemoradiation: preliminary results of a prospective study. Eur J Radiol. 2003;45:214-222. [PubMed] [Cited in This Article: ] |
| 10. | DeVries AF, Kremser C, Hein PA, Griebel J, Krezcy A, Ofner D, Pfeiffer KP, Lukas P, Judmaier W. Tumor microcirculation and diffusion predict therapy outcome for primary rectal carcinoma. Int J Radiat Oncol Biol Phys. 2003;56:958-965. [PubMed] [Cited in This Article: ] |
| 11. | Kremser C, Judmaier W, Hein P, Griebel J, Lukas P, de Vries A. Preliminary results on the influence of chemoradiation on apparent diffusion coefficients of primary rectal carcinoma measured by magnetic resonance imaging. Strahlenther Onkol. 2003;179:641-649. [PubMed] [Cited in This Article: ] |
| 12. | Sun YS, Zhang XP, Tang L, Ji JF, Gu J, Cai Y, Zhang XY. Locally advanced rectal carcinoma treated with preoperative chemotherapy and radiation therapy: preliminary analysis of diffusion-weighted MR imaging for early detection of tumor histopathologic downstaging. Radiology. 2010;254:170-178. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 228] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Dzik-Jurasz A, Domenig C, George M, Wolber J, Padhani A, Brown G, Doran S. Diffusion MRI for prediction of response of rectal cancer to chemoradiation. Lancet. 2002;360:307-308. [PubMed] [Cited in This Article: ] |
| 14. | Roth Y, Tichler T, Kostenich G, Ruiz-Cabello J, Maier SE, Cohen JS, Orenstein A, Mardor Y. High-b-value diffusion-weighted MR imaging for pretreatment prediction and early monitoring of tumor response to therapy in mice. Radiology. 2004;232:685-692. [PubMed] [Cited in This Article: ] |
| 15. | Amthauer H, Denecke T, Rau B, Hildebrandt B, Hünerbein M, Ruf J, Schneider U, Gutberlet M, Schlag PM, Felix R. Response prediction by FDG-PET after neoadjuvant radiochemotherapy and combined regional hyperthermia of rectal cancer: correlation with endorectal ultrasound and histopathology. Eur J Nucl Med Mol Imaging. 2004;31:811-819. [PubMed] [Cited in This Article: ] |
| 16. | Kalff V, Duong C, Drummond EG, Matthews JP, Hicks RJ. Findings on 18F-FDG PET scans after neoadjuvant chemoradiation provides prognostic stratification in patients with locally advanced rectal carcinoma subsequently treated by radical surgery. J Nucl Med. 2006;47:14-22. [PubMed] [Cited in This Article: ] |
| 17. | Gu J, Khong PL, Wang S, Chan Q, Law W, Zhang J. Quantitative assessment of diffusion-weighted MR imaging in patients with primary rectal cancer: correlation with FDG-PET/CT. Mol Imaging Biol. 2011;13:1020-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] [Cited in This Article: ] |
| 19. | Janssen MH, Ollers MC, Riedl RG, van den Bogaard J, Buijsen J, van Stiphout RG, Aerts HJ, Lambin P, Lammering G. Accurate prediction of pathological rectal tumor response after two weeks of preoperative radiochemotherapy using (18)F-fluorodeoxyglucose-positron emission tomography-computed tomography imaging. Int J Radiat Oncol Biol Phys. 2010;77:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Capirci C, Rubello D, Chierichetti F, Crepaldi G, Carpi A, Nicolini A, Mandoliti G, Polico C. Restaging after neoadjuvant chemoradiotherapy for rectal adenocarcinoma: role of F18-FDG PET. Biomed Pharmacother. 2004;58:451-457. [PubMed] [Cited in This Article: ] |
| 21. | Kristiansen C, Loft A, Berthelsen AK, Graff J, Lindebjerg J, Bisgaard C, Jakobsen A. PET/CT and histopathologic response to preoperative chemoradiation therapy in locally advanced rectal cancer. Dis Colon Rectum. 2008;51:21-25. [PubMed] [Cited in This Article: ] |
