Published online Apr 26, 2022. doi: 10.4330/wjc.v14.i4.206
Peer-review started: March 18, 2021
First decision: July 18, 2021
Revised: August 28, 2022
Accepted: March 27, 2022
Article in press: March 27, 2022
Published online: April 26, 2022
Cardiac myxomas are common primary neoplasms of the heart. They are biologically benign but “functionally malignant” because of the potential for embolization. They arise most commonly from the left atrium, but no chambers of the heart are immune. They may be sporadic in the majority but also familial as a part of the Carney complex. Two morphological forms exist: polypoid and papillary. Polypoid myxomas often present with obstructive features, while the papillary forms are more prone to embolization. Histogenesis is still controversial; the current view centres around origin from the primitive pluripotent mesenchymal cells. They may be of giant proportion, be calcified or get infected. Clinical presentation typically involves the triad of intracardiac obstruction, embolic events and constitutional symptoms. Precordial examination findings may simulate those of mitral or tricuspid stenosis. The presence of tumour plop and change of the physical findings with changing position may help differentiation between the two. Echocardiography is the investigation of choice. Echogenic polypoid or papillary mobile mass within the atrial cavity remaining attached to the interatrial septum through a stalk are the tell-tale echocardiographic features. Cardiac magnetic resonance and computed tomographic scanning may have incremental diagnostic value. Histopathological examination reveals abundant loose myxoid stroma with scattered round, polygonal or stellate cells with dense irregular nuclei. Genetic testing may detect mutations in the PRKAR1A gene in the familial form of cardiac myxoma, i.e. the Carney complex. Surgical excision is the mainstay of treatment with low operative mortality, excellent postoperative survival and low recurrence rate. The current trend favours minimal-access surgery with or without robotic assistance. Physicians should have appropriate preparedness to make a timely diagnosis and enthusiastic treatment to avoid potentially fatal complications.
Core Tip: Cardiac myxomas are biologically benign but “functionally malignant”. They can cause life-threatening embolic events. Associated constitutional symptoms may mimic those of inflammatory or connective tissue disorders. Timely diagnosis is of utmost importance because it offers a scope for definitive treatment, i.e. surgical excision. Cardiac myxoma is a relatively rare diagnosis, so physicians should have appropriate preparedness to deal with this entity. This review article has summarised the available information, offered practical tips and highlighted the recent advances.
- Citation: Islam AKMM. Cardiac myxomas: A narrative review. World J Cardiol 2022; 14(4): 206-219
- URL: https://www.wjgnet.com/1949-8462/full/v14/i4/206.htm
- DOI: https://dx.doi.org/10.4330/wjc.v14.i4.206
Cardiac myxomas are primary neoplasms of the heart. Despite a preference for the left atrium, it can involve any of the cardiac chambers. The unusual feature of cardiac myxoma is that it has the biological potential to embolise and grow at the site of embolization[1], causing organ infarction. Timely diagnosis and treatment are essential for the prevention of sometimes life-threatening complications. Though a well-known entity, some aspects of cardiac myxoma are still evolving.
Cardiac myxoma is a rare disease; however, the exact prevalence is unknown. The reported prevalence is 0.03% in the general population[2]. Annual incidence of cardiac myxoma may be 0.5 to 1 case per million individuals[3,4]. A recently published Spanish study revealed a higher incidence; age-adjusted incidence was 1.6 per million population adjusting to the world population as a reference and 2.1 per million adjusting to the European population[5]. Myxomas are the commonest primary cardiac tumour constituting 50% to 85% of benign ones[6-8]. Middle-aged persons are commonly affected, but no age is immune. The tumour has a female preponderance with a female-to-male ratio of approximately 3:1[3,4]. Two epidemiological forms of cardiac myxoma exist: sporadic and familial. The former is far more common than the latter, constituting about 95% of cases[6].
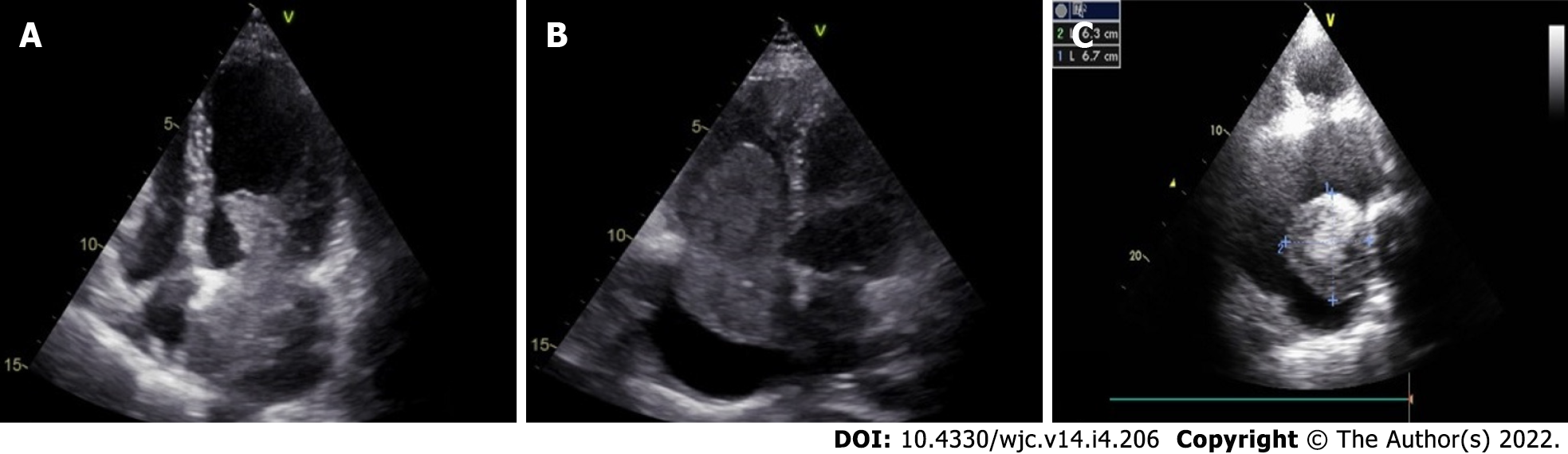
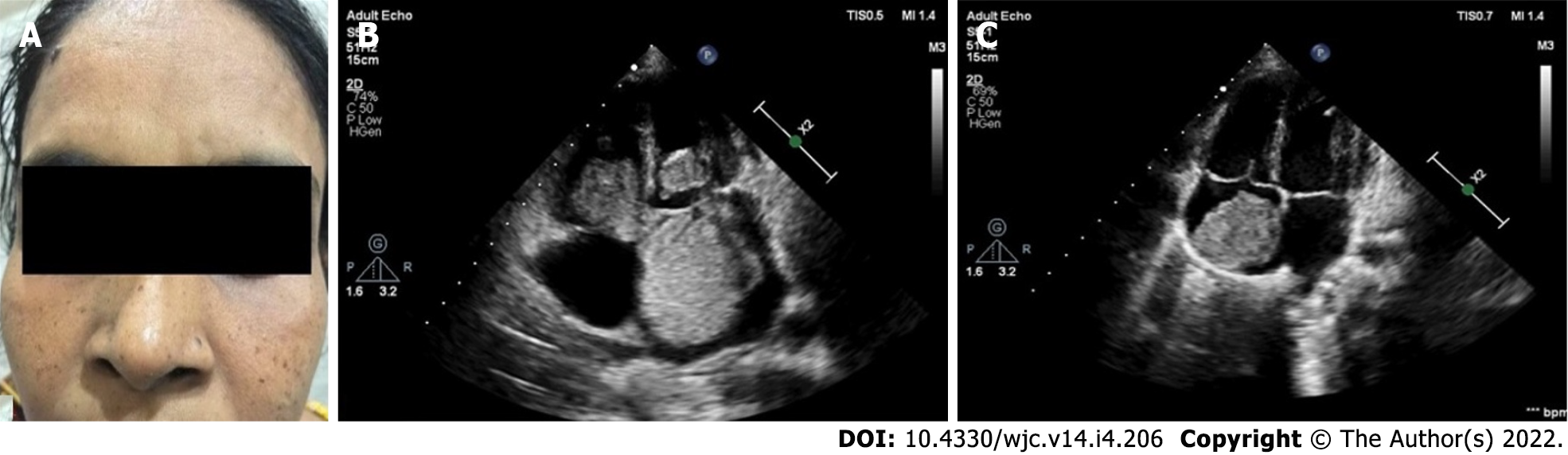
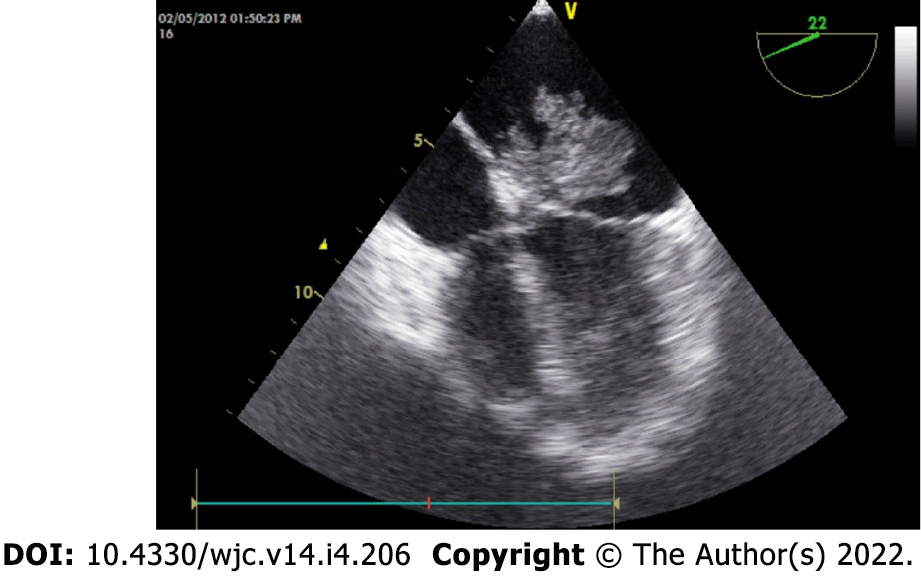
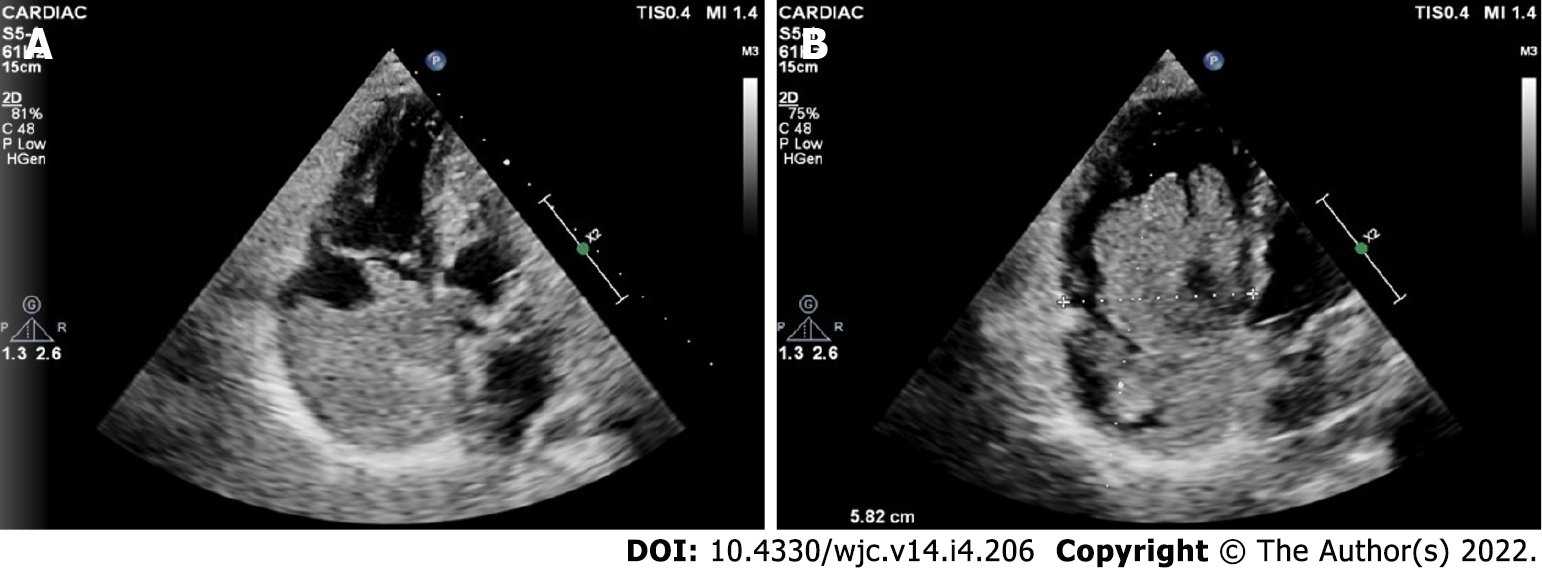
Cardiac myxomas can affect any chamber of the heart, but the left atrium is most commonly affected. Sites affected include the following: left atrium (75%); right atrium (15%-20%); left ventricle (3%-4%); and right ventricle (3%-4%)[9-12]. Regarding origin, myxoma has a predilection for limbus fossa ovalis of the interatrial septum. However, it can arise from the posterior atrial wall, anterior atrial wall and atrial appendage (Figure 1). The sporadic myxomas are usually single and bear these characteristics. On the other hand, familial myxomas may be multiple, multicentric and arising from atypical sites[13]. Less commonly, myxomas may be bi-atrial or multi-chamber; the latter may be part of the Carney complex[14-18] (Figures 2 and 3). Only rarely, myxomas affect the heart valves[7,19-21].
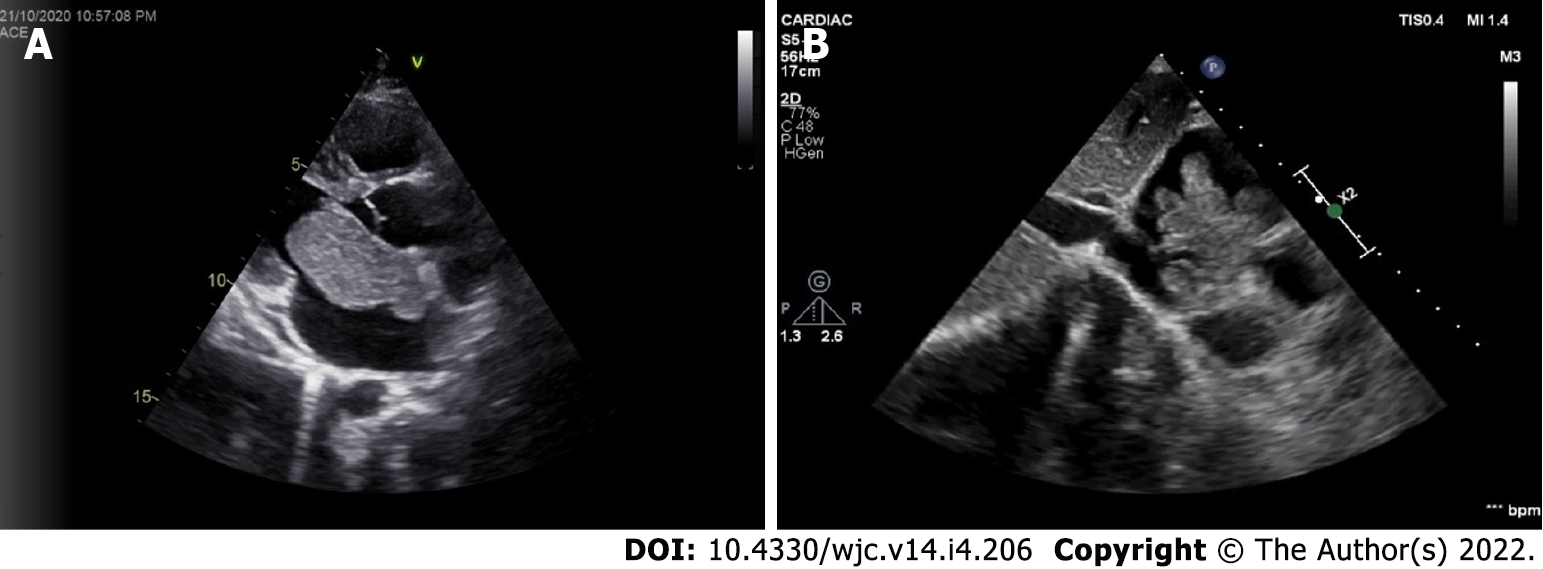
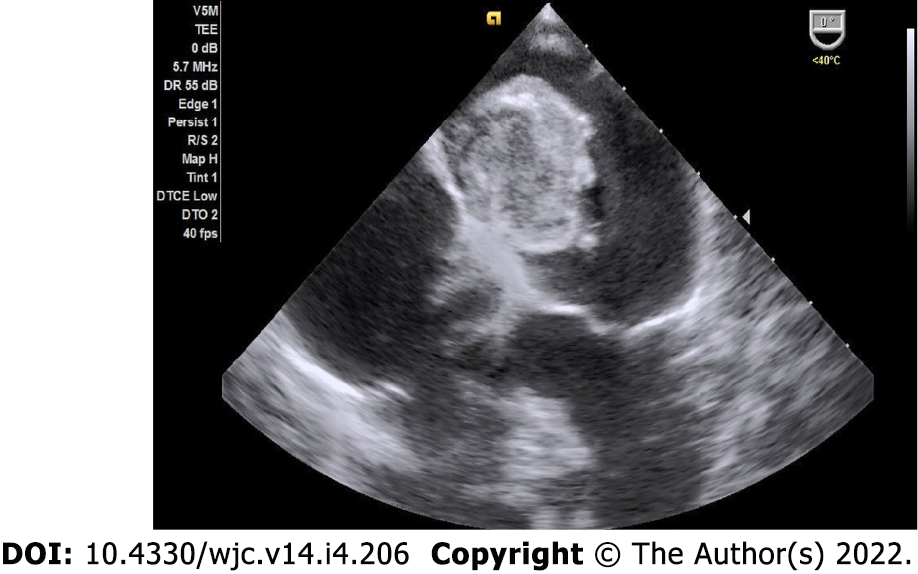
Myxomas may be ‘more solid’ polypoid in approximately two-thirds of cases or ‘softer’ papillary in one-third of cases[22] (Figure 4). The polypoid myxomas are generally pedunculated, more compact and have less tendency to undergo fragmentation and consequent embolisation[6]. On the other hand, papillary or villous myxomas are gelatinous, less compact, fragile and have a high potential for spontaneous fragmentation and embolisation to the central nervous system, kidney, spleen, extremities and coronary vessels (Figure 5).
