Published online Oct 26, 2021. doi: 10.4330/wjc.v13.i10.546
Peer-review started: May 8, 2021
First decision: June 29, 2021
Revised: July 10, 2021
Accepted: August 13, 2021
Article in press: August 13, 2021
Published online: October 26, 2021
Computed tomography (CT) is emerging as a prominent diagnostic modality in the field of cardiovascular imaging. Artificial intelligence (AI) is making significant strides in the field of information technology, the commercial industry, and health care. Machine learning (ML), a branch of AI, can optimize the performance of CT and augment the assessment of coronary artery disease. These ML platforms can automate multiple tasks, perform calculations, and integrate information from a variety of data sources. In this review article, we explore the ML in CT imaging.
Core Tip: Machine learning (ML), a subset of artificial intelligence, contains multiple algorithms which include supervised, unsupervised, reinforcement and deep learning. These algorithms can greatly augment multiple aspects in computed tomography which include automated segmentation, diagnosis, and stratification based on risk. Outputs need to be carefully assessed by the medical team for any potential biases. For the future of computed tomography and cardiovascular imaging, ML algorithms need to be integrated in clinical care.
- Citation: Seetharam K, Bhat P, Orris M, Prabhu H, Shah J, Asti D, Chawla P, Mir T. Artificial intelligence and machine learning in cardiovascular computed tomography. World J Cardiol 2021; 13(10): 546-555
- URL: https://www.wjgnet.com/1949-8462/full/v13/i10/546.htm
- DOI: https://dx.doi.org/10.4330/wjc.v13.i10.546
In this digital era, distance is no longer a limiting factor and information is emanating from a variety of devices and sources[1]. These technological innovations have considerably transformed our perception, culture, and our daily lifestyles[2]. Similarly, many of these changes have trickled downwards in healthcare and are especially apparent in the field of cardiovascular imaging. Over the last 10 years, the field of computed tomography (CT) has expanded tremendously with significant changes in diagnostic performance and prognostic implications in coronary artery disease[3,4]. Coronary CT angiography (CTA) is now heralded as an established diagnostic modality in the evaluation of coronary artery disease (CAD)[4,5]. With each year, data arising from each imaging scan is increasing exponentially in intricacy and size[6]. As we approach this technological ceiling, the sheer complexity of this information will supersede the analytic capabilities of conventional statistical software[7].
Artificial Intelligence (AI) refers to a set of actions that can mimic human cognitive thinking and decision making[8]. Machine learning (ML), a branch of AI, can extrapolate hidden characteristics or relationships present in vast expanses of data[2]. It can analyze data from a multitude of sources and link the information in user-friendly approaches[9]. In addition, it can automate several processes and perform many calculations[10]. With the application of ML algorithms in CT for cardiology, it can elevate the modality to unprecedented new heights which can improve the quality of patient care. In our review, we evaluate recent advances and progression of ML in cardiac CT over recent years.
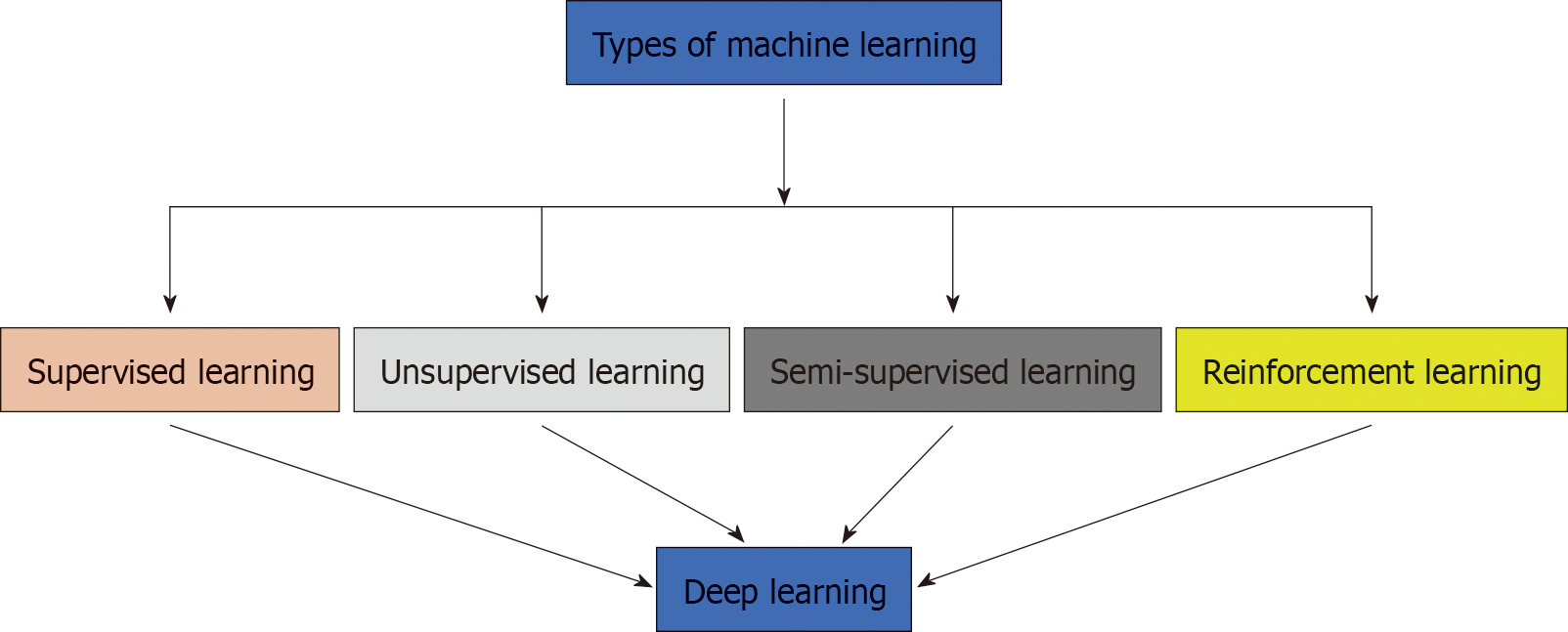
ML is an aggregate term which collectively encompasses a wide variety of analytical algorithms[11]. They can be simply divided into supervised learning, unsupervised learning, semi-supervised learning, deep learning and reinforcement learning[12,13] (Figure 1 and Table 1). Supervised learning requires labeled datasets or domains within the dataset to perform analytical actions[14]. Unsupervised learning does not require labels within a dataset and can analyze information in a very independent manner. For discussion purposes, it can be referred to as agnostic[2,15]. Hierarchical clustering, a type of unsupervised learning, can identify and distinguish new phenotypes within various cardiac diseases[2]. It has gained significant traction recently. Semi-supervised learning is a hybrid approach that utilizes properties present within supervised and unsupervised learning[16]. Reinforcement learning uses definitive reward conditions for the ML architecture to perform certain functions. Nevertheless, frequently not used in the field of cardiology[7]. Multiple studies have been documented to show the potential of ML in CT and CTA (Table 2).
| Types of machine learning | Function | Examples |
| Supervised learning (55) | Contains labels and outcomes, deduces inferences for prediction purpose | Includes logistic regression, ridge regression, elastic net regression, Bayesian and artificial neural networks |
| Unsupervised learning (55) | No labels, independently detects significant relationships. | Includes hierarchical clustering, k- means clustering, principal component analysis |
| Semi-supervised learning (55) | Properties of both supervised and unsupervised learning | Utilized in image and speech recognition |
| Re-enforcement learning (55) | Utilizes reward function to execute tasks | Utilized in medical imaging, analytics, and prescription selection |
| Ref. | ML approach | Brief study description |
| ML derived CAC assessment | ||
| Al’Aref et al[24] | Multiple ML algorithm | To use CAC and clinical factors for CAD prediction |
| Tesche et al[26] | ML algorithm | To compare ML derived CT FFR and CAC in CT |
| Kay et al[27] | ML algorithm | To identify phenotypes of left ventricular hypertrophy in combination with CAC |
| ML derived CT FFR assessment | ||
| Zhou et al[31] | Multiple ML algorithms | To employ CT FFR for myocardial bridge formation prediction |
| Tang et al[32] | ML algorithm | To compare ML CT FFR, CTA and invasive angiography |
| Coenen et al[33] | Supervised learning | To identify CAD |
| ML derived evaluation of plaque characteristics | ||
| Dey et al[34] | ML algorithm | To generate ML derived scores from plaque characteristics |
| Hell et al[35] | ML algorithm | To predict cardiac death from plaque characteristics from CTA |
| ML derived evaluation of epicardial adipose tissue | ||
| Rodrigues et al[38] | ML algorithm | To segment and distinguish between different varieties of EAT |
| Commandeur et al[39] | Deep learning | To quantify EAT in CT |
| Otaki et al[40] | Supervised learning | To assess the relationship between EAT in CT and MFR in PET |
| Miscellaneous applications of ML in CT | ||
| Baskaran et al[41] | Deep learning | To assess automatic and manual assessment of left and right cardiac structure and function |
| Al’Aref et al[42] | Supervised learning | To identify culprit coronary lesions in CT |
| Beecy et al[43] | Deep learning | To detect acute ischemic stroke in CT |
| Oikonomou et al[44] | Supervised learning | To utilize perivascular fat for cardiac risk prediction |
| Eisenberg et al[45] | Deep learning | To evaluate epicardial tissue for MACE events |
Among all the available ML algorithms, deep learning is considered to have the most revolutionary potential[17]. In various sectors of commerce and industry, deep learning is being heavily utilized to unravel information within large troves of data[18]. From voice recognition software in Siri or Alexa to self-driving cars in google, deep learning is garnering significant interest[12]. The architecture of deep learning algorithms is similar to the arrangement of a human neuron[19,20]. It is structured in a series of layers, there is significant communication between the preceding and subsequent layers. It processes information in multiple layers and is more independent compared to other ML algorithms. As the complexity and size of the dataset increase, the performance of the algorithm improves significantly[21,22].
Coronary artery calcium (CAC) measurement is heralded as a fundamental metric in coronary CT because it serves as a pivotal predictor of mortality and cardiac complications[23]. The Agatston scoring method is the conventional approach utilized to quantify CAC in coronary CT[19]. Furthermore, the CAC plays a diagnostic role in medical management, the CAC scores can be used to stratify patients and monitor medical therapy. However, CAC measurement can be quite tedious due to underlying artifacts, image noise, an abundance of calcifications, interobserver variability, and other factors[24]. The application of ML can significantly elevate the potential of CAC in CT.
Al'Aref et al[24] applied an ML architecture incorporating clinical factors in the CONFIRM registry with CAC for calculating the probability of CAD with CTA in a total of 35821 patients. It clearly showcased excellent AUC for ML and (CAC) (0.881) to other conventional approaches in their study [ML independently (0.773), updated Diamond- Forrester Score (0.682) coronary calcium (0.886)]. Hou et al[25] assessed the role of supervised ML to evaluate pretest likelihood of CAD in CTA with 6274 individuals. Their ML algorithm demonstrated superior discriminative capacity for CAD occlusion in comparison to traditional scoring metrics such as Modified Diamond Forester scores and CAD consortium score (P < 0.001). Tesche et al[26] exhibited superior performance of ML derived CT fractional flow reserve (FFR) in comparison to CTA with CAC, substantial distinctions in capability were noted and with propionate increases in Agatston scores (P < 0.001). Kay et al[27] integrated various algorithmic frameworks with radiomics for identifying new phenotypic characteristics regarding left ventricular hypertrophy (LVH) severity in CT with (CAC) assessment. As a result, ML frameworks are found to be efficacious in identification of LVH.
Although CTA enables visual evaluation of a stenotic lesion, it lags behind invasive FFR for assessing the hemodynamic significance of coronary stenosis[28]. Coronary fractional flow reserve (CT-FFR) has become a suitable non-invasive modality for evaluating ischemic heart disease and chest pain[29]. Furthermore, it can perform this task without the requirement of additional medications or imaging. It provides functional and anatomic evaluation, this approach is steadily gaining momentum in CT imaging[30]. ML algorithms can calculate FFR in the absence of computational fluid dynamics and yield additional prognostic information[3]. It can substantially expand the arena of CT-FFR in CT imaging.
Zhou et al[31] evaluated CT fractional flow reserve (CT FFR) for estimating myocardial bridge formation by integrating several algorithms. Interestingly, the framework chose properties which contained superior AUC (0.75 ± 0.04) in comparison to clinical attributes (0.53 ± 0.09, P < 0.0001), or CT- FFR prosperities (0.62 ± 0.06, P = 0.0127). Tang et al[32] demonstrated that CT FFR with computational fluid dynamics was superior CTA and invasive angiography for detecting vessel-specific ischemia. This was particularly seen in intermediate lesions (P < 0.001 for all). Coenen et al[33] demonstrated excellent correlation between ML based CT FFR and deep learning in CAD (r = 0.997).
ML algorithms can provide additional insight regarding plaque characteristics in CAD and augment our understanding[2]. Dey et al[34] utilized a logitboost algorithm to produce an ML-derived risk score from plaque characteristics in CTA for 254 patients. The ML algorithm displayed a higher AUC (0.84) than individual CTA parameters including stenosis (0.76), total plaque volume (0.74), and low likelihood of CAD (P < 0.0006) (0.63). Hell et al[35] investigated the role of ML algorithms to predict cardiac death from coronary CTA through the utilization of plaque features in 2748 patients. The non-calcified plaque > 146 mm3 (P = 0.027), low density non-calcified plaque (P = 0.025), total plaque volume > 179 mm3, and CDD > 35% in any vessel were significantly associated with elevated risk of future cardiac death.
Cardiac CT is deemed as the gold standard for evaluation of epicardial adipose tissue (EAT) quantification and assessment. EAT is a layer of adipose surrounding the heart and the accompanying coronary arteries. In addition, EAT is significantly linked with various cardiovascular risk factors, atherosclerosis of the coronary arteries, and CAD[36,37]. The application of ML algorithms can automate the quantification of EAT and greatly reduce the time of manual measurements. This can translate into greater clinical implementation in coronary CT.
Rodrigues et al[38] applied ML algorithms for segmenting and differentiating types of fat in CT. The ML platform was able to achieve 98.4% mean accuracy and a DICE similarity index of 96.8%. Commandeur et al[39] utilized a deep learning algorithm for quantifying EAT in coronary CT. Strong agreement was observed between automatic and expert manual quantification with a mean DICE score coefficient of 0.823 and an excellent correlation of 0.923 with EAT volume. Otaki et al[40] utilized a boost ensemble machine learning algorithm for assessing the association of epicardial fat volume from myocardial flow reserve (MFR) in non- contrast CT in positive emission tomography (PET). The ML composite risk score substantially increased risk reclassification of impaired MFR to EAT volume or coronary calcium score (IDI = 0.19 and P = 0.007, IDI = 0.22 and P = 0.002).
In CT, ML has been applied in a variety of different situations with overwhelmingly positive results. Baskaran et al[41] assessed deep learning for assessing cardiovascular structures for CTA in 166 patients. The ML architecture corroborated in parallel to manual annotation in CTA for left ventricular volume (r = 0.98), right ventricular volume (r = 0.97) (P < 0.05). Al'Aref et al[42] utilized ML in CTA to detect precursor culprit lesion from patients with CAD. It exhibited a superior AUC for discriminating lesions in comparison to other ML derived frameworks (P < 0.01). Beecy et al[43] on CT for detecting acute ischemic stroke events. Interestingly, their AUC was 0.91 for automatic detection of infarction and had a 93% accuracy with interpretation of experienced physicians. Oikonomou et al[44] examined the capability of the random forest ML architecture from the radiomic profile of CTA derived coronary perivascular adipose tissue (PVAT) for identifying cardiac risk. It exceeded traditional risk stratification metrics for MACE prediction (P < 0.001). Eisenberg used deep learning for MACE prediction with EAT and other characteristics. The EAT in CT predicted MACE effectively (HR, 1.35, P < 0.01), inversely with attenuation (0.83, P = 0.01)[45].
Big data has emerged as a valuable resource that provides significant depth and understanding and is instrumental to the growth of ML in clinical medicine (Table 3)[5]. Due to size and magnitude, many important characteristics are often unnoticed by conventional approaches[6,46]. The implementation of AI with these immense expanses of data can yield additional information which can aid in medical management and clinical care.
Motwani et al[47] evaluated an ML framework to predict CAD in 10,030 patients for five-year mortality in comparison to traditional cardiac metrics in CT. Interestingly, the ML architecture exhibited a superior AUC (0.79) than CT severity scores (SSS = 0.64, SIS = 0.64, DI = 0.62) for five-year all-cause mortality prediction (P < 0.0001). Similarly, van Rosendael et al[48] utilized an ML framework in CT with 8844 patients for detecting major cardiovascular events encompassing various attributes in relation to severity scores for CAD prediction. The ML derived AUC (0.771) was significantly higher in CT than conventional scoring parametric systems (0.685-0.701) for anticipating major cardiovascular events, with a notable difference (P < 0.001). Han et al[49] assessed an ML-derived predictive capacity for all-cause mortality in 86155 patients. Notably, the AUC (0.82) noted to be higher than Framingham risk score and other traditional metrics (P < 0.05).
It must be emphasized with great importance that cardiovascular disease is heterogeneous in nature[50]. It cannot be perceived as straightforward because disease mechanisms have intricate interactions among molecular, genetic, and environmental factors[22]. The process is very dynamic, it truly reflects the essence of ML algorithms. ML can integrate this information from multiple sources and analyze it in a variety of approaches. This can lead to the development of various genetic markers which can help guide medical management and monitor responses after therapy[6,51]. Furthermore, we can tailor treatment regimens appropriate to the genetic constitutional makeup of an individual, ML algorithms will facilitate the growth of precision medicine[12].
In current times, mobile devices, smartphone apps, and wearable devices are part and parcel of our daily lifestyles[52]. Telemedicine and ML algorithms are clearly intertwined in cardiovascular imaging and CT[1]. The information from these devices can be integrated with various parameters in cardiovascular imaging to yield additional insight regarding various cardiovascular diseases. In many underserved regions of the world, these devices can provide medical care and help direct patients towards appropriate intervention[1,53]. ML algorithms can analyze this information in real-time and help expedite this process[1]. These algorithms can serve as a bridge between different types of technology and cardiovascular imaging.
Although several algorithms have significant potential in computed tomography, deep learning has the most overwhelming potential[54]. It captures information through hierarchical levels of abstraction. As the computational prowess of graphical processing units (GPUs) continue to progressively evolve in conjunction with big data, the relevance of deep learning in computed tomography is becoming imminent. It is very effective in robust tasks such as image classification, image segmentation, and identification of various cardiovascular structures in CT, CTA, and cardiovascular imaging[20]. Furthermore, it does not require extensive training. The accuracy can be achieved by elevating the capability of the network or increasing the training set. This is a stark distinction in comparison to other ML algorithms[55]. Other algorithms entail a significant number of observations, computations, manual labor, and training to achieve optimal efficiency.
Randomized clinical trials (RCTs) are the gold standard in clinical research. The integration of ML algorithms could prove to be exceeding useful if implemented appropriately. Numerous RCTs fail to reach completion due to several factors which could include improper study design, inadequate number of participants, or lack of funding[56]. The integration of ML algorithms during the early or intermediate stage of an RCT could provide an outlook of different outcomes[5]. This information could be used to restructure the RCT to obtain more successful outcomes. In addition, ML algorithms can enhance the randomization process in RCT[56].
Though ML algorithms offer a significant promise for the future, it is far from straightforward. Several issues need to be resolved for successful implementation in clinical medicine. The potential of false discovery can occur with small databases, there is not enough information to properly train the algorithm[55]. Unfortunately, AI lacks a moral compass[57]. In addition, several unintentional biases can emerge during the process and could alter interpretation. The “black box” nature has always been an enigmatic property of ML algorithms, this has impeded its adoption in the medical field[2]. Investigators must have a proper research concept and plan before embarking on any ML-related task. As a result, engineers, physicians, and other members of a research team must play an active role in every stage of the ML algorithm[15,58]. Adjustments can be made to the algorithm to deliver clinically relevant information.
For any ML algorithm to thrive and grow, large information or databases is mandatory[15]. Obtaining this information can be complex and tedious. Data needs to be shared among institutions to allow training of the ML model[15]. This might require multiple IRB approvals. Information also needs to be de-identified before it can be shared. Many of these tasks can be time-consuming. Many types of imaging systems are frequently used for storing cardiovascular images. Nevertheless, each institution has their own unique protocols and there are differences in the acquisition process as well[2]. Some form of data standardization is required to facilitate data sharing and ML algorithm growth. If more information can be publicly available, it would be beneficial.
ML algorithms will have limitless potential in cardiovascular imaging, this has been evidenced in the field of CT. It will cause multiple paradigm shifts which will have a revolutionary impact in the field of medicine. These frameworks will automate several tasks, perform calculations, and aid as a supplementary tool for medical diagnosis and prognostication. By performing multiple tasks, physicians will have more time to spend with patients and be more focused on proper medical management. ML will serve as a long-lasting bridge between physicians and technology in clinical medicine.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ankrah AO S-Editor: Ma YJ L-Editor: A P-Editor: Wang LYT
| 1. | Seetharam K, Kagiyama N, Sengupta PP. Application of mobile health, telemedicine and artificial intelligence to echocardiography. Echo Res Pract. 2019;6:R41-R52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 2. | Seetharam K, Brito D, Farjo PD, Sengupta PP. The Role of Artificial Intelligence in Cardiovascular Imaging: State of the Art Review. Front Cardiovasc Med. 2020;7:618849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Al'Aref SJ, Anchouche K, Singh G, Slomka PJ, Kolli KK, Kumar A, Pandey M, Maliakal G, van Rosendael AR, Beecy AN, Berman DS, Leipsic J, Nieman K, Andreini D, Pontone G, Schoepf UJ, Shaw LJ, Chang HJ, Narula J, Bax JJ, Guan Y, Min JK. Clinical applications of machine learning in cardiovascular disease and its relevance to cardiac imaging. Eur Heart J. 2019;40:1975-1986. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 4. | Abdelrahman KM, Chen MY, Dey AK, Virmani R, Finn AV, Khamis RY, Choi AD, Min JK, Williams MC, Buckler AJ, Taylor CA, Rogers C, Samady H, Antoniades C, Shaw LJ, Budoff MJ, Hoffmann U, Blankstein R, Narula J, Mehta NN. Coronary Computed Tomography Angiography From Clinical Uses to Emerging Technologies: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;76:1226-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 119] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 5. | Seetharam K, Min JK. Artificial Intelligence and Machine Learning in Cardiovascular Imaging. Methodist Debakey Cardiovasc J. 2020;16:263-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. Machine learning in cardiovascular medicine: are we there yet? Heart. 2018;104:1156-1164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 227] [Cited by in F6Publishing: 228] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 7. | Seetharam K, Raina S, Sengupta PP. The Role of Artificial Intelligence in Echocardiography. Curr Cardiol Rep. 2020;22:99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 8. | Hamet P, Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69S:S36-S40. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 728] [Cited by in F6Publishing: 607] [Article Influence: 86.7] [Reference Citation Analysis (0)] |
| 9. | Seetharam K, Sengupta PP, Bianco CM. Cardiac mechanics in heart failure with preserved ejection fraction. Echocardiography. 2020;37:1936-1943. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Kagiyama N, Shrestha S, Farjo PD, Sengupta PP. Artificial Intelligence: Practical Primer for Clinical Research in Cardiovascular Disease. J Am Heart Assoc. 2019;8:e012788. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 68] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 11. | Seetharam K, Shrestha S, Sengupta P. Artificial Intelligence in Cardiac Imaging. US Cardiol Rev. 2020;13:110-116. [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Johnson KW, Torres Soto J, Glicksberg BS, Shameer K, Miotto R, Ali M, Ashley E, Dudley JT. Artificial Intelligence in Cardiology. J Am Coll Cardiol. 2018;71:2668-2679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 448] [Cited by in F6Publishing: 447] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 13. | Krittanawong C, Zhang H, Wang Z, Aydar M, Kitai T. Artificial Intelligence in Precision Cardiovascular Medicine. J Am Coll Cardiol. 2017;69:2657-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 416] [Article Influence: 59.4] [Reference Citation Analysis (0)] |
| 14. | Seetharam K, Kagiyama N, Shrestha S, Sengupta PP. Clinical Inference From Cardiovascular Imaging: Paradigm Shift Towards Machine-Based Intelligent Platform. Curr Treat Options Cardiovasc Med. 2020;22:8. [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Seetharam K, Shresthra S, Mills JD, Sengupta PP. Artificial Intelligence in Nuclear Cardiology: Adding Value to Prognostication. Curr Cardiovasc Imag Rep. 2019;12. [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 16. | Sengupta PP, Shrestha S. Machine Learning for Data-Driven Discovery: The Rise and Relevance. JACC Cardiovasc Imaging. 2019;12:690-692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Bizopoulos P, Koutsouris D. Deep Learning in Cardiology. IEEE Rev Biomed Eng. 2019;12:168-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 18. | Krittanawong C, Johnson KW, Rosenson RS, Wang Z, Aydar M, Baber U, Min JK, Tang WHW, Halperin JL, Narayan SM. Deep learning for cardiovascular medicine: a practical primer. Eur Heart J. 2019;40:2058-2073. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 134] [Cited by in F6Publishing: 159] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 19. | Sengupta PP, Shrestha S, Zeb I. Solving coronary risk: time to feed machines some calcium (score) supplements. Eur Heart J. 2020;41:368-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Litjens G, Ciompi F, Wolterink JM, de Vos BD, Leiner T, Teuwen J, Išgum I. State-of-the-Art Deep Learning in Cardiovascular Image Analysis. JACC Cardiovasc Imaging. 2019;12:1549-1565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 21. | Shrestha S, Sengupta PP. Machine learning for nuclear cardiology: The way forward. J Nucl Cardiol. 2019;26:1755-1758. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 16] [Reference Citation Analysis (0)] |
| 22. | Shrestha S, Sengupta PP. The Mechanics of Machine Learning: From a Concept to Value. J Am Soc Echocardiogr. 2018;31:1285-1287. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Al'Aref SJ, Min JK. Cardiac CT: current practice and emerging applications. Heart. 2019;105:1597-1605. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Al'Aref SJ, Maliakal G, Singh G, van Rosendael AR, Ma X, Xu Z, Alawamlh OAH, Lee B, Pandey M, Achenbach S, Al-Mallah MH, Andreini D, Bax JJ, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, Cury RC, DeLago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Kim YJ, Leipsic JA, Maffei E, Marques H, Gonçalves PA, Pontone G, Raff GL, Rubinshtein R, Villines TC, Gransar H, Lu Y, Jones EC, Peña JM, Lin FY, Min JK, Shaw LJ. Machine learning of clinical variables and coronary artery calcium scoring for the prediction of obstructive coronary artery disease on coronary computed tomography angiography: analysis from the CONFIRM registry. Eur Heart J. 2020;41:359-367. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 115] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 25. | Hou ZH, Lu B, Li ZN, An YQ, Gao Y, Yin WH, Liang S, Zhang RG. Machine Learning for Pretest Probability of Obstructive Coronary Stenosis in Symptomatic Patients. JACC Cardiovasc Imaging. 2019;12:2584-2586. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 26. | Tesche C, Otani K, De Cecco CN, Coenen A, De Geer J, Kruk M, Kim YH, Albrecht MH, Baumann S, Renker M, Bayer RR, Duguay TM, Litwin SE, Varga-Szemes A, Steinberg DH, Yang DH, Kepka C, Persson A, Nieman K, Schoepf UJ. Influence of Coronary Calcium on Diagnostic Performance of Machine Learning CT-FFR: Results From MACHINE Registry. JACC Cardiovasc Imaging. 2020;13:760-770. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 27. | Kay FU, Abbara S, Joshi PH, Garg S, Khera A, Peshock RM. Identification of High-Risk Left Ventricular Hypertrophy on Calcium Scoring Cardiac Computed Tomography Scans: Validation in the DHS. Circ Cardiovasc Imaging. 2020;13:e009678. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Pijls NH, Van Gelder B, Van der Voort P, Peels K, Bracke FA, Bonnier HJ, el Gamal MI. Fractional flow reserve. A useful index to evaluate the influence of an epicardial coronary stenosis on myocardial blood flow. Circulation. 1995;92:3183-3193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 589] [Cited by in F6Publishing: 535] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 29. | Leipsic J, Weir-McCall J, Blanke P. FFRCT for Complex Coronary Artery Disease Treatment Planning: New Opportunities. Interv Cardiol. 2018;13:126-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Hirshfeld JW Jr, Nathan AS. QFR and FFRCT: Accurate Enough? JACC Cardiovasc Interv. 2019;12:2060-2063. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Zhou F, Tang CX, Schoepf UJ, Tesche C, Rollins JD, Liu H, Zhou CS, Yan J, Lu MJ, Lu GM, Ni QQ, Zhang LJ. Machine Learning Using CT-FFR Predicts Proximal Atherosclerotic Plaque Formation Associated With LAD Myocardial Bridging. JACC Cardiovasc Imaging. 2019;12:1591-1593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Tang CX, Liu CY, Lu MJ, Schoepf UJ, Tesche C, Bayer RR 2nd, Hudson HT Jr, Zhang XL, Li JH, Wang YN, Zhou CS, Zhang JY, Yu MM, Hou Y, Zheng MW, Zhang B, Zhang DM, Yi Y, Ren Y, Li CW, Zhao X, Lu GM, Hu XH, Xu L, Zhang LJ. CT FFR for Ischemia-Specific CAD With a New Computational Fluid Dynamics Algorithm: A Chinese Multicenter Study. JACC Cardiovasc Imaging. 2020;13:980-990. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 64] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 33. | Coenen A, Kim YH, Kruk M, Tesche C, De Geer J, Kurata A, Lubbers ML, Daemen J, Itu L, Rapaka S, Sharma P, Schwemmer C, Persson A, Schoepf UJ, Kepka C, Hyun Yang D, Nieman K. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography–Based Fractional Flow Reserve. Circ Cardiovasc Imag. 2018;11:e007217. [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 231] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 34. | Dey D, Gaur S, Ovrehus KA, Slomka PJ, Betancur J, Goeller M, Hell MM, Gransar H, Berman DS, Achenbach S, Botker HE, Jensen JM, Lassen JF, Norgaard BL. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol. 2018;28:2655-2664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 35. | Hell MM, Dey D, Marwan M, Achenbach S, Schmid J, Schuhbaeck A. Non-invasive prediction of hemodynamically significant coronary artery stenoses by contrast density difference in coronary CT angiography. Eur J Radiol. 2015;84:1502-1508. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605-613. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 741] [Cited by in F6Publishing: 768] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 37. | de Vos AM, Prokop M, Roos CJ, Meijs MF, van der Schouw YT, Rutten A, Gorter PM, Cramer MJ, Doevendans PA, Rensing BJ, Bartelink ML, Velthuis BK, Mosterd A, Bots ML. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur Heart J. 2008;29:777-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 164] [Cited by in F6Publishing: 174] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 38. | Rodrigues ÉO, Morais FF, Morais NA, Conci LS, Neto LV, Conci A. A novel approach for the automated segmentation and volume quantification of cardiac fats on computed tomography. Comput Methods Programs Biomed. 2016;123:109-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Commandeur F, Goeller M, Betancur J, Cadet S, Doris M, Chen X, Berman DS, Slomka PJ, Tamarappoo BK, Dey D. Deep Learning for Quantification of Epicardial and Thoracic Adipose Tissue From Non-Contrast CT. IEEE Trans Med Imaging. 2018;37:1835-1846. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 40. | Otaki Y, Hell M, Slomka PJ, Schuhbaeck A, Gransar H, Huber B, Nakazato R, Germano G, Hayes SW, Thomson LE, Friedman JD, Achenbach S, Berman DS, Dey D. Relationship of epicardial fat volume from noncontrast CT with impaired myocardial flow reserve by positron emission tomography. J Cardiovasc Comput Tomogr. 2015;9:303-309. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 41. | Baskaran L, Maliakal G, Al'Aref SJ, Singh G, Xu Z, Michalak K, Dolan K, Gianni U, van Rosendael A, van den Hoogen I, Han D, Stuijfzand W, Pandey M, Lee BC, Lin F, Pontone G, Knaapen P, Marques H, Bax J, Berman D, Chang HJ, Shaw LJ, Min JK. Identification and Quantification of Cardiovascular Structures From CCTA: An End-to-End, Rapid, Pixel-Wise, Deep-Learning Method. JACC Cardiovasc Imaging. 2020;13:1163-1171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 42. | Al'Aref SJ, Singh G, Choi JW, Xu Z, Maliakal G, van Rosendael AR, Lee BC, Fatima Z, Andreini D, Bax JJ, Cademartiri F, Chinnaiyan K, Chow BJW, Conte E, Cury RC, Feuchtner G, Hadamitzky M, Kim YJ, Lee SE, Leipsic JA, Maffei E, Marques H, Plank F, Pontone G, Raff GL, Villines TC, Weirich HG, Cho I, Danad I, Han D, Heo R, Lee JH, Rizvi A, Stuijfzand WJ, Gransar H, Lu Y, Sung JM, Park HB, Berman DS, Budoff MJ, Samady H, Stone PH, Virmani R, Narula J, Chang HJ, Lin FY, Baskaran L, Shaw LJ, Min JK. A Boosted Ensemble Algorithm for Determination of Plaque Stability in High-Risk Patients on Coronary CTA. JACC Cardiovasc Imaging. 2020;13:2162-2173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 43. | Beecy AN, Chang Q, Anchouche K, Baskaran L, Elmore K, Kolli K, Wang H, Al'Aref S, Peña JM, Knight-Greenfield A, Patel P, Sun P, Zhang T, Kamel H, Gupta A, Min JK. A Novel Deep Learning Approach for Automated Diagnosis of Acute Ischemic Infarction on Computed Tomography. JACC Cardiovasc Imaging. 2018;11:1723-1725. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 44. | Oikonomou EK, Williams MC, Kotanidis CP, Desai MY, Marwan M, Antonopoulos AS, Thomas KE, Thomas S, Akoumianakis I, Fan LM, Kesavan S, Herdman L, Alashi A, Centeno EH, Lyasheva M, Griffin BP, Flamm SD, Shirodaria C, Sabharwal N, Kelion A, Dweck MR, Van Beek EJR, Deanfield J, Hopewell JC, Neubauer S, Channon KM, Achenbach S, Newby DE, Antoniades C. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur Heart J. 2019;40:3529-3543. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 246] [Cited by in F6Publishing: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 45. | Eisenberg E, McElhinney PA, Commandeur F, Chen X, Cadet S, Goeller M, Razipour A, Gransar H, Cantu S, Miller RJH, Slomka PJ, Wong ND, Rozanski A, Achenbach S, Tamarappoo BK, Berman DS, Dey D. Deep Learning-Based Quantification of Epicardial Adipose Tissue Volume and Attenuation Predicts Major Adverse Cardiovascular Events in Asymptomatic Subjects. Circ Cardiovasc Imaging. 2020;13:e009829. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 46. | Shameer K, Johnson KW, Glicksberg BS, Dudley JT, Sengupta PP. The whole is greater than the sum of its parts: combining classical statistical and machine intelligence methods in medicine. Heart. 2018;104:1228. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, Andreini D, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJ, Cury RC, Delago A, Gomez M, Gransar H, Hadamitzky M, Hausleiter J, Hindoyan N, Feuchtner G, Kaufmann PA, Kim YJ, Leipsic J, Lin FY, Maffei E, Marques H, Pontone G, Raff G, Rubinshtein R, Shaw LJ, Stehli J, Villines TC, Dunning A, Min JK, Slomka PJ. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38:500-507. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 112] [Cited by in F6Publishing: 222] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 48. | van Rosendael AR, Maliakal G, Kolli KK, Beecy A, Al'Aref SJ, Dwivedi A, Singh G, Panday M, Kumar A, Ma X, Achenbach S, Al-Mallah MH, Andreini D, Bax JJ, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, Cury RC, DeLago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Kim YJ, Leipsic JA, Maffei E, Marques H, Pontone G, Raff GL, Rubinshtein R, Shaw LJ, Villines TC, Gransar H, Lu Y, Jones EC, Peña JM, Lin FY, Min JK. Maximization of the usage of coronary CTA derived plaque information using a machine learning based algorithm to improve risk stratification; insights from the CONFIRM registry. J Cardiovasc Comput Tomogr. 2018;12:204-209. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 49. | Han D, Beecy A, Anchouche K, Gransar H, Dunham PC, Lee JH, Achenbach S, Al-Mallah MH, Andreini D, Berman DS, Bax JJ, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Chinnaiyan K, Chow BJW, Cury RC, DeLago A, Feuchtner G, Hadamitzky M, Hausleiter J, Kaufmann PA, Kim YJ, Leipsic JA, Maffei E, Marques H, de Araújo Gonçalves P, Pontone G, Raff GL, Rubinshtein R, Villines TC, Lu Y, Peña JM, Shaw LJ, Min JK, Lin FY. Risk Reclassification With Coronary Computed Tomography Angiography-Visualized Nonobstructive Coronary Artery Disease According to 2018 American College of Cardiology/American Heart Association Cholesterol Guidelines (from the Coronary Computed Tomography Angiography Evaluation for Clinical Outcomes : An International Multicenter Registry [CONFIRM]). Am J Cardiol. 2019;124:1397-1405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Shrestha S, Sengupta PP. Imaging Heart Failure With Artificial Intelligence: Improving the Realism of Synthetic Wisdom. Circ Cardiovasc Imaging. 2018;11:e007723. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 51. | Dey D, Slomka PJ, Leeson P, Comaniciu D, Shrestha S, Sengupta PP, Marwick TH. Artificial Intelligence in Cardiovascular Imaging: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:1317-1335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 312] [Cited by in F6Publishing: 301] [Article Influence: 60.2] [Reference Citation Analysis (0)] |
| 52. | Bhavnani SP, Narula J, Sengupta PP. Mobile technology and the digitization of healthcare. Eur Heart J. 2016;37:1428-1438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 203] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 53. | Bhavnani SP, Sola S, Adams D, Venkateshvaran A, Dash PK, Sengupta PP; ASEF-VALUES Investigators. A Randomized Trial of Pocket-Echocardiography Integrated Mobile Health Device Assessments in Modern Structural Heart Disease Clinics. JACC Cardiovasc Imaging. 2018;11:546-557. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 38] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 54. | Sabharwal NK. Could Deep Learning Change Our Working Lives? JACC Cardiovasc Imaging. 2018;11:1664-1665. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Seetharam K, Shrestha S, Sengupta PP. Artificial Intelligence in Cardiovascular Medicine. Curr Treat Options Cardiovasc Med. 2019;21:25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 56. | Krittanawong C, Johnson KW, Tang WW. How artificial intelligence could redefine clinical trials in cardiovascular medicine: lessons learned from oncology. Per Med. 2019;16:83-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Bostrom N, Yudkowsky E. The ethics of artificial intelligence. The Cambridge Handbook of Artificial Intelligence. Cambridge University Press, 2014. [DOI] [Cited in This Article: ] |
| 58. | Sengupta PP, Shrestha S, Berthon B, Messas E, Donal E, Tison GH, Min JK, D'hooge J, Voigt JU, Dudley J, Verjans JW, Shameer K, Johnson K, Lovstakken L, Tabassian M, Piccirilli M, Pernot M, Yanamala N, Duchateau N, Kagiyama N, Bernard O, Slomka P, Deo R, Arnaout R. Proposed Requirements for Cardiovascular Imaging-Related Machine Learning Evaluation (PRIME): A Checklist: Reviewed by the American College of Cardiology Healthcare Innovation Council. JACC Cardiovasc Imaging. 2020;13:2017-2035. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |