Published online Sep 26, 2010. doi: 10.4330/wjc.v2.i9.257
Revised: September 14, 2010
Accepted: September 21, 2010
Published online: September 26, 2010
Drug-eluting balloons (DEBs) represent an enhancement of the therapeutic repertoire for the interventional cardiologist. The therapeutic concept of DEBs is promising, notably on the basis of initial studies in patients with diffuse in-stent restenosis (ISR). At present, however, a number of questions regarding long-term efficacy and safety remain, specifically in indications other than diffuse ISR. The results of the evaluation of different substances, balloon systems and clinical indications will determine the long-term success of DEBs.
- Citation: Joost A, Kurowski V, Radke PW. Drug eluting balloons for the treatment of coronary artery disease: What can we expect? World J Cardiol 2010; 2(9): 257-261
- URL: https://www.wjgnet.com/1949-8462/full/v2/i9/257.htm
- DOI: https://dx.doi.org/10.4330/wjc.v2.i9.257
Ever since the broad clinical introduction of angioplasty and subsequentially coronary stent implantation in the 1990s, in-stent restenosis (ISR) has become and remains one of the major limitations of this treatment modality. In the era of “plain old balloon angioplasty (POBA)”, restenosis occurred as a result of elastic recoil and negative vascular remodeling. However, other factors such as neointima proliferation may contribute to the POBA-induced restenosis to some extent. These processes have been reduced using coronary stents during percutaneous coronary intervention (PCI). However, the proliferation and migration of vascular smooth muscle cells and myofibroblasts, together with the production of extracellular matrix, resulted in a new form of restenosis: ISR.
The development of drug-eluting stents (DES) markedly reduced the rate of ISR both in randomized clinical trials and so called “all-comer” evaluations. As compared to bare metal stent (BMS) usage, the implantation of DES, however, is associated with late stent-thrombosis and the need of a long-term dual antiplatelet therapy (DAPT), which in the majority of cases comprises acetylsalicylic acid and clopidogrel. Although the incidence of ISR has declined as a result of increased usage of DES worldwide, the issue of ISR will continue to be notable because of the increasing numbers of patients treated with PCIs. As over 3 million PCIs and consecutive implantation of one or more stents are performed worldwide every year, a clinically relevant rate of restenosis of 5%-10% in these patients may lead to at least 150 000 to 300 000 recurrent interventions per year.
Current treatment alternatives for ISR include plain angioplasty using conventional or cutting balloons, reimplantation of BMS or DES, rotablation, atherectomy or brachytherapy. The latter ones, however, have not proven to be more effective than balloon angioplasty alone. The implantation of DES, clearly, has become a common treatment modality, specifically in long restenotic lesions. The recurrent ISR-restenosis rate (“re-restenosis”), however, remains above 20%[1-3].
Balloon-based concepts for prevention and effective therapy of ISR are attractive for a number of reasons. Possible advantages include: (1) homogeneous drug absorption into the vessel tissue; (2) the highest concentration of the drug at the time of intervention and therefore at the beginning of the neointimal proliferation; (3) the absence of polymers, with a consequent reduction in chronic inflammation and the risk of late stent thrombosis; (4) the preservation of the original coronary anatomy particularly in small vessels and bifurcation lesions; (5) a reduction in the duration of DAPT; and (6) drug application in special situations where stent implantation is not allowed or wanted[4]. Paclitaxel plays an exceptional role in the context of local drug therapy because of its lipophilic properties, short absorption time after contact with the artery wall and prolonged duration of antiproliferative effects of up to several days[5]. Sound preclinical data specifically exist regarding the application of paclitaxel using the radio-contrast agent Iobromid[6]. This kind of application has been used for Paccocath® technology in the first-in-man study[7] within the PEPCAD-trial program.