Myxomas are considered biologically benign but “functionally malignant” tumours. They usually remain localised to the site of origin. They have a well-documented potential for fragmentation and embolisation. Besides this, metastasis to different locations, including the brain, sternum, spine and pelvis, has been described[23-29].
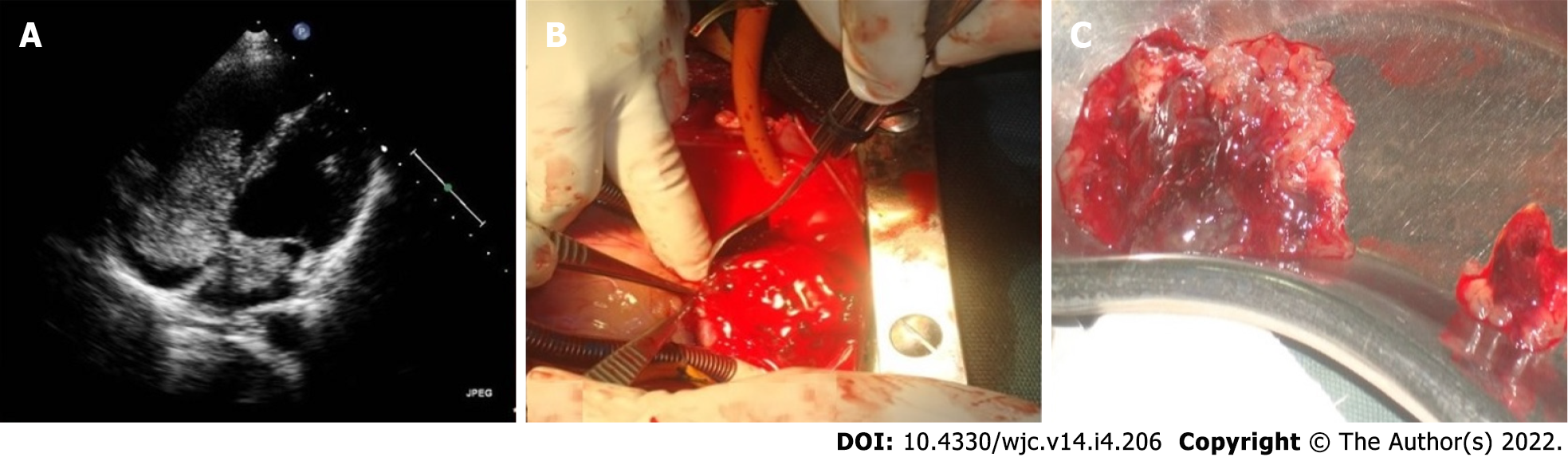
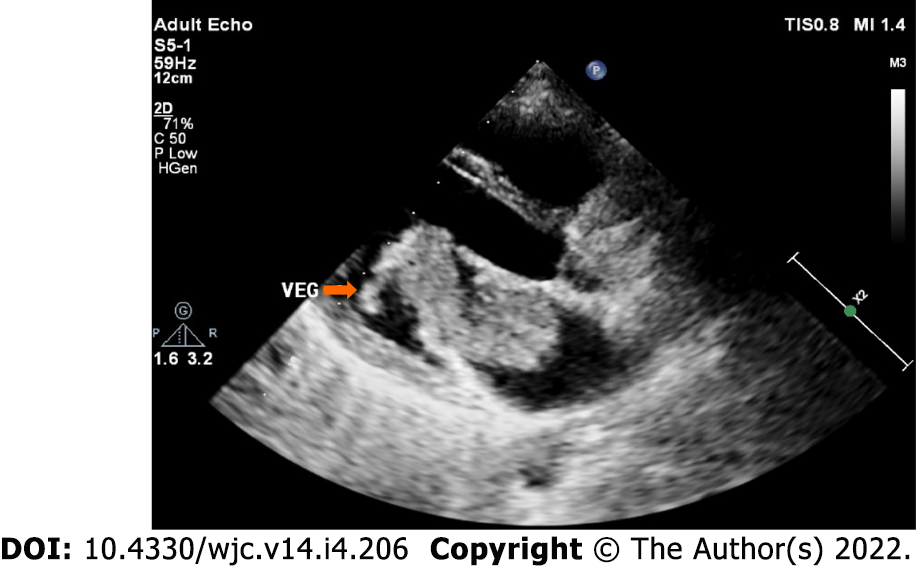
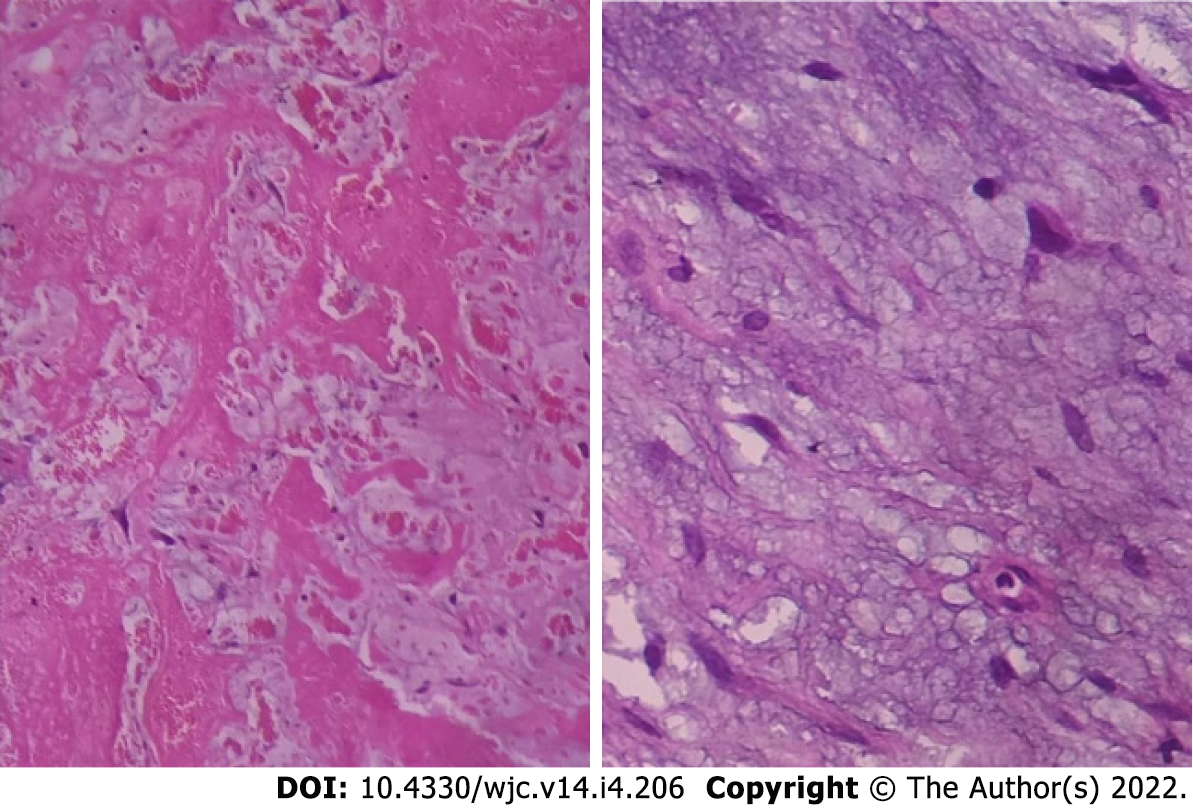
Myxomas may be enormous, occupying significant parts of the concerned cavity, sometimes termed “giant myxoma” (Figure 6). Rarely, they undergo calcification or osseous metaplasia[30-32] (Figure 7). Occasionally, they get infected[33-35] (Figure 8).
Histologically cardiac myxomas are mainly composed of stellated fusiform and polygonal cells immersed in an amorphous myxoid stroma[36] (Figure 9). Multinucleated cells are also observed. The cells are shaped and structured in chained rings or nests around the capillaries[37]. The surface of the tumour is often layered by flattened endothelium, while the tumour mass is infused liberally by thin-walled vessels lacking pericytes.
Immunohistochemically, a wide array of biological molecules has been found to be related to the cardiac myxomas, including CD31, CD34, CD56, FVIIIAg, S-100 protein, calretinin, vimentin, desmin, smooth muscle myosin, α1 antitrypsin and alpha 1-antichymotrypsin[38].
The histogenesis of myxoma is poorly understood; however, the current opinion favours origin from primitive pluripotent mesenchymal cells. Genes encoding heart precursor markers may get reactivated and expressed in cardiac myxoma cells leading to differentiation along endothelial/endocardial lines[39]. Previously, myxomas were thought to arise from Prichard structures, the microscopic endocardial/endothelial structures lined by plump endothelial cells, located in the fossa ovalis[40,41]. Origin from neuroendocrine tissue was also proposed.
The clinical presentation of cardiac myxoma depends on their location, size and mobility and is typified by the triad of intracardiac obstruction, embolisation and constitutional symptoms[6]. In a French series of 112 cases of cardiac myxoma, intracardiac obstruction in the form of mitral valve obstruction was the commonest manifestation (67%), followed by embolisation (29%) and constitutional symptoms (34%)[22].
Intracardiac obstruction: Intracardiac obstruction is common in polypoid myxoma. Because of preferential location, mitral valve pseudo-obstruction is the typical presentation[42-48]. Pulmonary hypertension may be present[43,47]. Valvular obstruction may even lead to syncope[46,49].
Right atrial myxomas may obstruct the tricuspid valve, the manifestation of which may be heart failure[50,51] or even collapse[52].
Embolisation: Embolisation is typically a feature of papillary-type myxomas because of their loose consistency and fragility. Overall, embolism occurs in 30% to 40% of patients with myxomas[6]. The site of embolisation depends on the location of the tumours. Left atrial myxomas commonly embolise to the brain, causing ischaemic stroke and occasionally visual loss. Coronary, renal and limb arteries may also be affected. In a retrospective study of 162 patients with cardiac myxomas surgically treated between 1998 and 2014 in China, the embolic event was observed in 33 patients (20.4%), in the brain (15.43%), limb (3.70%), pulmonary (0.62%) and coronary (0.62%)[53]. Tumour location (atypical), macroscopic appearance (irregular surface), mean platelet volume and high platelet count were strong risk factors for embolic events in patients with cardiac myxomas in this study. Cerebral embolisation is relatively common, leading to ischaemic stroke[54,55] and cerebral aneurysm formation[56]. Retinal artery occlusion and consequent visual loss have also been reported[57-60]. Coronary embolisation is a rare but well-documented and potentially fatal complication of cardiac myxomas[61-64]. Systemic embolisation may affect multiple sites, e.g., coronaries, viscera and limbs[65-67]. Right-sided myxomas are less prone to embolisation. However, right atrial myxoma, when present, may cause a pulmonary embolism[68-73].
Cardiac myxomas may even embolise peroperatively, leading to complications. Right atrial myxoma was reported to embolise during surgical excision, causing pulmonary embolism and cardiogenic shock and subsequent recovery after removal of the tumour embolus from the pulmonary artery[74].
Constitutional symptoms: Cardiac myxomas are commonly associated with constitutional symptoms mimicking inflammatory or connective tissue disorders[75-77]. These symptoms are more common in women than in men, in right-sided myxomas than in left-sided ones and in large and multicentric myxomas[78]. Malaise, anorexia, fever, arthralgia and weight loss are common. The underlying pathophysiology may be releasing cytokines from the tumour, especially interleukin-6 (IL-6)[79]. In fact, IL-6 may be a more sensitive biomarker than C-reactive protein in predicting the inflammatory status of patients with cardiac myxomas. Sessile, irregular and voluminous tumours tend to be associated with higher circulating IL-6 levels[80]. Myxomas occasionally present with pyrexia of unknown origin[79,81]. They may mimic bronchial asthma[82] or pulmonary tuberculosis[14]. Rarely, myxomas are associated with pleural effusion[14,83,84].
Infected myxoma: Occasionally, myxomas get infected, presenting with high fever and multiple embolic events[85-88] (Figure 8).
Cardiac myxoma in pregnancy: Occasionally, cardiac myxomas are diagnosed for the first time in pregnancy, mostly by echocardiography. Favourable maternal and foeto-neonatal outcomes with surgical management of cardiac myxoma in the pregnant patients have been reported in a recent review of 44 articles with 51 patients[89].
Cardiac myxomas are typified by the triad of intracardiac obstruction, embolic manifestations and constitutional symptoms. However, because of heterogeneity in location, size, morphology and histopathology, they may remain entirely asymptomatic, present with classical manifestations or produce life-threatening emergency of systemic embolisation or even sudden cardiac death[90]. As little as 10% to as high as 50% of the myxomas may be diagnosed incidentally during clinical evaluation[91,92]. General examination may reveal cachexia, fever, cyanosis, clubbing or rash. Neck veins may be engorged, and there may be a prominent A wave in the jugular venous pulse. Precordial findings may mimic mitral stenosis. The first heart sound (S1) may be loud and widely split because of the delay in the closure of the mitral valve due to the prolapse of the tumour into the mitral valve orifice. The pulmonary component of the second heart sound (P2) may be normal or loud depending on the presence of pulmonary hypertension. The characteristic “tumour plop” is a low-pitched early diastolic sound just after the S2. It may be confused with the opening snap of rheumatic mitral stenosis; however, the latter is high-pitched. It may be followed by a low-pitched diastolic murmur. The tumour plop is produced by the impact of the myxoma against the endocardial wall or when its excursion is halted. Also, a third heart sound (S3), fourth heart sound (S4) or a diastolic murmur of functional mitral or tricuspid stenosis may be audible. Occasionally, a systolic murmur of mitral or tricuspid regurgitation may be present. The auscultatory findings of cardiac myxomas characteristically change with changes in the position of the patient.
Echocardiography is the critical investigation for the diagnosis of cardiac myxomas. Other imaging modalities like computed tomography (CT) scanning and magnetic resonance imaging play an ancillary role. Chest X-ray and electrocardiography are of limited value. Haematological investigations are also routinely done. Histopathology confirms the diagnosis. Genetic testing plays a vital role in familial cases of myxomas.
Transthoracic echocardiography is the most practical investigation and often yields adequate information necessary for surgical resection. It makes the diagnosis and determines the location, size and shape of the tumour and its connections. Transesophageal echocardiography has a higher sensitivity and specificity and can detect small tumours, tumours located at atypical locations and possible multichamber myxomas[93-97].
Three-dimensional transthoracic echocardiography and transoesophageal echocardiography have also been used[98]. In a European study, transoesophageal echocardiography was superior to transthoracic echocardiography for myxoma detection (100% vs 95%) and attachment point identification (95.2% vs 64.5%)[99].
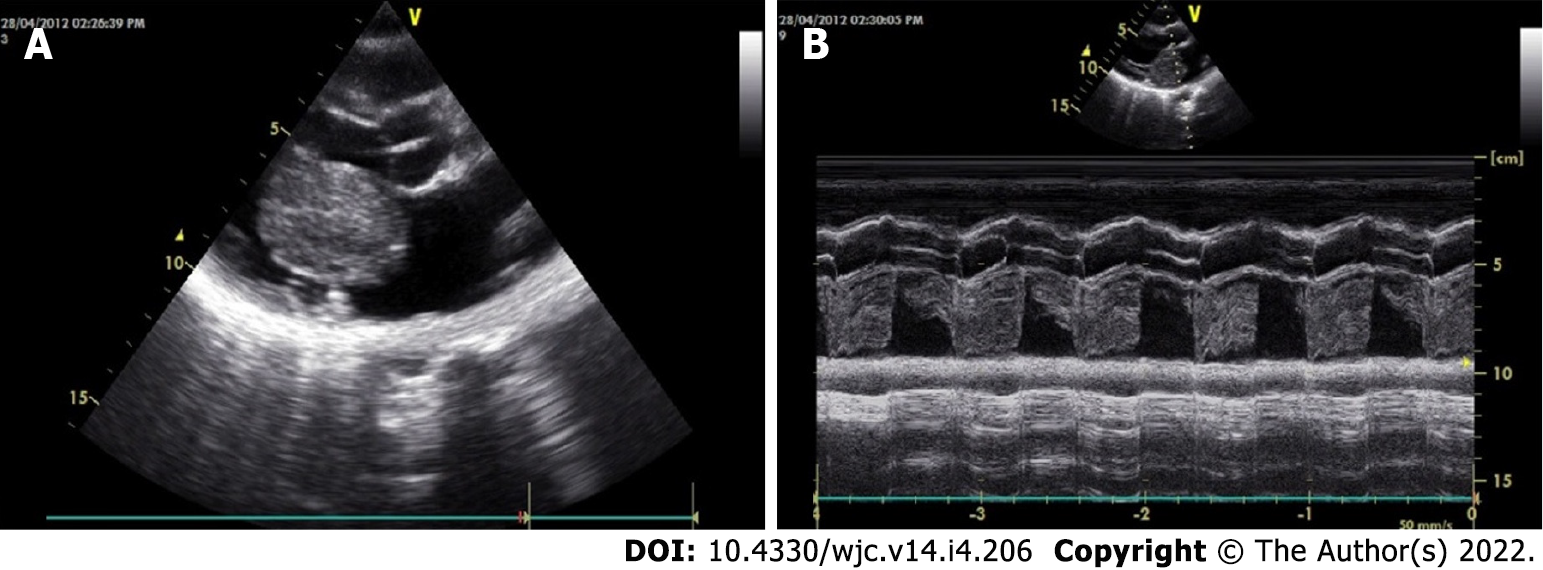
The classical features of an atrial myxoma in echocardiography include polypoid or papillary mass attached to the interatrial septum through a stalk and moving to and fro into the cavity, sometimes protruding into the corresponding ventricular cavity across the atrioventricular valve (Figure 10). Occasionally, the tumour mass may get areas of liquefaction or calcification. Doppler echocardiography shows the hemodynamic consequences of atrial myxoma.
During echocardiography, differentiation between myxoma and thrombus is of crucial importance. Myxomas typically have a stalk, a preference for limbus fossa ovalis of the atrial septum for stalk attachment and characteristic mobility. On the other hand, the thrombus is usually situated in the posterior portion of the atrium, has a preference for the atrial appendage, has a layered appearance and is most commonly seen in the presence of valvular mitral stenosis, atrial fibrillation and spontaneous echo contrast.
Contrast echocardiography can aid in the differential diagnosis of intracardiac masses based on perfusion of the mass. Malignant tumours are frequently highly vascular and present greater contrast enhancement than the adjacent myocardium, whereas myxomas demonstrate partial perfusion with lesser contrast enhancement than the adjoining myocardium. Thrombi, being avascular, show a complete absence of perfusion[100-102].
Despite the invaluable role of echocardiography and other imaging modalities, histopathological examination is the gold standard test for confirmation of the diagnosis of cardiac myxomas. A recently published study from Korea shows that out of 265 cases with an echocardiographic diagnosis of cardiac myxomas, 174 (65.7%) were surgically confirmed as myxomas. Compared with cardiac myxomas, other tumours were smaller and more frequently found in non-atrial sites[103].
ECG findings are nonspecific. Atrial enlargement or ventricular hypertrophy may be present. In contrast to the findings in mitral valve disease, atrial fibrillation is uncommon[6].
Chest skiagram has a limited role. Occasionally, it can present features of mitral stenosis, e.g., straightening of the left cardiac border and double contour of the right cardiac border and only rarely tumour calcification. Signs of pulmonary hypertension may be present.
Magnetic resonance imaging provides helpful information about the myxoma size, shape, surface characteristics and even its mobility on cine magnetic resonance gradient echo. The most frequent presentation is a mass isointense at T1-weighted and hyperintense at T2-weighted imaging with foci of hypointensity at one or two of these sequences[104]. Also, tissue characteristics can be used to differentiate a tumour from a thrombus.
CT scanning, generally, is not useful for the diagnosis of cardiac myxomas because it cannot reliably differentiate between myxomas and thrombi[105]. Typically, myxomas appear homogenous and isodense or as a slightly hypodense mass on non-contrast CT scanning, which does not show enhancement after iodinated contrast injection[106]. However, CT is the preferred technique to detect calcification, which is encountered in 10%–30% of cases[106,107].
Fluorodeoxyglucose positron emission tomography scanning is not typically indicated in the evaluation for myxoma[108].
Angiocardiography is seldom used for diagnosis of cardiac myxomas because of availability of non-invasive investigation modalities especially echocardiography. Also, manipulation of a catheter during angiocardiography carries a high risk of embolisation of tumour fragments[109,110]. In angiocardiography, cardiac myxomas typically appear as filling defects. In cases of left atrial myxoma, the levophase of a pulmonary angiogram may outline a radiolucent mass within the left atrium.
Genetic testing for mutations in the PRKAR1A gene is increasingly used for diagnostic certainty of Carney complex.
Erythrocyte sedimentation rate and C-reactive protein are generally elevated. Anaemia may be present. IL-6 rises especially when constitutional symptoms dominate.
Cardiac myxoma needs surgical excision often on an emergency basis. This is to reduce the risk of embolisation of the tumour. Surgery is otherwise simple. The root of the stalk and the full thickness of the adjacent interatrial septum is excised, and the consequent atrial septal defect is closed accordingly. Data published over the past decades show excellent overall outcomes in operative mortality, short- and long-term survival and tumour recurrence[4,101-118]. Surgical excision of 23 myxomas in Turkey between 2010 and 2017 showed excellent outcomes with no early or late mortality[112]. A 16-year single centre study from China reported no need for secondary surgery in 97.4% ± 2.5% of cases after 10 years. Overall, the actuarial survival was 98.4% ± 1.6% at 5 years and 96.0% ± 2.8% at 10 years[113].
A similar encouraging outcome was observed in Italy; surgical excision of 98 cardiac myxomas between 1990 and 2007 showed 3% operative mortality. Actuarial survival was 98%, 98% and 89% at 5, 10 and 15 years, respectively. There was only one recurrence 68 mo after the first surgery[115].
In a smaller series of 18 patients treated surgically over 5 years in the United Kingdom, no death occurred within 30 d post-procedure[116]. Follow-up of surgical treatment of cardiac myxomas in Germany showed no in-hospital deaths. Out of 57 patients, 52 were alive at a median follow-up of 7.5 years[117]. Between 2002 and 2008, 34 cardiac myxomas were operated on in a single centre in Pakistan; 32 patients survived the surgery, 2 patients died over a median follow-up of 34 mo, and 1 patient had recurrence after 27 mo[118]. Twenty-four years of experience in 49 patients from Austria revealed relatively low early mortality of 2.0% and late mortality of 6.1%. The long-term prognosis was excellent, with an actuarial survival rate of 0.74. The rate of reoperations was low, with 2.0% after 24 years[4]. In a recently published small study from Bangladesh, all 20 patients with cardiac myxoma survived the surgery, and 1 patient presented with recurrence 28 mo after the surgery[111]. In recent years, cardiac myxomas have been excised successfully by minimally invasive surgery with or without robotic assistance[119-122]. Robotic surgery has been associated with early restoration of normal quality of life and early return to employment[121].
Utmost caution is warranted during surgical excision of cardiac myxomas because of their potential for embolisation peroperatively[74].
Familial cardiac myxoma is rare and tend to form a syndrome, e.g., Carney complex. They can usually be distinguished from the sporadic forms by the presentation at a younger age, the unusual location and multicentricity of the lesions and the presence of rare pathological conditions. In addition, a higher rate of recurrent lesions is usually associated with the familial forms of this disease. Carney complex is a rare multiple neoplasia syndrome, characterised by pigmented lesions of the skin and mucosa, cardiac and extra-cardiac myxomatous tumours and multiple endocrine and non-endocrine neoplasms (Figure 3)[123,124]. It is inherited as an autosomal-dominant disorder in three-fourths of the cases. In the remaining one-fourth, it occurs sporadically as a result of a de novo genetic mutation[125]. The disease is caused by inactivating mutations or large deletions of the PRKAR1A gene located at 17q22–24 coding for the regulatory subunit type I alpha of protein kinase A (PKA) gene[126]. Myxomatous tumours also occur in the skin and breast. Lentigines, blue nevus and cutaneous myxoma are the common skin manifestations (Figure 3). Primary pigmented nodular adrenocortical disease and thyroid nodules are examples of endocrinopathies[127,128].
The Carney complex is diagnosed by the diagnostic criteria defined by Stratakis et al[124] (Table 1). Making the diagnosis, required either: (1) Two of the twelve manifestations of the disease listed; or (2) One of the twelve manifestations and one of the supplemental criteria. Genetic testing for mutations in the PRKAR1A gene confirms the diagnosis. For management, cardiac myxoma needs surgical excision. Primary pigmented nodular adrenocortical disease and pituitary adenomas are managed surgically, or the latter can be managed with somatostatin analogues[129,130]. Prognosis is good at present; however, lifelong follow-up is indicated[126].
| Diagnostic criteria |
| Spotty skin pigmentation with a typical distribution (lips, conjunctiva and inner or outer canthi, vaginal and penile mucosa) |
| Myxoma (cutaneous and mucosal)1 |
| Cardiac myxoma1 |
| Breast myxomatosis1 or fat-suppressed magnetic resonance imaging findings suggestive of this diagnosis |
| Primary pigmented nodular adrenocortical disease1 or paradoxical positive response of urinary glucocorticosteroids to dexamethasone administration during Liddle’s test |
| Acromegaly due to growth hormone-producing adenoma1 |
| Large cell calcifying Sertoli cell tumour1 or characteristic calcification on testicular ultrasonography |
| Thyroid carcinoma1 or multiple, hypoechoic nodules on thyroid ultrasonography, in a young patient |
| Psammomatous melanotic schwannoma1 |
| Blue nevus, epithelioid blue nevus (multiple)1 |
| Breast ductal adenoma (multiple)1 |
| Osteochondromyxoma1 |
| Supplemental criteria: |
| Affected first-degree relative |
| Inactivating mutation of thePRKAR1A gene |
Cardiac myxomas are the commonest neoplasm of the heart. They are primarily sporadic but may be familial as Carney complex. Though histologically benign, myxomas are prone to cause intracardiac obstruction and embolisation. Associated constitutional features may mimic inflammatory and connective tissue disorders creating diagnostic dilemmas. Echocardiography is a versatile tool for making the diagnosis and choosing the optimum management strategy. Surgery is the mainstay of treatment with an excellent prognosis. Long-term follow-up is often needed to look for recurrence. Physicians should have appropriate preparedness to diagnose this uncommon entity. Only timely diagnosis and prompt surgery can reduce the morbidity and mortality of cardiac myxoma patients.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: Bangladesh
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Papadopoulos KG, Greece S-Editor: Ma YJ L-Editor: Filipodia P-Editor: Ma YJ
| 1. | Velez Torres JM, Martinez Duarte E, Diaz-Perez JA, Rosenberg AE. Cardiac Myxoma: Review and Update of Contemporary Immunohistochemical Markers and Molecular Pathology. Adv Anat Pathol. 2020;27:380-384. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 2. | Grebenc ML, Rosado de Christenson ML, Burke AP, Green CE, Galvin JR. Primary cardiac and pericardial neoplasms: radiologic-pathologic correlation. Radiographics. 2000;20:1073-103; quiz 1110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 390] [Cited by in F6Publishing: 331] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Yoon DH, Roberts W. Sex distribution in cardiac myxomas. Am J Cardiol. 2002;90:563-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Keeling IM, Oberwalder P, Anelli-Monti M, Schuchlenz H, Demel U, Tilz GP, Rehak P, Rigler B. Cardiac myxomas: 24 years of experience in 49 patients. Eur J Cardiothorac Surg. 2002;22:971-977. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 170] [Cited by in F6Publishing: 170] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 5. | Pérez-Andreu J, Arribas Leal JM, Gervase G, Rivera-Caravaca JM, Cánovas López S, Marín F. Epidemiology of Cardiac Myxoma in a Spanish Population. A 30-year Surgical Series. Rev Esp Cardiol (Engl Ed). 2019;72:685-686. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Reynen K. Cardiac myxomas. N Engl J Med. 1995;333:1610-1617. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 878] [Cited by in F6Publishing: 791] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 7. | Wold LE, Lie JT. Cardiac myxomas: a clinicopathologic profile. Am J Pathol. 1980;101:219-240. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 8. | GOLDBERG HP, GLENN F, DOTTER CT, STEINBERG I. Myxoma of the left atrium; diagnosis made during life with operative and post-mortem findings. Circulation. 1952;6:762-767. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 139] [Cited by in F6Publishing: 146] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Hall RJ, Cooley DA, McAllister HA Jr, Frazier OH. Neoplastic heart disease. In: Hurst JW, ed. The heart, arteries and veins. 7th ed. New York: McGraw-Hill, 1990:1382-403. [Cited in This Article: ] |
| 10. | Silverman NA. Primary cardiac tumors. Ann Surg. 1980;191:127-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 308] [Cited by in F6Publishing: 329] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 11. | McAllister HA, Fenoglio JJ. Tumors of the cardiovascular system. Atlas of tumor pathology. 2nd Series. Fascicle 15. Washington, DC: Armed Forces Institute of Pathology, 1978:1-20. [Cited in This Article: ] |
| 12. | Meller J, Teichholz LE, Pichard AD, Matta R, Litwak R, Herman MV, Massie KF. Left ventricular myxoma: echocardiographic diagnosis and review of the literature. Am J Med. 1977;63:816-823. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 68] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | McCarthy PM, Piehler JM, Schaff HV, Pluth JR, Orszulak TA, Vidaillet HJ Jr, Carney JA. The significance of multiple, recurrent, and "complex" cardiac myxomas. J Thorac Cardiovasc Surg. 1986;91:389-396. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Islam AKMM, Islam KS, Ananya KF, Uddin MJ. A Tuberculosis Case Is Discovered to Be Carney Complex Instead! CASE (Phila). 2020;4:369-373. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Islam AM, Rahman MT, Paul TK. Biatrial myxoma in a young male patient. Korean Circ J. 2011;41:770-771. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Dittmann H, Voelker W, Karsch KR, Seipel L. Bilateral atrial myxomas detected by transesophageal two-dimensional echocardiography. Am Heart J. 1989;118:172-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Tway KP, Shah AA, Rahimtoola SH. Multiple bilateral myxomas demonstrated by two-dimensional echocardiography. Am J Med. 1981;71:896-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Balk AH, Wagenaar SS, Bruschke AV. Bilateral cardiac myxomas and peripheral myxomas in a patient with recent myocardial infarction. Am J Cardiol. 1979;44:767-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Sharma SC, Kulkarni A, Bhargava V, Modak A, Lashkare DV. Myxoma of tricuspid valve. J Thorac Cardiovasc Surg. 1991;101:938-940. [PubMed] [Cited in This Article: ] |
| 20. | Blondeau P. Primary cardiac tumors--French studies of 533 cases. Thorac Cardiovasc Surg. 1990;38 Suppl 2:192-195. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 133] [Cited by in F6Publishing: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Sandrasagra FA, Oliver WA, English TA. Myxoma of the mitral valve. Br Heart J. 1979;42:221-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 22. | Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001;80:159-172. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 550] [Cited by in F6Publishing: 615] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 23. | Wan Y, Du H, Zhang L, Guo S, Xu L, Li Y, He H, Zhou L, Chen Y, Mao L, Jin H, Hu B. Multiple cerebral metastases and metastatic aneurysms in patients with left atrial Myxoma: a case report. BMC Neurol. 2019;19:249. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 24. | Brinjikji W, Morris JM, Brown RD, Thielen KR, Wald JT, Giannini C, Cloft HJ, Wood CP. Neuroimaging Findings in Cardiac Myxoma Patients: A Single-Center Case Series of 47 Patients. Cerebrovasc Dis. 2015;40:35-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 25. | Zhan R, Ji T, Fan Z, Pan D. Perplexing imaging manifestations of multiple metastatic intracranial lesions associated with atrial myxoma. J Craniofac Surg. 2013;24:651-652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Raza E, Kamal AK. Recurrent non-aneurysmal, metastatic intraparenchymal haemorrhages following resection of atrial myxoma - case report and literature review. BMJ Case Rep. 2012;2012. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Moiyadi AV, Moiyadi AA, Sampath S, Kalpana SR, Mahadevan A, Shankar SK, Srikanth SG. Intracranial metastasis from a glandular variant of atrial myxoma. Acta Neurochir (Wien). 2007;149:1157-1162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Hirsch BE, Sehkar L, Kamerer DB. Metastatic atrial myxoma to the temporal bone: case report. Am J Otol. 1991;12:207-209. [PubMed] [Cited in This Article: ] |
| 29. | Okada N, Yamamura T, Kitano Y, Nakamura T, Kamido H, Matsuzawa Y, Katayama S, Ohara K. Metastasizing atrial myxoma: a case with multiple subcutaneous tumours. Br J Dermatol. 1986;115:239-242. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | Cervetti MR, Camporrotondo M, Clusa NM, Navia D. Advanced calcification of a left atrial myxoma. Eur J Cardiothorac Surg. 2020;57:196. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Singh SK, Kumar A, Tewarson V, Rani AK, Chandra S, Puri A. Calcified left atrial myxoma with osseous metaplasia. Indian J Chest Dis Allied Sci. 2012;54:201-203. [PubMed] [Cited in This Article: ] |
| 32. | Weiss SL, Russell HM, Lay A, Backer CL. Calcified right atrial myxoma in an adolescent. World J Pediatr Congenit Heart Surg. 2011;2:523-525. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Patel N, Arkonac D, Aoi S, Finkielstein D. Infective Endocarditis of a Left Ventricular Myxoma in a Heroin User. Tex Heart Inst J. 2019;46:215-218. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 34. | Yuan SM. Infected Cardiac Myxoma: an Updated Review. Braz J Cardiovasc Surg. 2015;30:571-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 35. | Revankar SG, Clark RA. Infected cardiac myxoma. Case report and literature review. Medicine (Baltimore). 1998;77:337-344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 46] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 36. | Burke AP, Tazeelar H, Gomez-Roman J, Loire R, Araoz PA. Benign tumours of pluripotent mesenchyme. In: Travis WD, Brambilla E, Muller-Hermelink HK, Harris CC, eds. World Health Organization tumours of the lung, pleura, thymus and heart. Lyon: IARC Press, 2004; 260-265. [Cited in This Article: ] |
| 37. | Altundag MB, Ertas G, Ucer AR, Durmus S, Abanuz H, Calikoğlu T, Ozbagi K, Demirkasimoglu A, Kaya B, Bakkal BH, Altundag K. Brain metastasis of cardiac myxoma: case report and review of the literature. J Neurooncol. 2005;75:181-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Hernández-Bringas O, Ortiz-Hidalgo C. [Histopathological and immunohistochemical features of cardiac myxomas]. Arch Cardiol Mex. 2013;83:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 39. | Di Vito A, Mignogna C, Donato G. The mysterious pathways of cardiac myxomas: a review of histogenesis, pathogenesis and pathology. Histopathology. 2015;66:321-332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | PRICHARD RW. Tumors of the heart; review of the subject and report of 150 cases. AMA Arch Pathol. 1951;51:98-128. [PubMed] [Cited in This Article: ] |
| 41. | Krikler DM, Rode J, Davies MJ, Woolf N, Moss E. Atrial myxoma: a tumour in search of its origins. Br Heart J. 1992;67:89-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 35] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Theodoropoulos KC, Masoero G, Pagnano G, Walker N, Papachristidis A, Monaghan MJ. Mitral pseudostenosis due to a large left atrial myxoma. J Geriatr Cardiol. 2018;15:244-245. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 43. | Shimizu Y, Itoda Y, Higashikuni Y, Kadowaki Y, Saito A, Fujita H, Yamashita H, Watanabe M, Ono M, Komuro I. Giant left atrial myxoma that caused mitral valve obstruction and pulmonary hypertension. Int J Cardiol. 2015;199:38-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Avakian SD, Takada JY, Mansur Ade P. Giant obstructive left atrial myxoma resembling mitral valve stenosis. Clinics (Sao Paulo). 2012;67:853-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 45. | Baztarrica GP, Salvaggio F, Porcile R. [Severe mitral valve stenosis caused by giant left atrial myxoma]. G Ital Cardiol (Rome). 2012;13:71-72. [PubMed] [Cited in This Article: ] |
| 46. | Strecker T, Agaimy A. Giant left atrial myxoma causing drop attacks by prolapsing into the mitral valve. Int J Clin Exp Pathol. 2012;5:996-999. [PubMed] [Cited in This Article: ] |
| 47. | Buyukates M, Aktunc E. Giant left atrial myxoma causing mitral valve obstruction and pulmonary hypertension. Can J Surg. 2008;51:E97-E98. [PubMed] [Cited in This Article: ] |
| 48. | Thangaroopan M, Chiu B, Thangaroopan N, Mullen J, Kasa L. Large left atrial myxoma causing mitral valve obstruction: diagnosis by computed tomography. J Card Surg. 2006;21:508-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Rajani AR, Muaz RN, Govindaswamy PR, Mian MH. Arrhythmias are not to blame for all cardiac syncope patients: left atrial myxoma causing syncope in a middle-aged man. BMJ Case Rep. 2015;2015. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Agstam S, Kumar B, Dahiya N, Guleria VS. Giant right atrial myxoma presenting as right heart failure: a rare manifestation. BMJ Case Rep. 2020;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 51. | Alamri Y, Lau YY, Lainchbury J. Large right atrial myxoma presenting with heart failure. ANZ J Surg. 2019;89:1341-1342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Agrawal R, Sharma A, Nath RK, Pandit BN. Massive right atrial myxoma presenting as congestive heart failure: an unusual presentation of a rare tumour. BMJ Case Rep. 2018;2018. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 53. | He DK, Zhang YF, Liang Y, Ye SX, Wang C, Kang B, Wang ZN. Risk factors for embolism in cardiac myxoma: a retrospective analysis. Med Sci Monit. 2015;21:1146-1154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 35] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Ihsen Z, Hela M, Khadija M, Zouhayer J. Cerebral embolism complicating left atrial myxoma: a case report. Pan Afr Med J. 2016;24:140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 55. | Al-Mateen M, Hood M, Trippel D, Insalaco SJ, Otto RK, Vitikainen KJ. Cerebral embolism from atrial myxoma in pediatric patients. Pediatrics. 2003;112:e162-e167. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 34] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 56. | Alrohimi A, Putko BN, Jeffery D, Van Dijk R, Chow M, McCombe JA. Cerebral Aneurysm in Association with Left Atrial Myxoma. Can J Neurol Sci. 2019;46:637-639. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Galvez-Ruiz A, Galindo-Ferreiro A, Lehner AJ, Kozac I. Clinical presentation of multiple cerebral emboli and central retinal artery occlusion (CRAO) as signs of cardiac myxoma. Saudi J Ophthalmol. 2018;32:151-155. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Shin YT, Kim JH, Park DH, Shin JP, Kim IT. Central Retinal Artery Occlusion by Left Atrial Myxoma. Korean J Ophthalmol. 2017;31:88-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 59. | Kim JH, Youn HJ, Jung MH, Oh CY, Ahn SH, Cho WH, Lee JH, Lee YS, Hyun HJ. Retinal artery occlusion by left atrial myxoma misdiagnosed as thrombus. Springerplus. 2016;5:352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 60. | Yu Y, Zhu Y, Dong A, Su Z. Retinal artery occlusion as the manifestation of left atrial myxoma: a case report. BMC Ophthalmol. 2014;14:164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 61. | Peters MJ, Tuwairqi KW, Farah MG. A Case of Infected Left Atrial Myxoma Presenting as ST-Elevation Myocardial Infarction (STEMI). Am J Case Rep. 2019;20:1930-1935. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 62. | Chaudhuri AA, Simmons C Jr, Ellison D, Hemp J, Chung K. Atrial Myxoma Presenting as Myocardial Infarction Diagnosed by Echocardiography, Managed Endoscopically with Robot-Assisted Surgery. Cureus. 2016;8:e484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 63. | Marta L, Peres M, Alves M, Ferreira da Silva G. [Giant left atrial myxoma presenting as acute myocardial infarction]. Rev Port Cardiol. 2012;31:815-819. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 64. | Braun S, Schrötter H, Reynen K, Schwencke C, Strasser RH. Myocardial infarction as complication of left atrial myxoma. Int J Cardiol. 2005;101:115-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 65. | Latifi AN, Ibe U, Gnanaraj J. A case report of atrial myxoma presenting with systemic embolization and myocardial infarction. Eur Heart J Case Rep. 2019;3:ytz104. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 66. | Wu Y, Fu XM, Liao XB, Zhou X. Stroke and peripheral embolisms in a pediatric patient with giant atrial myxoma: Case report and review of current literature. Medicine (Baltimore). 2018;97:e11653. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Bernatchez J, Gaudreault V, Vincent G, Rheaume P. Left Atrial Myxoma Presenting as an Embolic Shower: A Case Report and Review of Literature. Ann Vasc Surg. 2018;53:266.e13-266.e20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Ma G, Wang D, He Y, Zhang R, Zhou Y, Ying K. Pulmonary embolism as the initial manifestation of right atrial myxoma: A case report and review of the literature. Medicine (Baltimore). 2019;98:e18386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 69. | Pandey AC, Carey JJ, Thompson JL. Right atrial myxoma presenting as a pulmonary embolism in a 32-year-old female. JRSM Cardiovasc Dis. 2019;8:2048004018817606. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Kurnicka K, Domienik-Karłowicz J, Ciurzyński M, Biederman A, Pruszczyk P. Right atrial myxoma with pulmonary embolism. Kardiol Pol. 2015;73:298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 71. | Aydın C, Taşal A, Ay Y, Vatankulu MA, Inan B, Bacaksız A. A giant right atrial villous myxoma with simultaneous pulmonary embolism. Int J Surg Case Rep. 2014;5:206-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 72. | Subban V, Lakshmanan A, Sethurathinam R, Ajit MS. Right atrial myxoma--an unusual cause of pulmonary embolism. J Card Surg. 2012;27:604. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 73. | Cheema U, Thomas J. A giant right atrial myxoma presenting as acute pulmonary emboli. Eur Heart J Cardiovasc Imaging. 2012;13:799. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Papadopoulos K, Alexiou C, Ozden Tok O, Vannan MA. Intraoperative embolism of a right atrial myxoma: a case report. Eur Heart J Case Rep. 2020;4:1-4. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Garcia-Carretero R, Naranjo-Mansilla G, Luna-Heredia E, Arias-Baldo P, Beamonte-Vela BN. Incidental Finding of a Left Atrial Myxoma While Characterising an Autoimmune Disease. J Crit Care Med (Targu Mures). 2018;4:64-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 76. | Moreno-Ariño M, Ortiz-Santamaria V, Deudero Infante A, Ayats Delgado M, Novell Teixidó F. A classic mimicker of systemic vasculitis. Reumatol Clin. 2016;12:103-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 77. | Lazaros G, Masoura C, Brili S, Stavropoulos G, Kafiri G, Stefanadis C. Large left atrial myxoma in an oligosymptomatic young woman. Hellenic J Cardiol. 2013;54:60-63. [PubMed] [Cited in This Article: ] |
| 78. | Endo A, Ohtahara A, Kinugawa T, Ogino K, Hisatome I, Shigemasa C. Characteristics of cardiac myxoma with constitutional signs: a multicenter study in Japan. Clin Cardiol. 2002;25:367-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 79. | Gavrielatos G, Letsas KP, Pappas LK, Dedeilias P, Sioras E, Kardaras F. Large left atrial myxoma presented as fever of unknown origin: a challenging diagnosis and a review of the literature. Cardiovasc Pathol. 2007;16:365-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 80. | Yuan SM, Lin HZ. Predictors of Normalization of Circulating Interleukin-6 after Cardiac Myxoma Resection. Braz J Cardiovasc Surg. 2019;34:22-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 81. | Lin JN, Lai CH, Lu LF, Lin HH. Fever of unknown origin from a left atrial myxoma: an immunologic basis and cytokine association. South Med J. 2011;104:360-362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 82. | Zuwasti U, Quarrie R, Allen E, Haas C. Severe functional mitral stenosis due to a left atrial myxoma masquerading as asthma. BMJ Case Rep. 2020;13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 5] [Reference Citation Analysis (0)] |
| 83. | Cakar MA, Arslan C, Yildiz A, Vatan MB, Gunduz H. Left atrial myxoma with pleural effusion. J Clin Med Res. 2009;1:297-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 84. | Strâmbu I, Ilieşiu A, Creţul R, Stoicescu IP. [Recurrent pleural effusion revealing a left atrial myxoma]. Pneumologia. 2002;51:54-58. [PubMed] [Cited in This Article: ] |
| 85. | Yalta K, Ozturk C, Yalta T, Yetkin E. Systemic Inflammation in the Setting of Cardiac Myxomas: an Overview of Clinical and Practical Considerations. Korean Circ J. 2021;51:784-786. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 86. | Nagata T, Totsugawa T, Katayama K, Kuinose M, Yoshitaka H, Uesugi T. Infected cardiac myxoma. J Card Surg. 2013;28:682-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 87. | Leone S, dell'aquila G, Giglio S, Magliocca M, Maio P, Nigro FS, Pacifico P, De Chiara G, Acone N. Infected atrial myxoma: case report and literature review. Infez Med. 2007;15:256-261. [PubMed] [Cited in This Article: ] |
| 88. | Uchino K, Mochida Y, Ebina T, Tobe M, Kobayashi S, Yano Y, Kobayashi T, Nakazawa I, Ishikawa T, Kimura K, Takanashi Y, Umemura S. Infected left atrial myxoma. Intern Med. 2002;41:957-960. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Yuan SM. Cardiac myxoma in pregnancy: a comprehensive review. Rev Bras Cir Cardiovasc. 2015;30:386-394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 90. | Rios RE, Burmeister DB, Bean EW. Complications of atrial myxoma. Am J Emerg Med. 2016;34:2465.e1-2465.e2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 91. | Gošev I, Paić F, Durić Z, Gošev M, Ivčević S, Jakuš FB, Biočina B. Cardiac myxoma the great imitators: comprehensive histopathological and molecular approach. Int J Cardiol. 2013;164:7-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 55] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 92. | Beghetti M, Gow RM, Haney I, Mawson J, Williams WG, Freedom RM. Pediatric primary benign cardiac tumors: a 15-year review. Am Heart J. 1997;134:1107-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 231] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 93. | Obeid AI, Marvasti M, Parker F, Rosenberg J. Comparison of transthoracic and transesophageal echocardiography in diagnosis of left atrial myxoma. Am J Cardiol. 1989;63:1006-1008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 88] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 94. | Gadhinglajkar S, Sreedhar R. Intraoperative evaluation of left atrial myxoma using real-time 3D transesophageal echocardiography. Ann Card Anaesth. 2010;13:180-181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 95. | Gadhinglajkar S, Sreedhar R. Utility of transoesophageal echocardiography during surgery on left atrial myxoma. Ann Card Anaesth. 2008;11:142-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 96. | Srivastava R, Hsiung MC, Fadel A, Nanda NC. Transesophageal echocardiographic demonstration of biatrial myxoma. Echocardiography. 2004;21:187-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 97. | Pérez de Isla L, de Castro R, Zamorano JL, Almería C, Moreno R, Moreno M, Lima P, García Fernández MA. Diagnosis and treatment of cardiac myxomas by transesophageal echocardiography. Am J Cardiol. 2002;90:1419-1421. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 98. | Tolstrup K, Shiota T, Gurudevan S, Luthringer D, Luo H, Siegel RJ. Left atrial myxomas: correlation of two-dimensional and live three-dimensional transesophageal echocardiography with the clinical and pathologic findings. J Am Soc Echocardiogr. 2011;24:618-624. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 99. | Engberding R, Daniel WG, Erbel R, Kasper W, Lestuzzi C, Curtius JM, Sutherland GR, Lambertz H, von Hehn A, Lesbre JP. Diagnosis of heart tumours by transoesophageal echocardiography: a multicentre study in 154 patients. European Cooperative Study Group. Eur Heart J. 1993;14:1223-1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 136] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 100. | Bhattacharyya S, Khattar R, Senior R. Characterisation of intra-cardiac masses by myocardial contrast echocardiography. Int J Cardiol. 2013;163:e11-e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Uenishi EK, Caldas MA, Saroute AN, Tsutsui JM, Piotto GH, Falcão SN, Mathias W Jr. Contrast echocardiography for the evaluation of tumors and thrombi. Arq Bras Cardiol. 2008;91:e48-e52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Kirkpatrick JN, Wong T, Bednarz JE, Spencer KT, Sugeng L, Ward RP, DeCara JM, Weinert L, Krausz T, Lang RM. Differential diagnosis of cardiac masses using contrast echocardiographic perfusion imaging. J Am Coll Cardiol. 2004;43:1412-1419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 103. | Lee SH, Park JS, Park JH, Chin JY, Yoon WS, Kim HY, Cho JY, Kim KH, Kim WH. Comparison of Clinical and Echocardiographic Characteristics between Cardiac Myxomas and Masses Mimicking Myxoma. Korean Circ J. 2020;50:822-832. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | Colin GC, Dymarkowski S, Gerber B, Michoux N, Bogaert J. Cardiac myxoma imaging features and tissue characteristics at cardiovascular magnetic resonance. Int J Cardiol. 2016;202:950-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Scheffel H, Baumueller S, Stolzmann P, Leschka S, Plass A, Alkadhi H, Schertler T. Atrial myxomas and thrombi: comparison of imaging features on CT. AJR Am J Roentgenol. 2009;192:639-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 92] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 106. | Shin W, Choe YH, Kim SM, Song IY, Kim SS. Detection of cardiac myxomas with non-contrast chest CT. Acta Radiol. 2014;55:273-278. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 107. | Grebenc ML, Rosado-de-Christenson ML, Green CE, Burke AP, Galvin JR. Cardiac myxoma: imaging features in 83 patients. Radiographics. 2002;22:673-689. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 108. | Colin GC, Gerber BL, Amzulescu M, Bogaert J. Cardiac myxoma: a contemporary multimodality imaging review. Int J Cardiovasc Imaging. 2018;34:1789-1808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 109. | Wagner WL, Manz M, Steudel A, Kirchhoff PG, Lüderitz B. [Noninvasive diagnosis of bilocular left atrial and right ventricular myxoma of the heart]. Z Kardiol. 1988;77:251-255. [PubMed] [Cited in This Article: ] |
| 110. | Pindyck F, Peirce EC 2nd, Baron MG, Lukban SB. Embolization of left atrial myxoma after transseptal cardiac cathertization. Am J Cardiol. 1972;30:569-571. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 45] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 111. | Hosain N, Quaium Chowdhury MA, Maruf MF, Chowdhury MR, Barua S, Rahman M. Surgical Treatment of Atrial Myxomas: Outstanding Outcome of a Treacherous Tumor. CJC Open. 2021;3:354-360. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 112. | Gür AK, Aykaç MC. Surgical Treatment of Cardiac Myxomas: A 23-Case Experience. Heart Surg Forum. 2018;21:E370-E374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 113. | Lin Y, Xiao J, Chen J, Hong J, Peng H, Kang B, Wu L, Wang Z. Treating cardiac myxomas: a 16-year Chinese single-center study. J Cardiovasc Med (Hagerstown). 2016;17:44-53. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 114. | Vroomen M, Houthuizen P, Khamooshian A, Soliman Hamad MA, van Straten AH. Long-term follow-up of 82 patients after surgical excision of atrial myxomas. Interact Cardiovasc Thorac Surg. 2015;21:183-188. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 115. | Garatti A, Nano G, Canziani A, Gagliardotto P, Mossuto E, Frigiola A, Menicanti L. Surgical excision of cardiac myxomas: twenty years experience at a single institution. Ann Thorac Surg. 2012;93:825-831. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 116. | Owers CE, Vaughan P, Braidley PC, Wilkinson GA, Locke TJ, Cooper GJ, Briffa NP, Hopkinson DN, Sarkar PK. Atrial myxomas: a single unit's experience in the modern era. Heart Surg Forum. 2011;14:E105-E109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 117. | Kuroczyński W, Peivandi AA, Ewald P, Pruefer D, Heinemann M, Vahl CF. Cardiac myxomas: short- and long-term follow-up. Cardiol J. 2009;16:447-454. [PubMed] [Cited in This Article: ] |
| 118. | Khan MA, Khan AA, Waseem M. Surgical experience with cardiac myxomas. J Ayub Med Coll Abbottabad. 2008;20:76-79. [PubMed] [Cited in This Article: ] |
| 119. | Dang QH, Le NT, Tran DD, Ngo TH. Totally Endoscopic Resection of Giant Left Atrial Myxoma Without Robotic Assistance. Innovations (Phila). 2018;13:136-139. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 120. | Onan B, Kahraman Z, Erturk M, Erkanli K. Robotic resection of giant left ventricular myxoma causing outflow tract obstruction. J Card Surg. 2017;32:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 121. | Yang M, Yao M, Wang G, Xiao C, Wu Y, Zhang H, Gao C. Comparison of postoperative quality of life for patients who undergo atrial myxoma excision with robotically assisted versus conventional surgery. J Thorac Cardiovasc Surg. 2015;150:152-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 122. | Schilling J, Engel AM, Hassan M, Smith JM. Robotic excision of atrial myxoma. J Card Surg. 2012;27:423-426. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 123. | Sandrini F, Stratakis C. Clinical and molecular genetics of Carney complex. Mol Genet Metab. 2003;78:83-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 124. | Stratakis CA, Kirschner LS, Carney JA. Clinical and molecular features of the Carney complex: diagnostic criteria and recommendations for patient evaluation. J Clin Endocrinol Metab. 2001;86:4041-4046. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 377] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 125. | Kirschner LS, Carney JA, Pack SD, Taymans SE, Giatzakis C, Cho YS, Cho-Chung YS, Stratakis CA. Mutations of the gene encoding the protein kinase A type I-alpha regulatory subunit in patients with the Carney complex. Nat Genet. 2000;26:89-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 844] [Cited by in F6Publishing: 706] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 126. | Correa R, Salpea P, Stratakis CA. Carney complex: an update. Eur J Endocrinol. 2015;173:M85-M97. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 127. | Stratakis CA, Raygada M. Carney Complex. 2003 Feb 5. In: GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–. [PubMed] [Cited in This Article: ] |
| 128. | Stratakis CA. Hereditary syndromes predisposing to endocrine tumors and their skin manifestations. Rev Endocr Metab Disord. 2016;17:381-388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 129. | Stratakis CA. Genetics of Carney complex and related familial lentiginoses, and other multiple tumor syndromes. Front Biosci. 2000;5:D353-D366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 130. | Watson JC, Stratakis CA, Bryant-Greenwood PK, Koch CA, Kirschner LS, Nguyen T, Carney JA, Oldfield EH. Neurosurgical implications of Carney complex. J Neurosurg. 2000;92:413-418. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 69] [Article Influence: 2.9] [Reference Citation Analysis (0)] |