Published online Dec 26, 2012. doi: 10.4330/wjc.v4.i12.341
Revised: October 20, 2012
Accepted: October 27, 2012
Published online: December 26, 2012
AIM: To examine the feasibility and reliability of measuring global and segmental longitudinal strain (LS) compared to visual assessment of wall motion (WM).
METHODS: Assessment of segmental (17 left ventricular segments) LS using automatic function imaging (AFI) in 55 patients (60.0 ± 8.7 years, 73% male) divided into 2 groups: group I included 35 patients with WM abnormalities and/or impaired ejection fraction and group II included 20 patients with normal WM and ejection fraction. Visual analysis of WM abnormalities was performed using 2-dimensional echocardiography (2DE) and WM score was calculated. Both modalities were analyzed by one expert reader at 2 different sessions.
RESULTS: Analysis of 935 left ventricular segments was completed in 94.1% and 96.3% by visual assessment and AFI, respectively. There was a strong positive linear relationship between the WM score and global LS in all patients. Intra-observer agreement for calculation of WM score was excellent for group I patients (kappa: 0.97) and very good for group II patients (kappa: 0.92). Intra-observer agreement for AFI showed excellent agreement with very small mean difference in both group I and II (-0.0 ± 2.3 and -0.0 ± 1.9, respectively).
CONCLUSION: The interpretation of global and segmental LS using AFI is a more feasible and reliable technique for the quantification of myocardial deformation than visual assessment of WM scores.
- Citation: Anwar AM. Global and segmental myocardial deformation by 2D speckle tracking compared to visual assessment. World J Cardiol 2012; 4(12): 341-346
- URL: https://www.wjgnet.com/1949-8462/full/v4/i12/341.htm
- DOI: https://dx.doi.org/10.4330/wjc.v4.i12.341
Echocardiographic evaluation of segmental and global myocardial function by visual assessment is the most used method[1]. The visual assessment relies mainly on myocardial radial performance, and is limited by high inter- and intra-observer variability. Furthermore, it provides a subjective evaluation of endocardial thickening and excursion[2]. Tissue Doppler imaging was expected to overcome these limitations, but its sensitivity to measure the longitudinal and radial deformation was limited due to the angle-dependancy with inability to differentiate between active and passive myocardial motion[3,4].
Speckle-tracking echocardiography (STE) is a new noninvasive imaging technique that quantitatively analyzes global and regional myocardial function. Its evaluation is based on tracking natural acoustic reflections and interference patterns within an ultrasound window[5]. A large amount of published data has described the accuracy and clinical applications of STE with the ability to elaborate the myocardial deformation in longitudinal, radial and circumferential directions[6-8].
Automated function imaging (AFI) is a novel algorithm calculating the myocardial deformation from ultrasound speckles that is used to measure myocardial strain. It tracks the percentage of wall lengthening and shortening which helps to assess myocardial deformation based on grayscale images, similar in concept to magnetic resonance imaging tagging. It presents an objective, semi-automatic, and angle-independent analysis of longitudinal peak systolic strain (LS) based on speckle tracking and provides a single bull’s-eye summary of the left ventricular (LV) segmental wall strain[9].
The objective of this study was to assess the feasibility and reliability of AFI compared with visual assessment by 2-dimensional echocardiography (2DE), regardless of underlying cardiac disorders.
The study included 55 consecutive patients who were referred for echocardiography for assessment of LV function and segmental wall motion (WM) abnormalities. The inclusion criteria were adequate image quality and sinus rhythm. Conventional 2DE was performed according to guidelines using a commercial ultrasound system (Vivid 7, GE Health Medical, Milwaukee, WI, United States) supported by a multi-frequency transducer (M3S 1.7/3.4 MHz). After the completion of the 2DE study, the 17 LV segments were assessed by visual analysis and AFI. Both visual analysis and AFI were performed by one experienced observer at two different settings. The first analysis was performed immediately after the end of each study. The second re-analysis of all studies was performed by the same observer after collection of cases. The interval between the 2 analysis was 1 mo. In each study, the observer was blinded to the previous readings.