| 22. | Suppiah A, Hunter IA, Cowley J, Garimella V, Cast J, Hartley JE, Monson JR. Magnetic resonance imaging accuracy in assessing tumour down-staging following chemoradiation in rectal cancer. Colorectal Dis. 2009;11:249-253. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 23. | Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99:109-112. [PubMed] [Cited in This Article: ] |
| 24. | Cascini GL, Avallone A, Delrio P, Guida C, Tatangelo F, Marone P, Aloj L, De Martinis F, Comella P, Parisi V. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47:1241-1248. [PubMed] [Cited in This Article: ] |
| 25. | Janssen MH, Ollers MC, van Stiphout RG, Buijsen J, van den Bogaard J, de Ruysscher D, Lambin P, Lammering G. Evaluation of early metabolic responses in rectal cancer during combined radiochemotherapy or radiotherapy alone: sequential FDG-PET-CT findings. Radiother Oncol. 2010;94:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Herrmann K, Bundschuh RA, Rosenberg R, Schmidt S, Praus C, Souvatzoglou M, Becker K, Schuster T, Essler M, Wieder HA. Comparison of different SUV-based methods for response prediction to neoadjuvant radiochemotherapy in locally advanced rectal cancer by FDG-PET and MRI. Mol Imaging Biol. 2011;13:1011-1019. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Rosenberg R, Herrmann K, Gertler R, Künzli B, Essler M, Lordick F, Becker K, Schuster T, Geinitz H, Maak M. The predictive value of metabolic response to preoperative radiochemotherapy in locally advanced rectal cancer measured by PET/CT. Int J Colorectal Dis. 2009;24:191-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 28. | Lambrecht M, Deroose C, Roels S, Vandecaveye V, Penninckx F, Sagaert X, van Cutsem E, de Keyzer F, Haustermans K. The use of FDG-PET/CT and diffusion-weighted magnetic resonance imaging for response prediction before, during and after preoperative chemoradiotherapy for rectal cancer. Acta Oncol. 2010;49:956-963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Shanmugan S, Arrangoiz R, Nitzkorski JR, Yu JQ, Li T, Cooper H, Konski A, Farma JM, Sigurdson ER. Predicting pathological response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer using 18FDG-PET/CT. Ann Surg Oncol. 2012;19:2178-2185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Sun W, Xu J, Hu W, Zhang Z, Shen W. The role of sequential 18(F) -FDG PET/CT in predicting tumour response after preoperative chemoradiation for rectal cancer. Colorectal Dis. 2013;15:e231-e238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Bampo C, Alessi A, Fantini S, Bertarelli G, de Braud F, Bombardieri E, Valvo F, Crippa F, Di Bartolomeo M, Mariani L. Is the standardized uptake value of FDG-PET/CT predictive of pathological complete response in locally advanced rectal cancer treated with capecitabine-based neoadjuvant chemoradiation? Oncology. 2013;84:191-199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Schiepers C, Haustermans K, Geboes K, Filez L, Bormans G, Penninckx F. The effect of preoperative radiation therapy on glucose utilization and cell kinetics in patients with primary rectal carcinoma. Cancer. 1999;85:803-811. [PubMed] [Cited in This Article: ] |
| 33. | Denecke T, Rau B, Hoffmann KT, Hildebrandt B, Ruf J, Gutberlet M, Hünerbein M, Felix R, Wust P, Amthauer H. Comparison of CT, MRI and FDG-PET in response prediction of patients with locally advanced rectal cancer after multimodal preoperative therapy: is there a benefit in using functional imaging? Eur Radiol. 2005;15:1658-1666. [PubMed] [Cited in This Article: ] |
| 34. | Haberkorn U, Strauss LG, Dimitrakopoulou A, Engenhart R, Oberdorfer F, Ostertag H, Romahn J, van Kaick G. PET studies of fluorodeoxyglucose metabolism in patients with recurrent colorectal tumors receiving radiotherapy. J Nucl Med. 1991;32:1485-1490. [PubMed] [Cited in This Article: ] |
