Published online Aug 26, 2014. doi: 10.4330/wjc.v6.i8.855
Revised: March 4, 2014
Accepted: June 10, 2014
Published online: August 26, 2014
Optimal management of patients with renal artery stenosis (RAS) is a subject of considerable controversy. There is incontrovertible evidence that renal artery stenosis has profound effects on the heart and cardiovascular system in addition to the kidney. Recent evidence indicates that restoration of blood flow alone does not improve renal or cardiovascular outcomes in patients with renal artery stenosis. A number of human and experimental studies have documented the clinical, hemodynamic, and histopathologic features in renal artery stenosis. New approaches to the treatment of renovascular hypertension due to RAS depend on better understanding of basic mechanisms underlying the development of chronic renal disease in these patients. Several groups have employed the two kidney one clip model of renovascular hypertension to define basic signaling mechanisms responsible for the development of chronic renal disease. Recent studies have underscored the importance of inflammation in the development and progression of renal damage in renal artery stenosis. In particular, interactions between the renin-angiotensin system, oxidative stress, and inflammation appear to play a critical role in this process. In this overview, results of recent studies to define basic pathways responsible for renal disease progression will be highlighted. These studies may provide the rationale for novel therapeutic approaches to treat patients with renovascular hypertension.
Core tip: Renovascular hypertension is a common public health problem, particularly in older patients with underlying atherosclerotic vascular disease. Recent studies have shown that restoration of blood flow in these patients fails to improve renal function or survival. Recent studies to define basic mechanisms underlying the development of chronic renal disease in renin angiotensin system (RAS) have shown that pro-inflammatory pathways may play a critical role in this process. Therapeutic approaches that target inflammatory pathways may provide the basis for novel and more effective treatments for patients with RAS.
- Citation: Al-Suraih M, Grande JP. Management of renal artery stenosis: What does the experimental evidence tell us? World J Cardiol 2014; 6(8): 855-860
- URL: https://www.wjgnet.com/1949-8462/full/v6/i8/855.htm
- DOI: https://dx.doi.org/10.4330/wjc.v6.i8.855
It is well recognized that hypertension is a major public health problem. The prevalence of hypertension is 29% in the United States; an additional 28% of adults have “prehypertension”[1]. Although the most common form of hypertension is “essential” hypertension, there is increasing recognition of secondary forms of hypertension that contribute to morbidity and mortality in patients with hypertension. Many of these cases have been identified through use of imaging modalities to assess patency of the coronary arteries. The prevalence of renovascular hypertension (RVH) is 7% in patients over 65 years of age[2]. In patients with coronary artery disease or aortoiliac disease, the prevalence of RVH is as high as 50%[3-5]. From 1991-1997, the annualized incidence of RVH as a cause of end stage renal disease increased by 12.4% per year, a larger increase than other causes of end stage renal disease[6]. Atherosclerosis is the most common etiology underlying RVH in this population[7-9]. In addition to chronic renal disease, atherosclerotic RVH contributes to cardiac morbidity and mortality[10]. For example, recent studies have shown that the overall 4-year survival of patients undergoing cardiac catheterization was 86% in patients without RAS but only 65% in those with RAS[11]. The extent of RAS also predicts survival, with 4-year survival of 89% in patients with RAS < 75% luminal occlusion, but only 57% in those with > 75% luminal occlusion[10,11]. Optimal treatment of these patients require the development of animal models to elucidate mechanisms of renal and cardiovascular disease progression.
The two kidney 1 clip (2K1C) model of renovascular hypertension, developed by Goldblatt, has been extensively employed to understand the pathogenesis of renovascular hypertension[12]. In his original model, dogs subjected to renal artery stenosis developed malignant hypertension, which caused extensive damage to the contralateral kidney. More recently, this model has been extended to other species, including mice, rats, and pigs[13-19]. In general, these animals do not develop malignant hypertension, and may thereby more accurately model human renal artery stenosis. In these animals, the stenotic kidney develops progressive atrophy, whereas the contralateral kidney develops hypertrophy but without major histopathologic alterations[14].