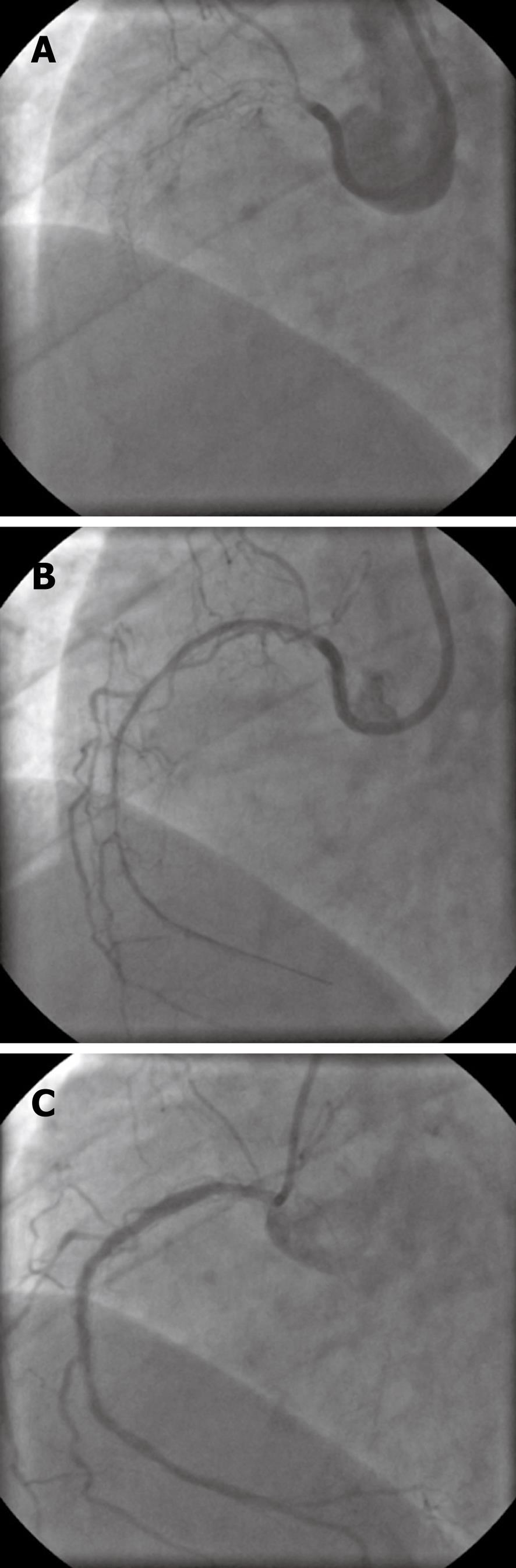
A 73-year-old woman with coronary 3-vessel-disease, insulin-dependent diabetes mellitus and hypercholesterolemia underwent PCI for multiple right coronary artery stenoses with implantation of 3 zotarolimus-eluting stents (total stent length 60 mm). One year later, she was readmitted with non-ST segment myocardial infarction as a result of long segment ISR which was treated percutaneous transluminal coronary angioplasty (PTCA), including dilatation with cutting balloons. Six months later the patient again presented with unstable angina. Control angiography revealed a subtotal long segment ISR (Figure 1A). Recanalization was performed by wire passage and PTCA using a low-diameter low-profile balloon (Figure 1B). Postdilatation was performed by means of a 3 mm cutting-balloon followed by 2 paclitaxel-eluting balloons (SeQuent® Please, 3.0 mm × 30 mm). Six months after drug-eluting balloon (DEB) treatment, control angiography showed only moderate recurrent neointimal proliferation (Figure 1C).
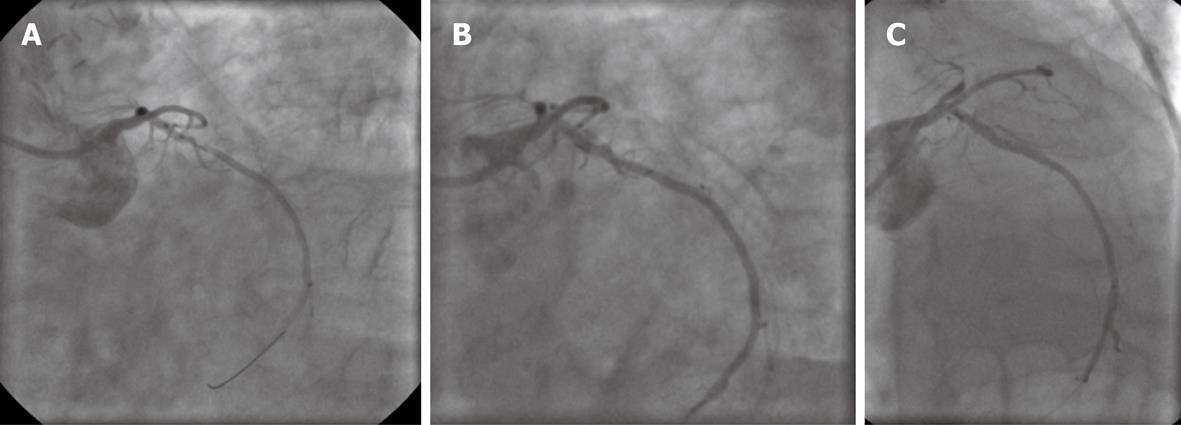
An 81-year-old man with known coronary artery disease, who underwent coronary artery bypass graft surgery 10 years previously, and had permanent atrial fibrillation (continuous warfarin therapy) and a history of peptic gastric ulcer as a result of former acetylsalicylic acid therapy was admitted to our hospital with unstable angina. Coronary angiography revealed open bypasses to the left anterior descending artery, first obtuse marginal and right coronary artery, and a proximal stenosis of the native left circumflex coronary artery (LCX) with a reference vessel diameter of 2.5 mm (Figure 2A). The LCX lesion was predilated with a 2 mm balloon followed by angioplasty using a paclitaxel-eluting balloon (SeQuent® Please, 2.5 mm × 20 mm). Postinterventional angiography showed a slight residual stenosis and a circumscript dissection without flow limitation (Figure 2B). DAPT with acetylsalicylic acid and clopidogrel was given for a period of 4 wk accompanied by continuation of warfarin (with a target international normalized ratio (INR) of 2 during the 4 wk of “triple therapy”, and a target INR of 3 thereafter). Because of the patient’s history of aspirin-associated ulcer, the proton pump inhibitor pantoprazol was administered along with DAPT. After PCI, the patient continued to be free of symptoms, and angiography after 6 mo showed a fair interventional result with no restenosis of LCX (Figure 2C).
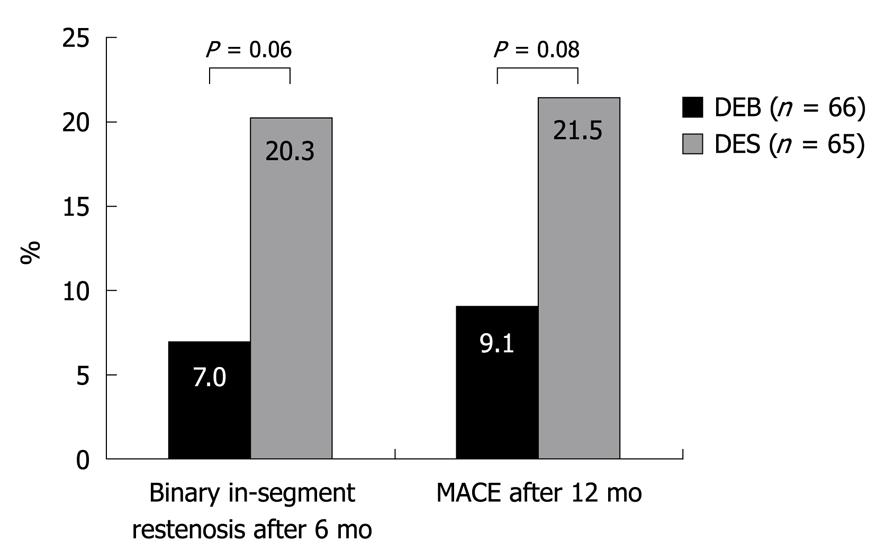
These case examples provide further information regarding potential indications for this new technique. Few data, however, have been published thus far regarding the efficacy of DEBs, particularly concerning the long-term efficacy and safety issues. Initial clinical trials have been performed using the Paccocath® technique. The Paccocath® ISR-I and ISR-II trials were designed as randomized, multicenter trials evaluating the efficacy of a DEB in comparison to an uncoated balloon in 108 patients with ISR. The use of the DEB reduced the late lumen loss, the rate of binary restenosis and the frequency of major adverse cardiac events[7,8]. The PEPCAD-II-trial, in contrast, evaluated the efficacy of the Paccocath® technique using a DEB (SeQuent® Please) in a randomized comparison with a paclitaxel-eluting stent (Taxus Liberté®) for the treatment of ISR in 131 patients[9]. The utilization of the DEB was associated with a lower rate of restenosis and a lower frequency of target lesion revascularization (Figure 3).
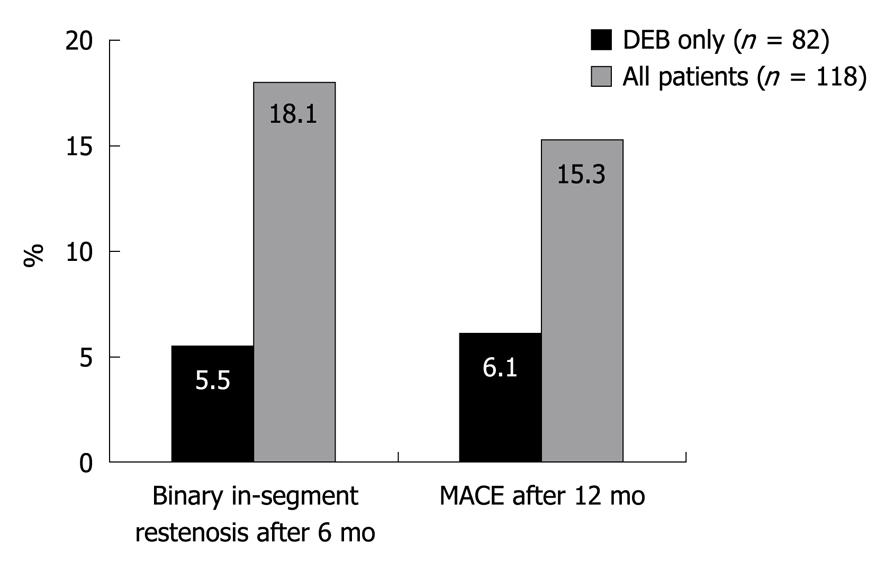
The efficacy of the SeQuent® Please DEB was also evaluated in 118 patients with de novo coronary stenosis < 2.8 mm in a non-randomized study[10]. The aim was to evaluate outcomes using a “DEB only” strategy and avoidance of any further stent implantation. Additional stents were only permitted in cases of dissection or acute recoil. The rate of binary restenosis was significantly lower in patients who were treated only with the DEB in comparison to patients who had to receive additional stents (Figure 4).
Bifurcation lesions are associated with a high risk of restenosis and thus represent a potential indication for DEBs. A non-randomized trial including 20 patients was able to document successful interventions of bifurcation lesions with promising mid-term results[11]. The largest randomized trial so far which evaluated the application of DEB in combination with BMS was the recently published PEPCAD-III trial. In this trial, the efficacy of treatment of de novo lesions in coronary arteries with a pre-crimped BMS on a DEB system (Coroflex® DEBlue) was compared to a sirolimus-eluting stent (Cypher®) in 637 patients. The results clearly showed that treatment with a “BMS on DEB” system was effective, but revealed less favorable results regarding the endpoint late lumen loss and frequency of target lesion revascularization than in the DES arm of the study[12]. At present, several ongoing trials are evaluating the application of DEB, e.g. in patients with diabetes mellitus, or for the treatment of chronic coronary occlusions, or for PCI of an ISR in DES. In addition to the treatment of coronary lesions, 2 randomized trials in 87 and 154 patients using DEBs for treatment of stenoses or occlusions of the femoropopliteal arteries in comparison to BMS or the addition of paclitaxel to the contrast media showed superior results for the DEB technology[13,14].
The concept of local drug delivery has been experimentally and clinically investigated for more than a decade[5]. The limitations of the currently used polymer-based DES (prolonged DAPT, late stent thrombosis) shifted the focus to DEB. At first glance, the DEB is apparently able to integrate several favorable characteristics. Preclinical in vivo data are only available for 2 systems[6,15,16]. The successful delivery of paclitaxel from the balloon into the vessel tissue and prolonged residence in the tissue was proven in experimental pig models. Other DEB systems currently used in the clinical context lack sound preclinical data. At present, however, it is also unclear what kind of drug coating on the balloon (e.g. hydrophilic Iopromid coating, microporous balloon surface, special balloon folding) or elution in the vessel tissue produces the most favorable safety-efficacy ratio. Regarding the clinical evaluation of DES, the last years clearly showed that a reliable assessment of safety and efficacy depends on major randomized trials and consecutive registry data with sufficient long-term follow-up periods (years rather than months). This approach should also be maintained in the context of DEBs as late adverse effects (e.g. as a result of delayed healing) may occur. At the moment, published data are only available for one balloon system (Paccocath®, Sequent® Please) regarding one indication (ISR) in comparison to lone dilatation or DES (Taxus®)[7,9]. The largest trial in the context of DEB in 637 patients with de novo lesions, the PEPCAD-III study, compared the combined usage of DEB and BMS to the sirolimus-eluting Cypher® stent and demonstrated better results for the DES concept[12].
The DEB represents an excellent therapeutic concept. However, this technique carries a number of unanswered questions and is being further evaluated in different clinical settings. Regarding product development, it is unclear which method of drug retention and elution and which drug is most favorable. It has to be proven in the clinical context whether use of DEBs beyond the indication in ISR (< 10% of our patients) compares favorably with current standard therapy. Furthermore, reliable data regarding the implantation of BMS for clinical reasons (e.g. for a dissection) after a DEB-dilatation are lacking. A thorough analysis of the PEPCAD-III study may give the answers (DEB-stent “mismatch”). Currently, additional stent implantation seems to improve the acute procedural outcome, but worsens the long-term outcome. In addition, long-term results when treating ISR in DES are uncertain. Taken together, expectations should not be too high regarding the broad application of DEB in the near future. At our center, we are also using the DEB primarily for the treatment of recurrent diffuse restenosis of BMS and, in some cases, of DES. The results of ongoing preclinical development and clinical evaluation of various DEB, however, will determine the long-term success of the concept of DEB. As a result of the rapid development in the field of stent technologies (e.g. degradable drug-eluting polymers, complete stent degradation, polymer-free eluting systems) and concomitant pharmacologic therapy, it is unclear whether DEBs will be used for more than niche indications in the long-term.
The DEB enhances the therapeutic repertoire of interventional cardiologists. The use of DEB especially for the treatment of ISR can prevent the implantation of further stents. First results from trials evaluating small vessels and bifurcation lesions may give rise to more indications for this technique, but results of randomized trials regarding these issues are still required. The use of DEBs in selected patients in combination with BMS may be considered as an alternative to BMS, mainly because of the possible reduction in the duration of DAPT. Regarding the broad utilization of DEBs, in terms of efficacy and especially safety, data from larger clinical trials and registries should be awaited.
Peer reviewers: Seung-Woon Rha, MD, PhD, FACC, FAHA, FESC, FSCAI, FAPSIC, Cardiovascular Center, Korea University Guro Hospital, 80, Guro-dong, Guro-gu, Seoul 152-703, South Korea; Hiroki Teragawa, MD, PhD, Department of Cardiovascular Medicine, Hiroshima University Graduate School of Biomedical Sciences, 1-2-3 Kasumi, Minami-ku, Hiroshima 734-8551, Japan
S- Editor Cheng JX L- Editor Cant MR E- Editor Zheng XM
| 1. | Kastrati A, Mehilli J, von Beckerath N, Dibra A, Hausleiter J, Pache J, Schühlen H, Schmitt C, Dirschinger J, Schömig A. Sirolimus-eluting stent or paclitaxel-eluting stent vs balloon angioplasty for prevention of recurrences in patients with coronary in-stent restenosis: a randomized controlled trial. JAMA. 2005;293:165-71. [Cited in This Article: ] |
| 2. | Stone GW, Ellis SG, O'Shaughnessy CD, Martin SL, Satler L, McGarry T, Turco MA, Kereiakes DJ, Kelley L, Popma JJ. Paclitaxel-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the TAXUS V ISR randomized trial. JAMA. 2006;295:1253-1263. [Cited in This Article: ] |
| 3. | Holmes DR Jr, Teirstein P, Satler L, Sketch M, O'Malley J, Popma JJ, Kuntz RE, Fitzgerald PJ, Wang H, Caramanica E. Sirolimus-eluting stents vs vascular brachytherapy for in-stent restenosis within bare-metal stents: the SISR randomized trial. JAMA. 2006;295:1264-1273. [Cited in This Article: ] |
| 4. | De Labriolle A, Pakala R, Bonello L, Lemesle G, Scheinowitz M, Waksman R. Paclitaxel-eluting balloon: from bench to bed. Catheter Cardiovasc Interv. 2009;73:643-652. [Cited in This Article: ] |
| 5. | Axel DI, Kunert W, Göggelmann C, Oberhoff M, Herdeg C, Küttner A, Wild DH, Brehm BR, Riessen R, Köveker G. Paclitaxel inhibits arterial smooth muscle cell proliferation and migration in vitro and in vivo using local drug delivery. Circulation. 1997;96:636-645. [Cited in This Article: ] |
| 6. | Scheller B, Speck U, Abramjuk C, Bernhardt U, Böhm M, Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110:810-814. [Cited in This Article: ] |
| 7. | Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U. Treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. N Engl J Med. 2006;355:2113-2124. [Cited in This Article: ] |
| 8. | Scheller B, Hehrlein C, Bocksch W, Rutsch W, Haghi D, Dietz U, Böhm M, Speck U. Two year follow-up after treatment of coronary in-stent restenosis with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2008;97:773-781. [Cited in This Article: ] |
| 9. | Unverdorben M, Vallbracht C, Cremers B, Heuer H, Hengstenberg C, Maikowski C, Werner GS, Antoni D, Kleber FX, Bocksch W. Paclitaxel-coated balloon catheter versus paclitaxel-coated stent for the treatment of coronary in-stent restenosis. Circulation. 2009;119:2986-2994. [Cited in This Article: ] |
| 10. | Unverdorben M, Kleber FX, Heuer H, Figulla HR, Vallbracht C, Leschke M, Cremers B, Hardt S, Buerke M, Ackermann H. Treatment of small coronary arteries with a paclitaxel-coated balloon catheter. Clin Res Cardiol. 2010;99:165-174. [Cited in This Article: ] |
| 11. | Fanggiday JC, Stella PR, Guyomi SH, Doevendans PA. Safety and efficacy of drug-eluting balloons in percutaneous treatment of bifurcation lesions: the DEBIUT (drug-eluting balloon in bifurcation Utrecht) registry. Catheter Cardiovasc Interv. 2008;71:629-635. [Cited in This Article: ] |
| 12. | Pöss J, Jacobshagen C, Ukena C, Böhm M. Hotlines and clinical trial updates presented at the German Cardiac Society Meeting 2010: FAIR-HF, CIPAMI, LIPSIA-NSTEMI, Handheld-BNP, PEPCAD III, remote ischaemic conditioning, CERTIFY, PreSCD-II, German Myocardial Infarction Registry, DiaRegis. Clin Res Cardiol. 2010;99:411-417. [Cited in This Article: ] |
| 13. | Werk M, Langner S, Reinkensmeier B, Boettcher HF, Tepe G, Dietz U, Hosten N, Hamm B, Speck U, Ricke J. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118:1358-1365. [Cited in This Article: ] |
| 14. | Tepe G, Zeller T, Albrecht T, Heller S, Schwarzwälder U, Beregi JP, Claussen CD, Oldenburg A, Scheller B, Speck U. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358:689-699. [Cited in This Article: ] |
| 15. | Cremers B, Biedermann M, Mahnkopf D, Böhm M, Scheller B. Comparison of two different paclitaxel-coated balloon catheters in the porcine coronary restenosis model. Clin Res Cardiol. 2009;98:325-330. [Cited in This Article: ] |
| 16. | Posa A, Hemetsberger R, Petnehazy O, Petrasi Z, Testor M, Glogar D, Gyöngyösi M. Attainment of local drug delivery with paclitaxel-eluting balloon in porcine coronary arteries. Coron Artery Dis. 2008;19:243-247. [Cited in This Article: ] |