| 35. | van Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, Jager PL, Hoekstra HJ, Elsinga PH. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45:695-700. [PubMed] [Cited in This Article: ] |
| 36. | Wieder HA, Geinitz H, Rosenberg R, Lordick F, Becker K, Stahl A, Rummeny E, Siewert JR, Schwaiger M, Stollfuss J. PET imaging with [18F]3’-deoxy-3’-fluorothymidine for prediction of response to neoadjuvant treatment in patients with rectal cancer. Eur J Nucl Med Mol Imaging. 2007;34:878-883. [PubMed] [Cited in This Article: ] |
| 37. | Jung SH, Heo SH, Kim JW, Jeong YY, Shin SS, Soung MG, Kim HR, Kang HK. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging. 2012;35:110-116. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 96] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Ha HI, Kim AY, Yu CS, Park SH, Ha HK. Locally advanced rectal cancer: diffusion-weighted MR tumour volumetry and the apparent diffusion coefficient for evaluating complete remission after preoperative chemoradiation therapy. Eur Radiol. 2013;23:3345-3353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Cai G, Xu Y, Zhu J, Gu WL, Zhang S, Ma XJ, Cai SJ, Zhang Z. Diffusion-weighted magnetic resonance imaging for predicting the response of rectal cancer to neoadjuvant concurrent chemoradiation. World J Gastroenterol. 2013;19:5520-5527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 37] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 40. | Birlik B, Obuz F, Elibol FD, Celik AO, Sokmen S, Terzi C, Sagol O, Sarioglu S, Gorken I, Oztop I. Diffusion-weighted MRI and MR- volumetry--in the evaluation of tumor response after preoperative chemoradiotherapy in patients with locally advanced rectal cancer. Magn Reson Imaging. 2015;33:201-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 41. | Kim YC, Lim JS, Keum KC, Kim KA, Myoung S, Shin SJ, Kim MJ, Kim NK, Suh J, Kim KW. Comparison of diffusion-weighted MRI and MR volumetry in the evaluation of early treatment outcomes after preoperative chemoradiotherapy for locally advanced rectal cancer. J Magn Reson Imaging. 2011;34:570-576. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Seierstad T, Folkvord S, Røe K, Flatmark K, Skretting A, Olsen DR. Early changes in apparent diffusion coefficient predict the quantitative antitumoral activity of capecitabine, oxaliplatin, and irradiation in HT29 xenografts in athymic nude mice. Neoplasia. 2007;9:392-400. [PubMed] [Cited in This Article: ] |
| 43. | Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging. 2012;35:1365-1371. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 44. | Genovesi D, Filippone A, Ausili Cèfaro G, Trignani M, Vinciguerra A, Augurio A, Di Tommaso M, Borzillo V, Sabatino F, Innocenti P. Diffusion-weighted magnetic resonance for prediction of response after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: preliminary results of a monoinstitutional prospective study. Eur J Surg Oncol. 2013;39:1071-1078. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 45. | Ippolito D, Monguzzi L, Guerra L, Deponti E, Gardani G, Messa C, Sironi S. Response to neoadjuvant therapy in locally advanced rectal cancer: assessment with diffusion-weighted MR imaging and 18FDG PET/CT. Abdom Imaging. 2012;37:1032-1040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 46. | Guerra L, Niespolo R, Di Pisa G, Ippolito D, De Ponti E, Terrevazzi S, Bovo G, Sironi S, Gardani G, Messa C. Change in glucose metabolism measured by 18F-FDG PET/CT as a predictor of histopathologic response to neoadjuvant treatment in rectal cancer. Abdom Imaging. 2011;36:38-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |