INTRODUCTION
Salivary gland tumours, despite being relatively uncommon, encompass a wide range of benign and malignant pathologies[1]. There can be crossovers between the appearance of benign and malignant lesions, there are rare subtypes and multiple pathologies can co-existing within a specimen[1]. The resulting unfamiliarity and diversity presents a diagnostic challenge to the general pathologist. Furthermore, the parotid gland presents additional challenges due to the contrasting treatments available, complex anatomy and relationship to the facial nerve.
As a rule, as the size of the salivary gland decreases, the chances of a lesion within that gland being malignant increases (roughly 25% parotid, 50% submandibular, 75% sublingual). Thus, around 75% of all parotid masses are benign[2]. Of benign parotid tumours, over 50% are pleomorphic adenomas[2,3]. Warthin’s tumour is the second most common benign tumour, usually found in older male patients and bilateral in 10%-15%[3]. Of the malignant tumours, the commonest are muco-epidemoid carcinoma, adenoid cystic carcinomas and metastases from squamous cell carcinoma or melanoma[2,3].
Traditionally, following presentation to surgical outpatients with a parotid mass, open surgical excision biopsy (SEB) was used as a method of obtaining a histological diagnosis. This fell out of favour in the 1980s due to the risk of infection, tumour seeding, facial nerve injury, sialocoele and fistula formation[4]. As a result, fine needle aspiration cytology (FNAC) gained popularity[5] and is still commonly used today. It is increasingly recognised that pre-operative (or pre-non-operative management) diagnosis of parotid lesions is essential. As has occurred in breast practice, the investigative pathway has evolved into a form of triple assessment with clinical, imaging and histological examination.
Ultrasound (US) is the primary imaging investigation of choice[6], with magnetic resonance imaging (MRI) reserved for large, complex or possibly malignant tumours[7,8]. Computed tomography can be of use where an inflammatory lesion such as an abscess is suspected or in malignant lesions where MRI is contraindicated. An accurate tissue diagnosis is required to inform decisions regarding management. Some benign lesions may be managed conservatively or non-operatively whereas others may require adjuvant therapies and in lesions where surgery is required, a tissue diagnosis would help with planning surgical technique. This could range from minimally invasive excision to an extensive dissection[2]. Furthermore, pre-treatment diagnosis is essential for appropriate patient consent for the various treatment options and prognosis.
As described below, FNAC has limitations and more recently a combined approach has emerged to obtain imaging and histological diagnosis: Ultrasound guided core biopsy (USCB). It is the purpose of this review to assess the evidence for the advantages and disadvantages of the techniques available.
FNAC
Since the 1980s the technique used most commonly for initial investigation has been FNAC[9]. This classically involves blind insertion of a needle into the parotid mass and aspiration by the clinician. It is a quick and safe sampling technique that can be performed readily in the outpatient setting. In the hands of a small number of experienced clinicians, FNAC can achieve high specificity and diagnostic accuracy (89% and 85%[2]). However, there are significant variations in the performance of FNAC within different practice settings as demonstrated in a recent meta-analysis[10]; it is associated with high levels of inadequate diagnoses and missed malignancies especially outside specialist centres[2]. Sensitivity for detecting malignancy has been reported between 70% and 80%[2,10] and non-diagnostic rates average at 14%-18%[11-13] but can be as high as 56%[2]. This is increasingly considered unacceptable and may have adverse effects on diagnosis, treatment and prognosis.
The diagnostic performance and specificity of FNAC can be improved significantly when used in conjunction with a cytologist or cytology technician-led service where the sample can be assessed immediately and lesions can be re-aspirated if required[14]. Image guidance also improves the yield of FNAC; US guidance increases diagnostic accuracy by enabling avoidance of necrotic or cystic regions and targeting of higher yield areas of the lesion for tissue extraction[12,14,15]. US guidance also allows the operator to confirm that the needle tip is inserted within the lesion.
Even when FNAC is optimised, parotid cytology still presents challenges to the pathologist. Cellular aspirate cannot be used for grading, staging or immunofluorescence and cannot assess the interaction with surrounding tissue. Reactive hyperplasia with atypia in lymph nodes adjacent to the salivary gland is often indistinguishable from lymphoma or squamous cell carcinoma metastases[11]. With Warthin’s tumour, cytological misinterpretation may occur due to a lack of characteristic features and overabundance of squamous metaplasia/atypia, mucoid/mucinous background, spindle-shaped cells and cystic/inflammatory debris[16]. Similarly, the presence of either cystic degeneration or squamous and mucinous metaplasia in the context of pleomorphic adenoma can lead to a false positive diagnosis of malignancy[17]. Ancillary cytology techniques including flow cytometry and in situ hybridisation can increase the diagnostic yield from FNAC but these are expensive and not widely available. There is also a shortage of trained cytology staff.
USCB
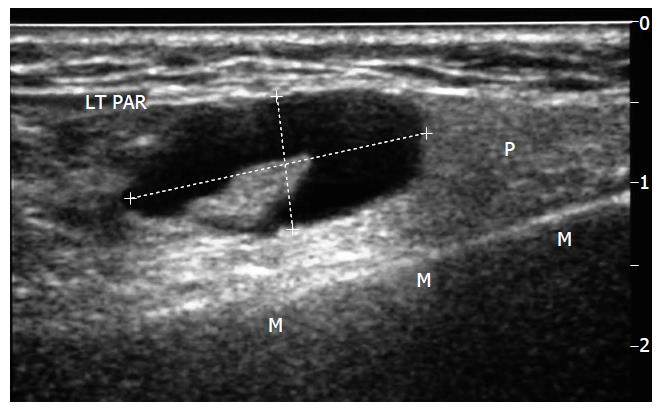
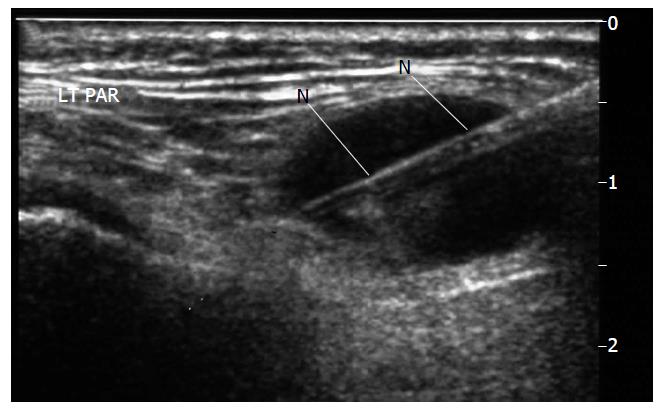
The perceived and real diagnostic limitations associated with FNAC have led operators to explore alternative biopsy techniques. USCB is a safe and successful technique for the diagnosis of parotid lesions where FNAC is non-diagnostic[18] and is developing into an established technique. The technique[18,19] requires an operator, usually a radiologist trained in head and neck US and biopsy techniques. The procedure requires local anaesthetic infiltration, a small skin incision and use of an automated spring-loaded biopsy device. The throw is deployed under US guidance. Figure 1 shows a parotid lesion on US scanning and Figure 2 shows the biopsy needle within the lesion. The core of tissue is then processed by paraffin section histology. Sections from the core can be used for grading, staging and immunohistochemical analysis, allowing planning of appropriate operative or non-operative management. Figures 3 and 4 show the results of a core biopsy macroscopically and microscopically respectively. Immunofluorescence analysis is particularly useful for haematological malignancies[18,19]. This technique is already well established in other areas of image guided biopsy including breast and abdomen[20] and is now widely accepted by histopathologists and oncologists as a sufficient technique to allow tumour grading and typing[21]. There will be a requirement for a wider pool of trained operators in order to disseminate the technique.
Figure 1 Longitudinal sonogram through left parotid gland (P) superficial lobe, mandible (M).
Demonstrates an atypical node, callipers-eccentric hilum displaced and heterogeneous cortex. LT PAR: Left Parotid.
Figure 2 Biopsy undertaken with spring loaded biopsy automated device, 22 mm throw, 18G needle (N).
Note needle deployed to traverse but not exit the lesion. LT PAR: Left Parotid.
Figure 3 Image of biopsy specimen taken in Figure 2.
18G-needle in biopsy tray with overlying needle overlying sheath retracted.
Figure 4 Low power image, H and E stain, of biopsy samples obtained in this case to give an idea of what material is available to the pathologist for reporting and immunohistochemistry - final diagnosis in this case was B cell non-hodgkins Lymphoma.
A recent meta-analysis incorporates 12 studies and provides substantial evidence supporting the potentially superior diagnostic yield of USCB over FNAC[22]. Although using a database of admittedly smaller studies when compared to FNAC, USCB showed a higher sensitivity (96%) and specificity (100%) compared to FNAC, a low complication rate (1.6% haematoma rate) and no variation in accuracy between locations[22]. The low complication rate is attributed to the ability of US to visualise the vessels and allow the operator to infer the position of facial nerve branches. Although only including a small number of centres, the authors felt the results could be replicated on a wider scale, possibly because of the standardisation of the procedure[22]. In agreement with this, a systematic observational clinico-pathological study demonstrated USCB of salivary gland lesions to have sensitivity 94%, specificity 100%, accuracy 96%, positive predictive value 100% and negative predictive value 90%[23]. A study by Eom et al[24] also showed that trainees had a significantly lower accuracy with fine needle aspiration than USCB when diagnosing malignant tumours when compared to USCB. Lymphomas diagnosed much more accurately using USCB as shown by Huang et al[25].
Despite generally favourable outcomes and diagnostic advantages, USCB is not yet considered the reference standard in parotid lesion biopsy and the optimum technique remains controversial. The potential problems with USCB include possible false negatives, complications of a slightly more invasive technique and tumour seeding. Haldar et al[2] describe 2 cases which were malignant clinically, on imaging and in the resected specimen but benign on USCB, i.e., false negatives. Also, some cases of well-differentiated malignancy require direct histological assessment of the whole excised lesion capsule to demonstrate invasiveness; USCB will miss this. This emphasises that USCB should not be used alone but as part of a triple assessment.
USCB is a slightly more invasive technique and cannot be used as part of a one-stop diagnostic clinic service as FNAC can. Bleeding and pain are potential complications following the technique. However it is thought the bleeding risk should be minimised by US allowing the operator to avoid vessels. Eom et al[24] as showed there were no complications requiring intervention or hospital admission. Tumour seeding and displaced epithelia are further potential complications. This is particularly relevant to pleomorphic adenoma which has recognised potential for tumour seeding[26]. However, the most recent meta-analysis did not describe any such events[22]. A comparative evaluation in 2013 which addressed tumour seeding post salivary gland biopsy[27] found only two cases following a 14G core needle biopsy; no cases were reported with needles of 18G or smaller. Interestingly, the same study also reported two cases of seeding post FNAC biopsy. However, the interval between seeding and resultant disease is variable and may be up to 20 years so longer term follow-up is needed for USCB. In a review of the literature on seeding of tumour cells Douville et al[28] conclude that there is a small risk of seeding, that needle size should be limited while maintaining diagnostic accuracy and that more follow-up is required to accurately assess the long term complications of core biopsy patients. One technique to potentially avoid seeding is to perform surgical excision of the biopsy tract but there is no evidence to support this routinely.
INTRA-OPERATIVE FROZEN SECTION
Another salivary gland biopsy technique is intra-operative frozen section (IOFS), which is used in a small number of specialist centres as an alternative to FNAC or where FNAC is not performing well. A surgical specimen is sent to the histopathology department prior to closure of the skin. It is frozen and examined immediately and results can then be telephoned to the surgeon within 20-30 min. This is useful where no pre-operative diagnosis is available, for example in deep parotid lesions not amenable to percutaneous biopsy or for lesions that are suspicious on clinical/imaging assessment but benign or considered non-representative on FNAC/USCB. IOFS can be employed to guide further surgical management while the patient is still on the operating table. A meta-analysis published in 2011 looking at data from 13 studies over a 25-year period describes 90% sensitivity, 99% specificity and good consistency of results across study centres[29].
IOFS mandates a full SEB and so cannot be recommended as a first-line diagnostic technique, particularly as patients cannot be consented appropriately. In addition, this service cannot be widely supported outside dedicated histopathology units, the histopathology unit may not be set up for it, there is a wide variety of complex lesions and most pathologists are reluctant to diagnose these on the table in the absence of further techniques including immunofluorescence. The pitfalls and failures outlined above for USCB also apply to IOFS. In general, IOFS can be used as an adjunct to the less invasive options described where diagnostic suspicion and uncertainty remain.
CONCLUSION
The wide spectrum of parotid gland pathology and complex gland anatomy with a variety of surgical options mean that accurate tissue diagnosis is an essential part of triple assessment to provide the appropriate care. There is no current referenced standard in the optimum method of biopsy assessment and the techniques described remain controversial. The continuing debate of cytology vs histology persists as the underlying issue. FNAC is an accepted and widely used technique under optimised circumstances. However, the diagnostic accuracy of a cellular aspirate is inherently lower, even under optimised circumstances, than the core of tissue which can be used for immunohistochemistry as is provided by USCB. USCB is also safe and well tolerated.
On balance and where available, USCB would seem the diagnostic biopsy technique of choice for parotid lesions. In institutions where FNAC has high rates of accuracy and is performing well, USCB should be considered as second line for non-diagnostic or equivocal samples. IOFS does not have a role as a first line diagnostic technique but may be reserved as an adjunct to equivocal FNAC/USCB or for lesions not amenable to percutaneous biopsy. With time and an increasing pool of trained operators, USCB is likely to supplant FNAC as the biopsy technique of choice, replicating that which has occurred already in breast practice. Continual evaluation of USCB outcome, clinical follow-up data and standardised operator training will be required.
P- Reviewer: Chen F, Tang GH S- Editor: Song XX L- Editor: A E- Editor: Li D