Visual analysis: Visual analysis of the contractile function of all the 17 segments was interpreted according to the American Society of Echocardiography criteria[10] using a four-point score: (1) normal; (2) hypokinetic; (3) akinetic; or (4) dyskinetic. The sum of the WM scores, averaged over the number of segments with interpretive scores, gave the WM score index.
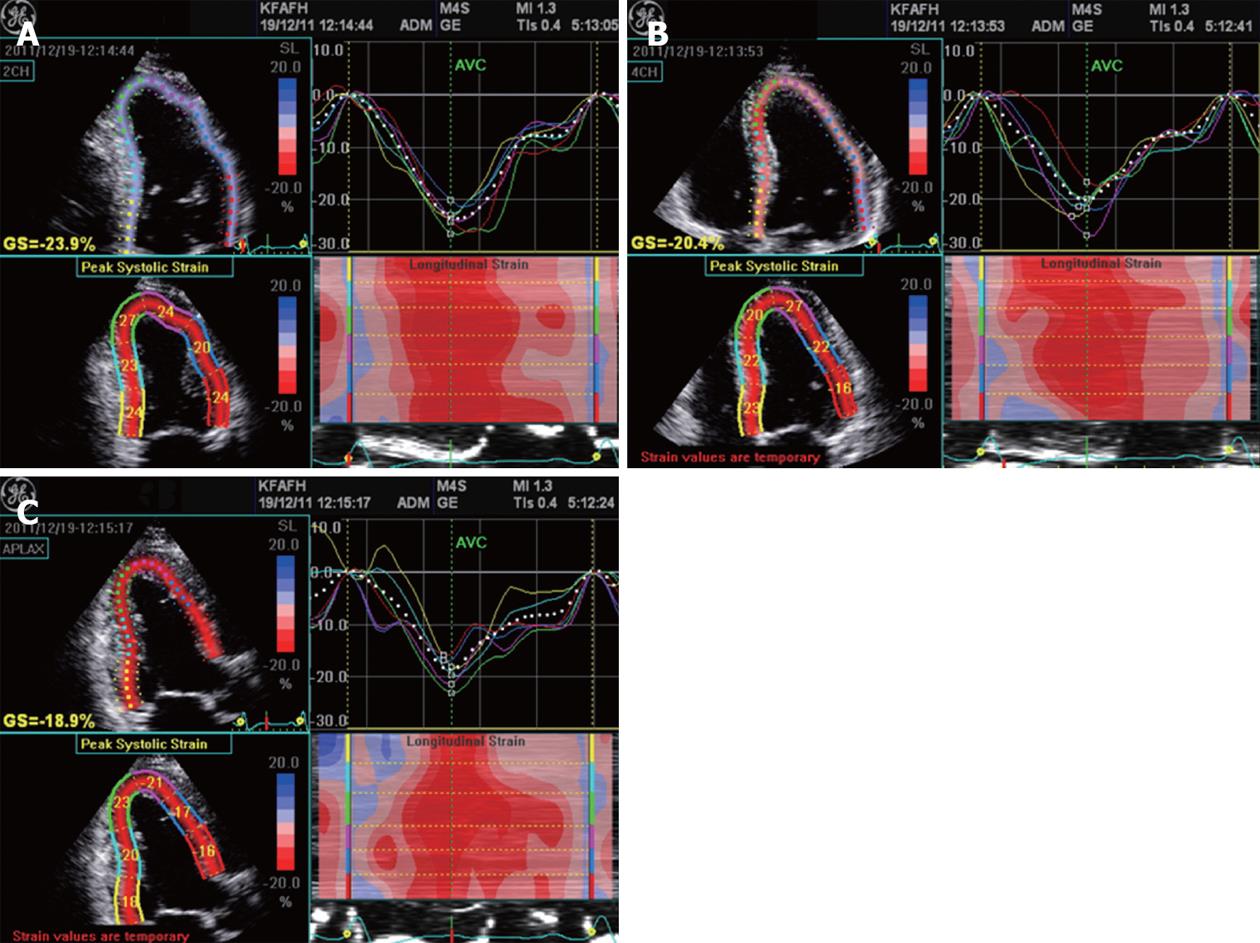
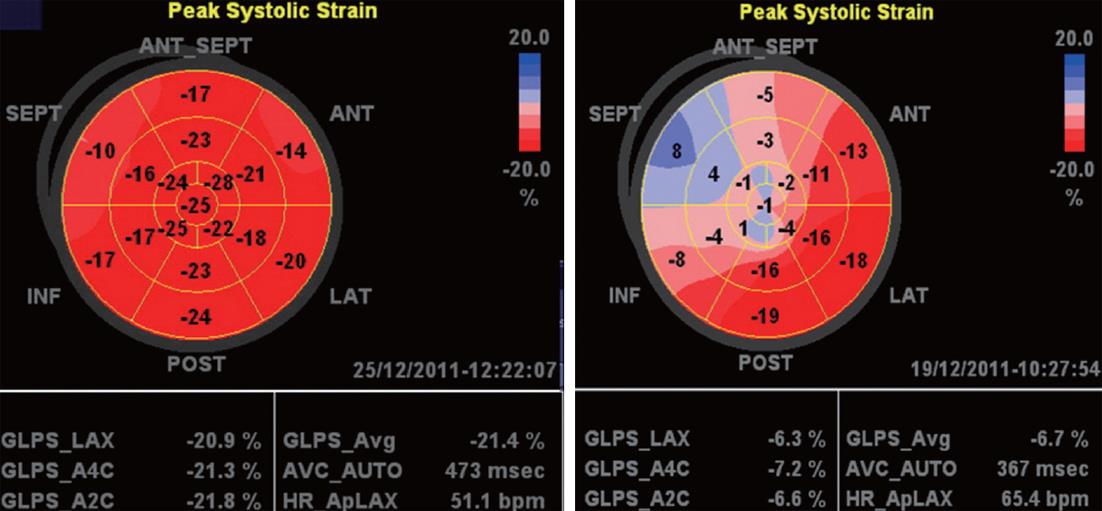
AFI protocol: The AFI was performed through an off-line analysis of 3 digitally stored 2D images (apical long-axis, 2- and 4-chamber) with high frame rates (> 60 frames/s) using commercial imaging analysis software (EchoPAC 6.1.0, GE Health Medical)[11]. The peak systolic strain values in a 17-segment LV model were used in the present study. End-systole was defined as aortic valve closure in the apical long-axis view by continuous Doppler wave recording. Automated delineation of endocardial borders was obtained through marking the mitral annulus level and at the apex on each digital loop. The area of interest was manually adjusted if automated delineation was not optimal. Segments with poor image acquisition or artifacts were excluded due to inability to measure LS. Segmental LS was calculated as the percentage of lengthening or shortening and the results for each plane were displayed (Figure 1). The results for all 3 planes were then combined in a single bull’s-eye summary (Figure 2). The sum of LS, averaged over the number of segments with interpretive scores, gave the global LS. The normal values of segmental and global LS were considered (-17.4% for females and -15.9% for males) based on the Hunt study in Norway[12] which presented the reference values in 1266 healthy individuals according to age and sex.
Statistical analysis was performed using the software package SPSS version 11.5 (SPSS Inc, Chicago IL, United States). All data obtained were presented as mean ± SD. Paired sample t test was performed to determine the difference in values between the repeated readings of visual analysis and AFI. The level of significance was set to P < 0.05. Intraobserver agreement for visual analysis score for each segment was estimated using kappa values and classified as excellent with a value of 0.93-1.0, very good 0.81-0.92, good 0.41-0.60, and poor ≤ 0.4[13]. Intraobserver agreement for global and segmental LS measurements was estimated according to the Bland and Altman method[14].
The study included 55 consecutive patients (60.0 ± 8.7 years, 73% male). Depending on 2DE calculation of LV ejection fraction and assessment of WM abnormalities, the patients were classified into 2 groups: group I included 35 patients with WM abnormalities and/or impaired LV ejection fraction (≤ 50%), and group II included 20 patients with normal WM and LV ejection fraction. Baseline criteria of both groups are displayed in Table 1. Clinical data of patients in group I showed a higher incidence of diabetes, hypertension, smoking and ischemic heart disease (55%, 40%, 45% and 55%, respectively) than for patients in group II (30%, 25%, 30% and 15%, respectively). Through analysis of LV by the 17-segment model, a total of 935 segments were assessed by both visual and AFI analysis techniques. Global LS was well correlated (R = 0.86, P < 0.0001) with WM score index in all patients, while the correlation with LV ejection fraction was less (R = 0.62, P < 0.01).
| Total (n = 55) | GroupI(n = 35) | Group II (n = 20) | P value | |
| Age | 59.4 ± 13.6 | 61.0 ± 9.9 | 57.7 ± 16.9 | 0.4 |
| Gender, n (%) | 0.8 | |||
| Male | 29 (72.5%) | 14 (70%) | 15 (75%) | |
| Female | 11 (27.5%) | 6 (30%) | 5 (25%) | |
| IVS (mm) | 10.3 ± 2.4 | 9.4 ± 2.1 | 11.3 ± 2.4 | 0.01 |
| PW (mm) | 9.8 ± 1.7 | 9.4 ± 1.5 | 10.2 ± 1.9 | 0.1 |
| LV mass | 245.4 ± 99.5 | 254.9 ± 108.9 | 235.3 ± 90.6 | 0.5 |
| LA diameter (mm) | 38.3 ± 6.6 | 40.2 ± 6.4 | 36.3 ± 6.3 | 0.07 |
| LVDD (mm) | 51.2 ± 9.8 | 55.2 ± 11.6 | 49.0 ± 5.1 | 0.009 |
| LVSD (mm) | 36.4 ± 11.9 | 41.3 ± 14.1 | 30.2 ± 5.3 | 0.008 |
| LV-FS (%) | 30.0 ± 11.1 | 26.4 ± 11.9 | 34.9 ± 7.1 | 0.03 |
| LVDV (mL) | 119.4 ± 65.7 | 137.9 ± 85.8 | 99.9 ± 20.8 | 0.07 |
| LVSV (mL) | 65.7 ± 60.8 | 87.3 ± 77.3 | 40.9 ± 20.1 | 0.02 |
| LV-EF (%) | 49.9 ± 15.0 | 41.9 ± 14.1 | 59.7 ± 8.1 | 0.0001 |
| WM score | 20.6 ± 6.4 | 24.9 ± 6.4 | 16.0 ± 0.0 | 0.0001 |
| WM score index | 1.3 ± 0.4 | 1.6 ± 0.4 | 1.0 ± 0.0 | 0.0001 |
| Global LS | -11.8 ± 6.7 | -9.3 ± 7.1 | -15.6 ± 3.7 | 0.02 |
Complete analysis was achieved in all patients within 3.5 ± 1.9 min. Complete scoring was obtained for 880 segments (94.1%), while it was not possible for 55 segments (5.9%) due to poor endocardial visualization. Distribution of the missed segments showed high incidence for basal antero-lateral (40%), basal anterior (20%) and apico-inferior (20%). The highest intra-observer correlation (R > 0.8, P < 0.0001) was obtained for analysis of mid segments of all walls, while the lowest correlation (R = 0.56, P = 0.01) was for both basal antero-lateral and basal anterior segments. Intra-observer agreement for calculation of WM score was excellent for group I patients (kappa value: 0.97) and very good for group II patients (kappa value: 0.92).
Complete analysis was achieved in all patients within 3.9 ± 2.1 min. Manual modification of the endocardial border was performed to get optimal delineation in 10 patients (18%). Calculation of LS was obtained for 900 segments (96.3%), while it was not obtained for 35 segments (3.7%). Distribution of the missed segments showed high incidence for basal antero-lateral (26%), apico-lateral (20%), apico-inferior (20%) and basal anterior (14%). The highest intra-observer correlation (≥ 0.83, P < 0.0001) was obtained for analysis of apico-lateral, apico-septal, apico-inferior, mid antero-lateral, mid infero-septal and mid antero-septal segments. The lowest correlation (R = 0.53, P = 0.008) was for the basal antero-lateral segment. Basal and apical anterior segments showed low correlation (R = 0.65, P = 0.001). Using the Bland and Altman method for intra-observer agreement showed excellent agreement with a very small mean difference in both groups of patients (Table 2). Comparison between both techniques is summarized in Table 3.
| Wall motion score | Speckle tracking (AFI) | |||||
| Total | GroupI | Group II | Total | GroupI | Group II | |
| (n = 55) | (n = 35) | (n = 20) | (n = 55) | (n = 35) | (n = 20) | |
| 1st reading | 20.6 ± 6.4 | 24.9 ± 6.4 | 16.0 ± 0.0 | -11.8 ± 6.7 | -9.3 ± 7.1 | -15.6 ± 3.7 |
| 2nd reading | 22.6 ± 8.7 | 27.3 ± 5.9 | 17.7 ± 2.9 | -13.6 ± 5.5 | -11.3 ± 5.6 | -15.6 ± 4.8 |
| Correlation | R = 0.73, P = 0.03 | R = 0.9, P < 0.0001 | ||||
| MD | 2.1 ± 5.9 | 2.4 ± 5.9 | 1.7 ± 2.9 | -0.02 ± 2.0 | -0.0 ± 2.3 | -0.0 ± 1.9 |
| Visual analysis | AFI | |
| Analysis time (min) | 3.5 ± 1.9 | 3.9 ± 2.1 |
| Complete analysis (%) | 94.10 | 96.30 |
| Intra-observer mean difference | ||
| Normal subjects | 1.7 ± 2.9 | -0.0 ± 1.9 |
| Abnormal subjects | 2.4 ± 5.9 | -0.0 ± 2.3 |
Based on global LS calculation, all patients in group I and 5 patients in group II had reduced global LS (-12.4% ± 2.1%). The remaining 15 patients in group II had normal global LS (-17.8% ± 1.6%). The 5 patients in group II with reduced global LS were older (62.1 ± 2.3 years), 4 males and 1 female. All were diabetic and hypertensive. 2DE showed borderline LV ejection fraction (51.2% ± 0.8%).
This study demonstrated that AFI analysis of global and segmental LS was well correlated with visual assessment of WM abnormalities. Compared to visual assessment, AFI showed very small intra-observer variability regardless of the presence or absence of WM abnormalities and impaired LV systolic function.
The visual interpretation of WM abnormalities with conventional 2DE is based on the assessment of myocardial thickening and endocardial excursion. This method is widely used for assessing LV global and segmental systolic function. However, it is observer-dependant and requires experience[1]. Several reports have described the reliability and feasibility of STE to evaluate global and segmental myocardial deformation throughout the cardiac cycle in both normal and abnormal subjects. It is able to investigate LV function (circumferential, radial and longitudinal) without angle dependency[15-17].
The AFI algorithm is a novel method based on 2D strain imaging which tracks acoustic pixels equally distributed within the myocardial wall. It enables the quantification of myocardial strain simultaneously in different LV segments with ultrasound beam angle-independency. Previous studies reported that AFI is a fast, simple and high reproducible tool for semi-automatic assessment of LV function that supports clinical decision-making[9,18]. The current study aimed to evaluate the reliability and feasibility of AFI to detect segmental WM asynergy compared with visual assessment.
In agreement with the studies of Reisner et al[18] and Munk et al[19], global LS was correlated better with WM score index than with LV ejection fraction (R = 0.86, P < 0.0001; R = 0.62, P < 0.01, respectively).This finding can be explained by the direct effect of LV volume and heart rate on calculation of ejection fraction, while this effect is minimal on WM score index and nil on global LS.
By AFI, complete analysis was obtained for 96.3% of segments while by visual analysis, it was less (94.1%). This was explained by the higher percentage of un-interpretable segments by visual analysis due to poor endocardial delineation, while AFI (which depends on objective tracking of pixels) originated predominantly from the sub-endocardial fibers[20]. Among the missed segments, basal antero-lateral and basal anterior had the higher incidence by both AFI and visual analysis, with tendency of better intra-observer correlation for AFI than visual analysis.
Theoretically speaking, the semi-automated calculation improves reproducibility of the technique when compared with objective assessment. This can explain the good intra-observer variability in measurement of global LS and LV rotation by 2D speckle tracking which was demonstrated in previous studies[18,21]. In the current study, excellent intra-observer correlation and agreement was shown by both techniques in normal subjects. In abnormal subjects, AFI showed better intra-observer correlation and agreement than visual assessment.
Reclassification of our patients based on global LS measurements showed that a considerable percentage (25%) of patients who were considered as normal due to absent segmental WM abnormalities had reduced global LS. This was in agreement with previous studies[19,22] which demonstrated that quantification of segmental and global LS may detect subclinical LV dysfunction earlier than visual analysis of WM in asymptomatic patients with apparently normal LV ejection fraction.
The number of patients included is relatively small; however, it was enough to reach the target of the study. Only one software application (AFI) was applied for measurement of LS; therefore, our results must be taken in consideration towards the AFI only and not for other available software.
The AFI algorithm enabled a comprehensive assessment of global and segmental LS of the LV. It overcame the subjective and semi-quantitative analysis of LV obtained by visual assessment and showed better intra-observer agreement in both normal and abnormal subjects.
Speckle-tracking echocardiography is a new noninvasive imaging technique that quantitatively analyzes global and regional myocardial function. Its evaluation is based on tracking natural acoustic reflections and interference patterns within an ultrasound window. It presents an objective, semi-automatic, and angle-independent analysis based on speckle tracking and provides a single bull’s-eye summary of the left ventricular segmental wall strain.
Many algorithms are available for calculation of myocardial deformation. All algorithms tracks the percentage of wall lengthening and shortening which helps to assess myocardial deformation based on grayscale images, similar in concept to magnetic resonance imaging tagging. The study aimed to assess the feasibility and reliability of (AFI) algorithm for assessment of longitudinal strain using speckle tracking compared with visual assessment by 2-dimensional echocardiography. The study applied this algorithm in both normal and abnormal cardiac conditions.
The study demonstrated that AFI analysis of global and segmental longitudinal strain (LS) was well correlated with visual assessment of wall motion (WM) abnormalities. Compared to visual assessment, AFI showed very small intra-observer variability regardless of the presence or absence of WM abnormalities and impaired left ventricular (LV) systolic function. The study also showed that a considerable percentage (25%) of patients who were considered as normal due to absent segmental WM abnormalities had reduced global LS which mean that detection of myocardial abnormalities can be detected early.
This study directs towards improvement of the analysis and assessment of LV wall abnormalities using longitudinal strain as it is a more feasible and reliable technique than visual assessment of WM scores.
This is an interesting article verifying the utility of Speckle tracking technique. The study is small but concludes that there is good correlation between visual estimates and AFI.
Peer reviewers: Manendra Pal Singh Chawla, Assistant Professor, Department of Electrical Engineering, Indian Institute of Technology, Roorkee 247667, India; Manish Prakash Gupta, MD, Department of Cardiology, UPENN, 11108 Arbor Pine Ave, Las Vegas, NV 89144, United States
S- Editor Cheng JX L- Editor Logan S E- Editor Li JY
| 1. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79-108. [Cited in This Article: ] |
| 2. | Sitia S, Tomasoni L, Turiel M. Speckle tracking echocardiography: A new approach to myocardial function. World J Cardiol. 2010;2:1-5. [PubMed] [Cited in This Article: ] |
| 3. | Perk G, Tunick PA, Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography--from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234-243. [PubMed] [Cited in This Article: ] |
| 4. | Nesbitt GC, Mankad S, Oh JK. Strain imaging in echocardiography: methods and clinical applications. Int J Cardiovasc Imaging. 2009;25 Suppl 1:9-22. [PubMed] [Cited in This Article: ] |
| 5. | Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, Nesser HJ, Khandheria B, Narula J, Sengupta PP. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23:351-369; quiz 453-455. [PubMed] [Cited in This Article: ] |
| 6. | Mondillo S, Galderisi M, Mele D, Cameli M, Lomoriello VS, Zacà V, Ballo P, D'Andrea A, Muraru D, Losi M. Speckle-tracking echocardiography: a new technique for assessing myocardial function. J Ultrasound Med. 2011;30:71-83. [PubMed] [Cited in This Article: ] |
| 7. | Lipiec P, Szymczyk E, Michalski B, Stefańczyk L, Woźniakowski B, Rotkiewicz A, Szymczyk K, Kasprzak JD. Echocardiographic quantitative analysis of resting myocardial function for the assessment of viability after myocardial infarction--comparison with magnetic resonance imaging. Kardiol Pol. 2011;69:915-922. [PubMed] [Cited in This Article: ] |
| 8. | Kusunose K, Yamada H, Nishio S, Mizuguchi Y, Choraku M, Maeda Y, Hosokawa S, Yamazaki N, Tomita N, Niki T. Validation of longitudinal peak systolic strain by speckle tracking echocardiography with visual assessment and myocardial perfusion SPECT in patients with regional asynergy. Circ J. 2011;75:141-147. [PubMed] [Cited in This Article: ] |
| 9. | Belghitia H, Brette S, Lafitte S, Reant P, Picard F, Serri K, Lafitte M, Courregelongue M, Dos Santos P, Douard H. Automated function imaging: a new operator-independent strain method for assessing left ventricular function. Arch Cardiovasc Dis. 2008;101:163-169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 85] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440-1463. [PubMed] [Cited in This Article: ] |
| 11. | Leitman M, Lysyansky P, Sidenko S, Shir V, Peleg E, Binenbaum M, Kaluski E, Krakover R, Vered Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J Am Soc Echocardiogr. 2004;17:1021-1029. [PubMed] [Cited in This Article: ] |
| 12. | Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176-183. [PubMed] [Cited in This Article: ] |
| 13. | Byrt T. How good is that agreement? Epidemiology. 1996;7:561. [PubMed] [Cited in This Article: ] |
| 14. | Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32742] [Cited by in F6Publishing: 31513] [Article Influence: 829.3] [Reference Citation Analysis (2)] |
| 15. | Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21:1207-1215. [PubMed] [Cited in This Article: ] |
| 16. | Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, Tahk SJ, Shin JH. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21:907-911. [PubMed] [Cited in This Article: ] |
| 17. | Lim P, Mitchell-Heggs L, Buakhamsri A, Thomas JD, Grimm RA. Impact of left ventricular size on tissue Doppler and longitudinal strain by speckle tracking for assessing wall motion and mechanical dyssynchrony in candidates for cardiac resynchronization therapy. J Am Soc Echocardiogr. 2009;22:695-701. [PubMed] [Cited in This Article: ] |
| 18. | Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630-633. [PubMed] [Cited in This Article: ] |
| 19. | Munk K, Andersen NH, Nielsen SS, Bibby BM, Bøtker HE, Nielsen TT, Poulsen SH. Global longitudinal strain by speckle tracking for infarct size estimation. Eur J Echocardiogr. 2011;12:156-165. [PubMed] [Cited in This Article: ] |
| 20. | Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1:366-376. [PubMed] [Cited in This Article: ] |
| 21. | van Dalen BM, Soliman OI, Vletter WB, Kauer F, van der Zwaan HB, ten Cate FJ, Geleijnse ML. Feasibility and reproducibility of left ventricular rotation parameters measured by speckle tracking echocardiography. Eur J Echocardiogr. 2009;10:669-676. [PubMed] [Cited in This Article: ] |
| 22. | Nakai H, Takeuchi M, Nishikage T, Lang RM, Otsuji Y. Subclinical left ventricular dysfunction in asymptomatic diabetic patients assessed by two–dimensional speckle tracking echocardiography: correlation with diabetic duration. Eur J Echocardiogr. 2009;10:926-932. [Cited in This Article: ] |