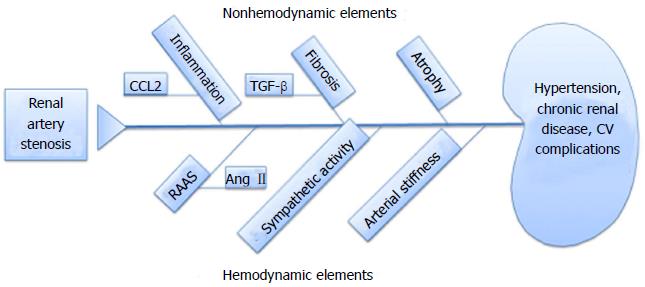
This model has allowed investigators to study the interrelationships between hemodynamic factors and non-hemodynamic factors responsible for the development of cardiovascular and renal disease (Figure 1). Hemodynamic factors include vasoactive effects mediated by activation of the renin-angiotensin-aldosterone system, increased sympathetic nervous activity, and increased arterial stiffness. Non-hemodynamic factors include the signaling pathways triggered by renal parenchymal cells and infiltrating inflammatory cells in the development and progression of renal and cardiovascular disease, and include chemokines, reactive oxygen species, and transforming growth factor β (ΤGFβ).
Most studies have focused on the role of renal hypo-perfusion and subsequent hypoxia on the development of chronic renal damage in the stenotic kidney. It is well recognized that reduced blood flow leads to intra-renal activation of the renin-angiotensin system, leading to elevated plasma levels of angiotensin II, a potent vasoconstrictor, and the development of systemic hypertension. However, several recent observations have called this paradigm into question. Recent imaging studies to assess renal oxygenation have suggested that the stenotic kidney is not hypoxic. It is recognized that the kidney receives far more blood than needed to support basic metabolic demands-indeed, renal tissue requires less than 10% of normal blood flow to support basic metabolic needs[20]. Furthermore, the kidney has the capacity to adapt to significant reduction in the diameter of renal artery with preservation of renal oxygenation[21]. In both human and experimental models, it appears that systemic activation of the renin-angiotensin system is transient, and that progression of renal and cardiovascular disease can occur without persistent elevation of plasma angiotensin II levels[22]. These observations have prompted investigations into basic signaling pathways triggered by renal artery stenosis that may be responsible for maintenance of systemic hypertension and the development of chronic renal disease.
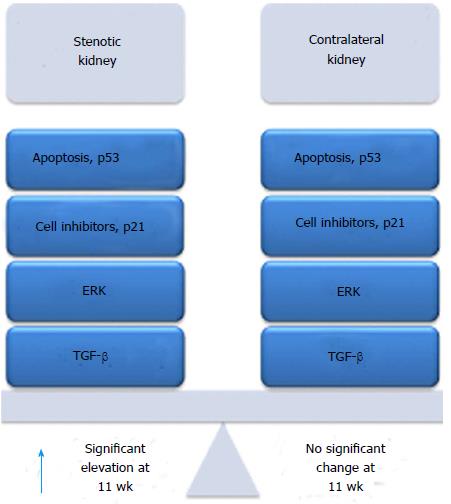
Although plasma angiotensin II levels may not remain elevated as cardiac and renal damage progress in renal artery stenosis, there is evidence for persistent activation of the intra-renal renin-angiotensin system. The kidney can produce all elements needed to completely activate the renin-angiotensin system, including renin, angiotensinogen, angiotensin converting enzyme, and angiotensin type 1 and type 2 receptor[23-25]. In the kidney, renin is expressed primarily by the juxtaglomerular cells. Angiotensinogen is expressed in proximal tubular epithelial cells and is secreted into tubular lumens. Angiotensin I is converted to Angiotensin II through action of ACE located on the apical brush border of tubular epithelium. We have shown that renal expression of Ren1 in the stenotic, but not contralateral, kidney persists in renal artery stenosis[26]. Based on these considerations, we embarked on a series of studies to compare signaling pathways activated in the stenotic and contralateral kidneys during the development and progression of renal damage in renal artery stenosis. A summary of our findings is highlighted in Figure 2.
In our initial studies, we correlated histopathologic alterations in the stenotic and contralateral kidneys at 2, 5, and 11 wk following renal artery stenosis surgery with signaling pathways that govern cell cycle regulation (cyclins D, E, A, and B; p21; p27), proliferation (PCNA, ERK, p38 MAPK), fibrosis (TGF-β; Smad2, Smad3, Smad4), and inflammation (MCP-1)[14]. The stenotic kidney showed progressive tubular atrophy, which was associated with interstitial fibrosis and inflammation, which closely recapitulates the histopathologic alterations observed in humans with advanced renal artery stenosis[27]. The contralateral kidney underwent compensatory enlargement, which was at least in part through hyperplasia. Compensatory enlargement in the contralateral kidney occurred in the absence of significant histopathologic alterations. We found that signaling pathways associated with cell cycle regulation, inflammation, and fibrosis were activated in both kidneys following induction of renal artery stenosis. However, these pathways were transiently activated in the contralateral kidney, returning to baseline levels by 11 wk, whereas they were progressively and persistently activated in the stenotic kidney.
A critical role for the p38 MAPK pathway in the development of renal atrophy was established in studies using the biochemical inhibitor SB203580[28]. The development of renal atrophy in the stenotic kidney was significantly decreased in mice treated with SB203580 at the time of renal artery stenosis surgery. Decreased atrophy was associated with reduced interstitial inflammatory infiltrates and decreased fibrosis. The p38 MAPK inhibitor had no significant effect on blood pressure or on plasma renin activity. Of note, treatment of mice with the ERK inhibitor U0126 did not prevent the development of renal atrophy, interstitial fibrosis, and interstitial inflammation (unpublished data).
In our previous studies, we demonstrated that TGF--β and its receptors (RI and RII) are persistently induced in the stenotic kidney of mice subjected to RAS. TGF-β has been implicated as a critical mediator of cell cycle regulation, inflammation, and fibrosis in other model systems[14,29-31]. The TGF-β signaling pathway interacts with a number of other signaling pathways, including the renin-angiotensin system and the MAPK pathways. TGF-β mediates fibrosis through interactions with Smad 2, Smad3, and Smad4. Although TGF-β knockout mice have high embryonic lethality and develop a systemic inflammatory syndrome shortly after birth, Smad3 knockout mice are viable and exhibit defects in TGF-β signaling[32].
We found that the stenotic kidneys of Smad3 knockout mice are almost completely protected from the development of interstitial fibrosis, tubular atrophy, and interstitial inflammation, despite an elevation of plasma renin activity and a reduction in blood flow of over 70%[22]. In an acute ischemia-reperfusion model, we showed that the kidneys of Smad3 knockout mice were resistant to the development of acute injury[33]. A similar protective effect has been observed in Smad3 mice subjected to unilateral ureteric obstruction.
Although we have shown that interruption of the p38 MAPK or Smad3-TGF-β signaling pathways prevent the development of renal atrophy, it is not clear how renal damage is initiated in this model. For this reason, we have conducted a series of studies to better understand the early signaling events and to correlate these with histopathologic alterations during the development of chronic renal disease in this model[26]. At 3 d following renal artery stenosis surgery, the stenotic kidney shows minimal histopathologic alterations. In particular, there is no evidence of acute injury to tubular epithelial cells, no significant interstitial fibrosis, tubular atrophy, or interstitial inflammation. Despite the normal appearance of the stenotic kidney, the tubular epithelial cells express markers of oxidative stress. It is recognized that the kidney expresses all components of the NADPH oxidase system[34] and that Ang II promotes ROS generation through activation of this membrane bound complex[34]. However, most studies of the interaction between Ang II activation and ROS generation have focused on later time points, after the initiation and development of chronic renal injury.
Influx of inflammatory cells, predominantly macrophages and lymphocytes, is first seen at 7 d following RAS surgery, a time point at which the kidney begins to develop tubular atrophy[26]. Influx of inflammatory cells is associated with induction of CCL2 (MCP-1) a potent chemoattractant protein. The relevance of this observation is underscored by studies demonstrating that increased production of CCL2 is associated with the influx of inflammatory cells in human renal artery stenosis[35,36] and that the development of chronic renal disease in RAS is associated with the influx of macrophages and T cells. Recent studies have suggested that signaling through CCL2 may play a critical role in the development and progression of both renal and cardiovascular disease in renal artery stenosis and other cardiovascular and renal diseases[37-39]. Both renal parenchymal cells and infiltrating inflammatory cells express CCR2, the primary receptor for CCL2. Through activation of this pathway, infiltrating inflammatory cells are capable of generating ROS and a number of chemokines which promote renal fibrosis.
We have observed increased expression of CCL2 at 3 d, prior to the influx of inflammatory cells, suggesting that renal parenchymal cells may be the source of this chemotactic chemokine[26]. Additional studies are required to verify this observation. It is not yet known whether blockade of CCL2-CCR2 signaling will prevent the development and/or progression of chronic renal disease in RAS.
Until recently, management of RAS was predicated on restoration of blood flow to the stenotic kidney. It was thought that this intervention would decrease local and systemic activation of the renin-angiotensin system, thereby restoring normal blood pressure and reducing both renal and cardiovascular morbidity and mortality. Recent studies have clearly demonstrated that restoration of blood flow through stenting fails to improve renal or cardiovascular outcomes, compared to medical therapy[40]. The mainstay of medical therapy involves blood pressure control, through use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, and/or other agents to reduce blood pressure[41]. There are concerns that aggressive blood pressure reduction may exacerbate damage to the stenotic kidney, although reduction of blood pressure is thought to improve overall renal function by protecting the contralateral kidney. Unfortunately, patients treated with medical therapy are still at risk for the development of progressive chronic renal disease, which in itself is a risk factor for the development of cardiovascular events. Recent studies have raised the possibility that therapies directed towards modulating the inflammatory response to chronic renal injury may have a role in management of patients with renal artery stenosis.
Optimal management of patients with RAS is limited by our lack of understanding of the events leading to the development of chronic renal damage in the stenotic kidney, and how these events contribute to cardiovascular morbidity and mortality. Inhibitors of P38 MAPK and of Smad3 signaling have been shown to prevent the development of chronic renal damage in experimental RAS. In addition to concerns regarding adverse effects of currently available compounds, there is no evidence that these agents can prevent the progression of chronic renal damage once clinical manifestations of renal artery stenosis become apparent. Similarly, human trials of antioxidant therapies to arrest the progression of systemic inflammatory conditions including atherosclerosis have been disappointing. Our recent observations, that generation of CCL2 and expression of CCR2 is an early event in RAS-an event which precedes the influx of inflammatory cells-merit additional study. In particular, it is not known whether abrogation of CCL2-CCR2 signaling with prevent the development of chronic renal disease in RAS or will arrest the progression of chronic renal disease once the disease becomes clinically apparent. Studies to address these important issues may provide the basis for changing the paradigm for treatment of renal artery stenosis from one that emphasizes restoration of renal blood flow to one that focuses on treatment of the inflammatory response to renal artery stenosis.
P- Reviewer: Kirali K, Mehta Y S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Ostchega Y, Yoon SS, Hughes J, Louis T. Hypertension awareness, treatment, and control--continued disparities in adults: United States, 2005-2006. NCHS Data Brief. 2008;1-8. [PubMed] [Cited in This Article: ] |
| 2. | Hansen KJ, Edwards MS, Craven TE, Cherr GS, Jackson SA, Appel RG, Burke GL, Dean RH. Prevalence of renovascular disease in the elderly: a population-based study. J Vasc Surg. 2002;36:443-451. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 347] [Cited by in F6Publishing: 291] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 3. | Iglesias JI, Hamburger RJ, Feldman L, Kaufman JS. The natural history of incidental renal artery stenosis in patients with aortoiliac vascular disease. Am J Med. 2000;109:642-647. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 60] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Valabhji J, Robinson S, Poulter C, Robinson AC, Kong C, Henzen C, Gedroyc WM, Feher MD, Elkeles RS. Prevalence of renal artery stenosis in subjects with type 2 diabetes and coexistent hypertension. Diabetes Care. 2000;23:539-543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 47] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Textor SC. Managing renal arterial disease and hypertension. Curr Opin Cardiol. 2003;18:260-267. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Fatica RA, Port FK, Young EW. Incidence trends and mortality in end-stage renal disease attributed to renovascular disease in the United States. Am J Kidney Dis. 2001;37:1184-1190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Textor SC. Atherosclerotic renal artery stenosis: flaws in estimated glomerular filtration rate and the problem of progressive kidney injury. Circ Cardiovasc Interv. 2011;4:213-215. [PubMed] [Cited in This Article: ] |
| 8. | Textor SC, Wilcox CS. Renal artery stenosis: a common, treatable cause of renal failure? Annu Rev Med. 2001;52:421-442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 91] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Garovic VD, Textor SC. Renovascular hypertension and ischemic nephropathy. Circulation. 2005;112:1362-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 195] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 10. | Conlon PJ, Athirakul K, Kovalik E, Schwab SJ, Crowley J, Stack R, McCants CB, Mark DB, Bashore TM, Albers F. Survival in renal vascular disease. J Am Soc Nephrol. 1998;9:252-256. [PubMed] [Cited in This Article: ] |
| 11. | Conlon PJ, Little MA, Pieper K, Mark DB. Severity of renal vascular disease predicts mortality in patients undergoing coronary angiography. Kidney Int. 2001;60:1490-1497. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 229] [Cited by in F6Publishing: 205] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 12. | Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : i. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1506] [Cited by in F6Publishing: 1263] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 13. | Gouvea SA, Bissoli NS, Moysés MR, Cicilini MA, Pires JG, Abreu GR. Activity of angiotensin-converting enzyme after treatment with L-arginine in renovascular hypertension. Clin Exp Hypertens. 2004;26:569-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 14. | Cheng J, Zhou W, Warner GM, Knudsen BE, Garovic VD, Gray CE, Lerman LO, Platt JL, Romero JC, Textor SC. Temporal analysis of signaling pathways activated in a murine model of two-kidney, one-clip hypertension. Am J Physiol Renal Physiol. 2009;297:F1055-F1068. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Chade AR, Rodriguez-Porcel M, Grande JP, Zhu X, Sica V, Napoli C, Sawamura T, Textor SC, Lerman A, Lerman LO. Mechanisms of renal structural alterations in combined hypercholesterolemia and renal artery stenosis. Arterioscler Thromb Vasc Biol. 2003;23:1295-1301. [PubMed] [Cited in This Article: ] |
| 16. | Eng E, Veniant M, Floege J, Fingerle J, Alpers CE, Menard J, Clozel JP, Johnson RJ. Renal proliferative and phenotypic changes in rats with two-kidney, one-clip Goldblatt hypertension. Am J Hypertens. 1994;7:177-185. [PubMed] [Cited in This Article: ] |
| 17. | Hocher B, Godes M, Olivier J, Weil J, Eschenhagen T, Slowinski T, Neumayer HH, Bauer C, Paul M, Pinto YM. Inhibition of left ventricular fibrosis by tranilast in rats with renovascular hypertension. J Hypertens. 2002;20:745-751. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Martinez-Maldonado M. Pathophysiology of renovascular hypertension. Hypertension. 1991;17:707-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 148] [Cited by in F6Publishing: 152] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Thöne-Reineke C, Olivier J, Godes M, Zart R, George I, Bauer C, Neumayer HH, Hocher B. Effects of angiotensin-converting enzyme inhibition and calcium channel blockade on cardiac apoptosis in rats with 2K1C (two-kidney/one-clip) renovascular hypertension. Clin Sci (Lond). 2003;104:79-85. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Lerman L, Textor SC. Pathophysiology of ischemic nephropathy. Urol Clin North Am. 2001;28:793-803, ix. [PubMed] [Cited in This Article: ] |
| 21. | Gloviczki ML, Glockner JF, Lerman LO, McKusick MA, Misra S, Grande JP, Textor SC. Preserved oxygenation despite reduced blood flow in poststenotic kidneys in human atherosclerotic renal artery stenosis. Hypertension. 2010;55:961-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Warner GM, Cheng J, Knudsen BE, Gray CE, Deibel A, Juskewitch JE, Lerman LO, Textor SC, Nath KA, Grande JP. Genetic deficiency of Smad3 protects the kidneys from atrophy and interstitial fibrosis in 2K1C hypertension. Am J Physiol Renal Physiol. 2012;302:F1455-F1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 23. | Raizada V, Skipper B, Luo W, Griffith J. Intracardiac and intrarenal renin-angiotensin systems: mechanisms of cardiovascular and renal effects. J Investig Med. 2007;55:341-359. [PubMed] [Cited in This Article: ] |
| 24. | Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251-287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 879] [Cited by in F6Publishing: 860] [Article Influence: 50.6] [Reference Citation Analysis (0)] |
| 25. | Lavoie JL, Sigmund CD. Minireview: overview of the renin-angiotensin system--an endocrine and paracrine system. Endocrinology. 2003;144:2179-2183. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 382] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 26. | Hartono SP, Knudsen BE, Zubair AS, Nath KA, Textor SJ, Lerman LO, Grande JP. Redox signaling is an early event in the pathogenesis of renovascular hypertension. Int J Mol Sci. 2013;14:18640-18656. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 27. | Keddis MT, Garovic VD, Bailey KR, Wood CM, Raissian Y, Grande JP. Ischaemic nephropathy secondary to atherosclerotic renal artery stenosis: clinical and histopathological correlates. Nephrol Dial Transplant. 2010;25:3615-3622. [PubMed] [Cited in This Article: ] |
| 28. | Wang D, Warner GM, Yin P, Knudsen BE, Cheng J, Butters KA, Lien KR, Gray CE, Garovic VD, Lerman LO. Inhibition of p38 MAPK attenuates renal atrophy and fibrosis in a murine renal artery stenosis model. Am J Physiol Renal Physiol. 2013;304:F938-F947. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 29. | Böttinger EP. TGF-beta in renal injury and disease. Semin Nephrol. 2007;27:309-320. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 269] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 30. | Cheng J, Grande JP. Transforming growth factor-B and kidney dysfunction. J Organ Dysfunct. 2009;5:182-192. [Cited in This Article: ] |
| 31. | Grande JP, Warner GM, Walker HJ, Yusufi AN, Cheng J, Gray CE, Kopp JB, Nath KA. TGF-beta1 is an autocrine mediator of renal tubular epithelial cell growth and collagen IV production. Exp Biol Med (Maywood). 2002;227:171-181. [PubMed] [Cited in This Article: ] |
| 32. | Owen CR, Yuan L, Basson MD. Smad3 knockout mice exhibit impaired intestinal mucosal healing. Lab Invest. 2008;88:1101-1109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 33. | Nath KA, Croatt AJ, Warner GM, Grande JP. Genetic deficiency of Smad3 protects against murine ischemic acute kidney injury. Am J Physiol Renal Physiol. 2011;301:F436-F442. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Liu J, Yang F, Yang XP, Jankowski M, Pagano PJ. NAD(P)H oxidase mediates angiotensin II-induced vascular macrophage infiltration and medial hypertrophy. Arterioscler Thromb Vasc Biol. 2003;23:776-782. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 149] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 35. | Eirin A, Gloviczki ML, Tang H, Gössl M, Jordan KL, Woollard JR, Lerman A, Grande JP, Textor SC, Lerman LO. Inflammatory and injury signals released from the post-stenotic human kidney. Eur Heart J. 2013;34:540-548a. [PubMed] [Cited in This Article: ] |
| 36. | Gloviczki ML, Keddis MT, Garovic VD, Friedman H, Herrmann S, McKusick MA, Misra S, Grande JP, Lerman LO, Textor SC. TGF expression and macrophage accumulation in atherosclerotic renal artery stenosis. Clin J Am Soc Nephrol. 2013;8:546-553. [PubMed] [Cited in This Article: ] |
| 37. | Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension. 2011;57:132-140. [PubMed] [Cited in This Article: ] |
| 38. | Stouffer GA, Pathak A, Rojas M. Unilateral renal artery stenosis causes a chronic vascular inflammatory response in ApoE-/- mice. Trans Am Clin Climatol Assoc. 2010;121:252-264; 264-266. [PubMed] [Cited in This Article: ] |
| 39. | Oliver E, McGillicuddy F, Phillips C, Toomey S, Roche HM. The role of inflammation and macrophage accumulation in the development of obesity-induced type 2 diabetes mellitus and the possible therapeutic effects of long-chain n-3 PUFA. Proc Nutr Soc. 2010;69:232-243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 93] [Cited by in F6Publishing: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 40. | Cooper CJ, Murphy TP, Cutlip DE, Jamerson K, Henrich W, Reid DM, Cohen DJ, Matsumoto AH, Steffes M, Jaff MR. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370:13-22. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 653] [Cited by in F6Publishing: 569] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 41. | Textor SC. Stable patients with atherosclerotic renal artery stenosis should be treated first with medical management. Am J Kidney Dis. 2003;42:858-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |