Published online Jul 26, 2015. doi: 10.4330/wjc.v7.i7.415
Peer-review started: January 16, 2015
First decision: March 20, 2015
Revised: June 2, 2015
Accepted: June 18, 2015
Article in press: June 19, 2015
Published online: July 26, 2015
AIM: To investigate if magnetic resonance (MR)-guided biopsy can improve the performance and safety of such procedures.
METHODS: A novel MR-compatible bioptome was evaluated in a series of in-vitro experiments in a 1.5T magnetic resonance imaging (MRI) system. The bioptome was inserted into explanted porcine and bovine hearts under real-time MR-guidance employing a steady state free precession sequence. The artifact produced by the metal element at the tip and the signal voids caused by the bioptome were visually tracked for navigation and allowed its constant and precise localization.
RESULTS: Cardiac structural elements and the target regions for the biopsy were clearly visible. Our method allowed a significantly better spatial visualization of the bioptoms tip compared to conventional X-ray guidance. The specific device design of the bioptome avoided inducible currents and therefore subsequent heating. The novel MR-compatible bioptome provided a superior cardiovascular magnetic resonance (imaging) soft-tissue visualization for MR-guided myocardial biopsies. Not at least the use of MRI guidance for endomyocardial biopsies completely avoided radiation exposure for both patients and interventionalists.
CONCLUSION: MRI-guided endomyocardial biopsies provide a better than conventional X-ray guided navigation and could therefore improve the specificity and reproducibility of cardiac biopsies in future studies.
Core tip: Myocardial biopsy is the method of choice for assessing tissue pathologies. Cardiac magnetic resonance imaging (MRI) provides a 3D visualization and discrimination of soft-tissue and could therefore enable a targeted specimen sampling. We developed a novel MR-compatible bioptome which was evaluated by in-vitro experiments in a 1.5T MRI system under real-time MR-guidance. MRI-guided endomyocardial biopsies provide a superior soft-tissue visualization, a better than conventional X-ray guided navigation and could therefore improve the specificity and reproducibility of cardiac biopsies in future studies. Not at least the use of MRI guidance for endomyocardial biopsies completely avoided radiation exposure for both patients and interventionalists.
-
Citation: Lossnitzer D, Seitz SA, Krautz B, Schnackenburg B, André F, Korosoglou G, Katus HA, Steen H. Feasibility of real-time magnetic resonance imaging-guided endomyocardial biopsies: An
in-vitro study. World J Cardiol 2015; 7(7): 415-422 - URL: https://www.wjgnet.com/1949-8462/full/v7/i7/415.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i7.415
Endomyocardial biopsy (EMB) is the “gold” standard diagnostic tool in the detection and classification of myocardial pathologies. It is recommended in the management and diagnosis of cardiomyopathies and inflammatory myocardial diseases[1-4].
Despite the benefits, EMB indications and their procedural management are controversially discussed. Reasons include EMB’s low sensitivity when conducted under fluoroscopic guidance[5,6] as well as its potential complications. Thus, EMB may cause pericardial tamponade, severe arrhythmias or structural damages of the tricuspid valve[1]. Since sensitivity of EMB is low, clinicians are forced to increase the amount of samples (> 6, according to the AHA/ACCF/ESC scientific statement[1]) while focal pathologies like fibrosis in different forms of myocarditis may remain undetected.
In the clinical routine, fluoroscopy is used to guide the EMB procedure, offering a high frame rate (≤ 30 fps) and high spatial resolution (2-3 line pairs/mm). However, this technique provides only two-dimensional projection images containing overlays of all anatomic structures in the X-ray beam in combination with a low soft-tissue contrast. Furthermore, it exposes both patient and interventionalist to a substantial radiation burden[7].
Cardiac magnetic resonance imaging (CMR) is a non-invasive modality which offers superior soft-tissue contrast, enables tissue characterization and allows the capture of specific slices with any required spatial orientations. Furthermore, contrast-enhanced CMR itself is able to visualize distinct pathologies like myocardial fibrosis, necrosis or inflammation and additionally provides macroscopic information, which may be complementary to that acquired by EMB[8]. Consequently, targeted myocardial biopsies under CMR guidance could reduce the number of required samples, increase specificity and sensitivity of the retrieved tissue samples and obviate radiation exposure. However, due to the very high static magnetic and radiofrequency (RF) electromagnetic fields, conventional metal bioptomes cannot be used in the MRI environment. First, the RF signals induce significant heating, mainly at the tip of metal wires leading to the necrosis of tissue[9,10], and secondly, metal devices lead to massive CMR image disturbance that could impair visualization of target areas. However, such problems can be overcome by MRI-compatible needles, which are already in use for real-time MR-guided tissue biopsies in static organs like breast, brain, liver, kidney or the prostate[11-14].
The localization and navigation of an invasive interventional instrument in the MRI system can be achieved with either active or passive tracking. While active tracking is more precise and faster since the bioptome’s position is always known, it requires substantial modifications and cost-intensive miniaturized electrical extensions within the instrument. Conversely, passive tracking requires only minor modifications of the device to assure an appropriate visualization because it is implemented solely on the MRI system or additional computer systems and uses only the acquired real-time images to determine the instrument’s position. This can be carried out manually by the operator, possibly supported by a software solution, but it requires a significant amount of operator training.
Therefore, we sought to develop and assess the feasibility of a novel MR-compatible bioptome for passive tracking in an in-vitro model to address these technical issues. Subsequently, we applied this method and performed endomyocardial biopsies in explanted animal heart models.
All experiments were carried out in a cylindrical 1.5T MRI system (Achieva 1.5T, Philips Medical Systems, Best, The Netherlands) using the built-in birdcage coils for excitation and a cardiac 32-channel receive coil. The images were obtained with standard real-time sequences which provides continuous scanning and tracking of the bioptome’s position in every possible angulation of three imaging planes within space (Table 1).
| Name | Type | SAR (W/kg) | Frame rate (fps) | Flip angle (degrees) | TR/TE (ms) | Resolution (mm) |
| Interactive | Balanced SSFP | 0.765 | 8 | 45 | 3.10/1.55 | 1.79 × 1.79 × 6 |
The major safety concern when using conventional metallic instruments that have dimensions in the range of the RF field’s wave length is the possibility of tissue heating. The B0-field of a 1.5T system correlates with a RF frequency of 64 MHz that corresponds then to a wavelength λAir of approx. 0.78 m in saline (0.9% NaCl), which is comparable to human body conditions. Therefore, characteristic RF wave lengths like λ/2 and λ/4 are well in the range of an outstretched bioptome, which could then lead to significant heating at the forceps of conventional endomyocardial bioptomes.
The shaft and the tip of the distal end of bioptome were constructed by using non ferromagnetic metals, synthetics and ceramic. The mechanical properties of the sample extraction mechanism at the tip of the MR bioptome were identical to a conventional device. It consisted of two spoon-shaped parts with sharp edges that could be pressed together and opened with a handle at the grip part. The spoons were coated with an MRI-visible marker to help determining the opening state. The device was engineered in close collaboration with H. + H. Maslanka GmbH, Tuttlingen, Germany.
The MR bioptome in the tested version did not have the capability of deflecting the tip for navigation purposes, which was compensated with a separate deflectable sheath (see below).
The sheath prototype we used was a guiding catheter developed for electrophysiology applications and provided by Imricor (Burnsville, MN). It had the ability to deflect and steer the MR bioptome during the procedure and was fully MR-compatible causing neither heating nor disturbing imaging artifacts[15]. It was open at the distal end and a port prevented the outflow of blood at the proximal side. Its inner lumen provided a tight fit of the MR bioptome, effectively preventing a reverse flow of blood. The level of deflection (up to 150 degrees) at the tip could be adjusted and maintained in two directions with a mechanism at the grip. The diameter was approximately 3.7 mm.
In this study, we focused on a passive tracking approach with MRI visible markers at the spoons and the distal end of the device but without the need of an additional coil at the bioptome’s tip and electronics inside the bioptome to evaluate the possibility of an affordable, easily available biopsy system as established in conventional EMB while benefiting from the imaging capabilities of MRI.
The necessary procedural adjustments of the corresponding image plane were conducted by the technician at the console while an MRI in-room monitor allowed the interventionalist to instruct the technician and navigate the bioptome. The MRI-control software provided an interface for real-time image visualization and parameter manipulation to allow a real time tracking of the bioptome within the phantom or heart.
All procedures involving animals were reviewed and approved by the Institutional Animal Care and Use Committee of the Regierungspräsidium Karlsruhe Case Number 35-9185.81/G-79/12.
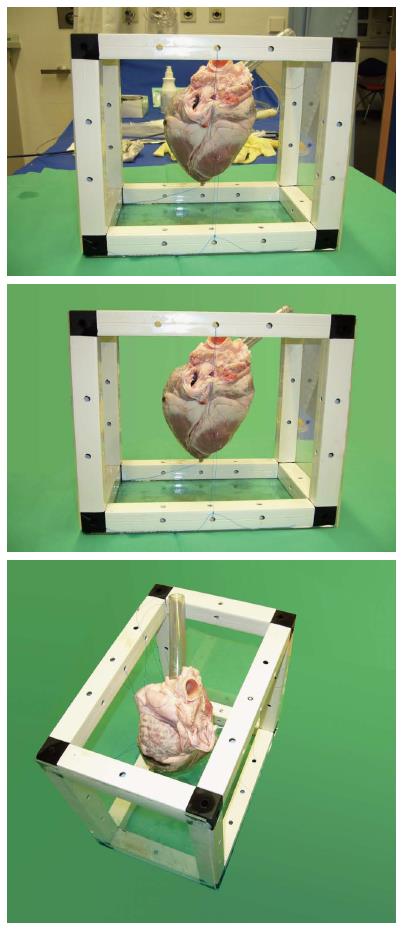
The most beneficial aspect of the MR-guided myocardial biopsy was the targeted and specific retrieval of tissue samples. To reproduce the complex anatomical structures present in a human heart, explanted porcine and bovine hearts were employed. With their anatomical properties being comparable to human hearts, they provided an appropriate test environment to evaluate the navigation features and the new MR biopsy system.
When a catheter is pushed forward in a living heart, crossing the valve plane can normally be achieved in ventricular diastole (right ventricular biopsy) or systole (left ventricular biopsy). In an explanted, ex-vivo heart, the valves are static and might permanently obstruct the anatomic path of the instrument. Since their presence was not of primary concern for the actual experiment, the valve cups were removed during the preparation of the models.
A transparent plastic tube was attached at the orifice of the superior vena cava (SVC) into the right atrium (ostium venae cavae superioris) to mimic the venous vasculature normally guiding the instrument in-vivo into the right atrium and ventricle (Figure 1). The blood flow was not simulated.
To be able to evaluate the success of the biopsy later, several target areas of the endocardium were marked with an injection of a mixture of india ink and gadolinium. The first served as a visual marker in the specimen, the latter mimicked the elevated levels of gadolinium deposition in scar or fibrotic tissue areas eligible for an EMB. In the employed interactive sequences, a gadolinium marked area would appear as slightly brighter spot compared to the surrounding tissue.
Due to the design and material composition of the MR bioptome, a heating problem was not expected. Nevertheless, the temperature was constantly monitored with a fiber optic-based thermometer (Fotemp, Optocon, Dresden, Germany) during the first experiment. One sensor was placed in the vicinity of the tip of the MR bioptome[16,17], the second recorded the overall temperature of the phantom filling.
During the course of the in-vitro experiments, the metal parts of the MR bioptome induced no heating. Only a general heating (< 1 °C) of the phantom due to the constant exposure to the RF field and the heat dissipated by the MRI system during operation was detected.
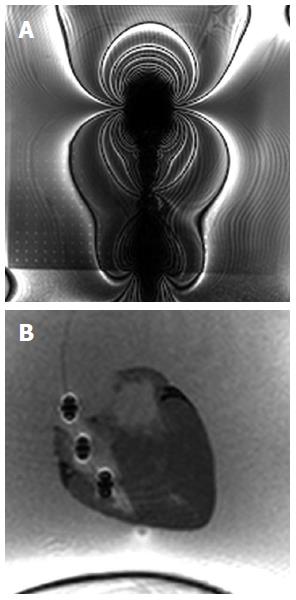
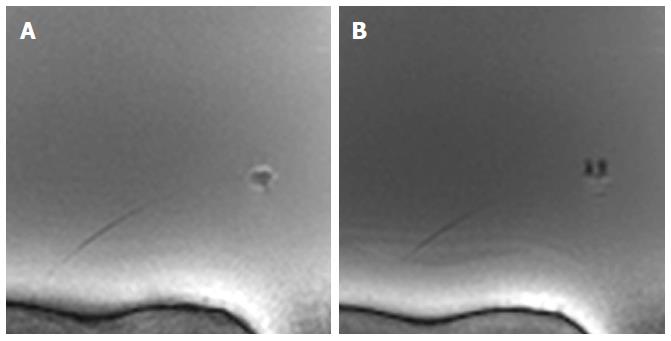
The new design of the MR bioptome effectively prevented the device from a negative interaction with the RF fields present during the MRI examination in terms of excessive artifacts. Only the coating of the sample cutting mechanism caused a small artifact at the tip (size approx. 2 mm × 2 mm, Figure 2), but this did not overlay the target structures in the heart (Figure 3). The shaft of the MR bioptome and the sheath caused a signal void compared to the surrounding tissue. At the same time signal voids supported the visual tracking of the tip of the MR bioptome when being pushed forward inside the sheath. The size of the artifact slightly increased when the tip left the isolating sheath and was exposed directly to the saline (Figure 3).
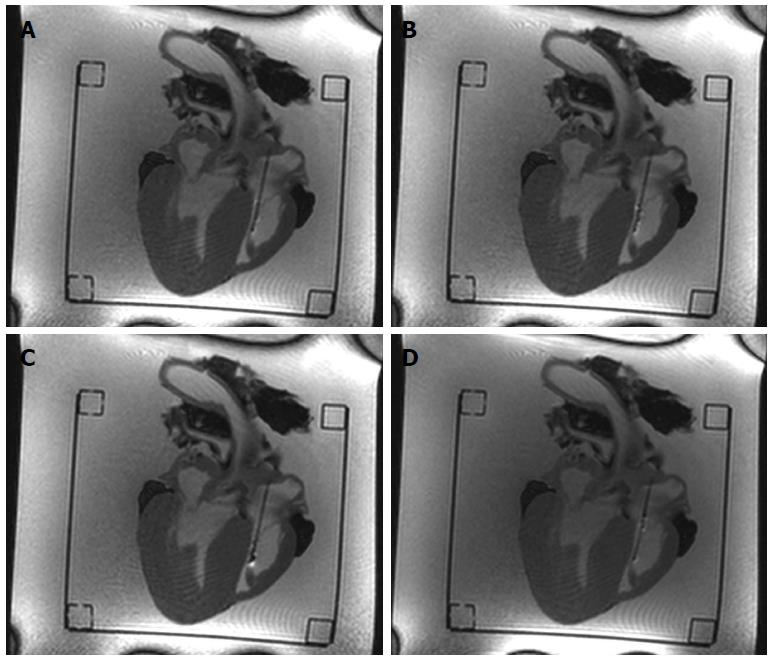
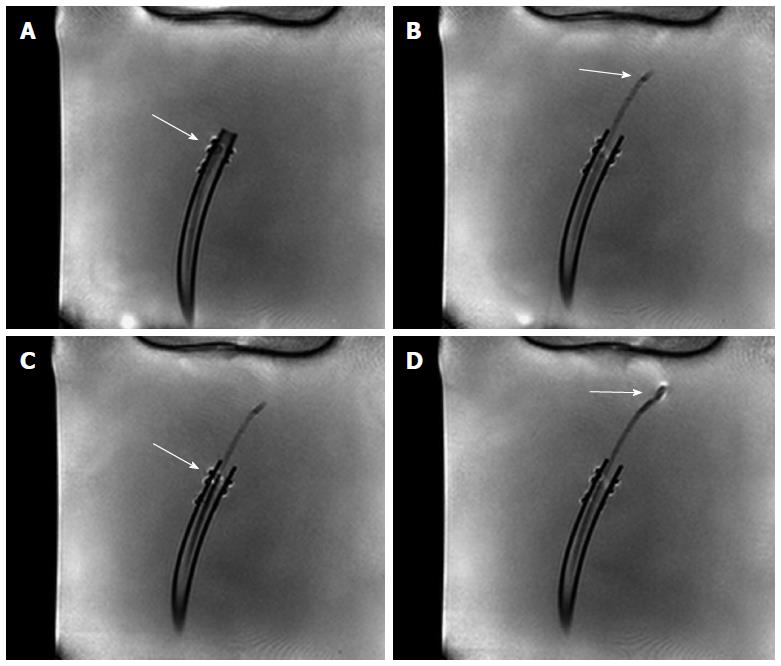
The opening and closing states of the sample cutter were slightly visible on the MRI images (Figures 4 and 5) when the bioptome was not moving. All maneuvers were carried out (1) in a saline (0.9% NaCl) filled but otherwise empty plastic container; and (2) in a porcine heart (Figure 6) submerged in the saline.
The passive, visual tracking of the MR bioptome/sheath system and especially the tip was easily feasible due to the static design of the experiment. As shown in Figure 5, a moving tip caused turbulences in its vicinity which resulted in an artifact similar to the opened sample cutting mechanism. As a consequence, only for a non-moving tip, the opening/closing state could be reliably determined.
The control interface of the scanner supported a quick re-adjustment of the current imaging plane into parallel and orthogonal planes to follow the bioptome’s movement. The achieved frame rate was approx. 4 fps for one slice when using an automatic continuous imaging mode. The visualization of orthogonal slices was only manually possible with manual plane re-adjustment causing the frame rate to decrease to approx. 0.3 fps to 0.25 fps.
In case of a jugular venous insertion, the interventionalist’s access to the MR bioptome would be realized from the rear end of the scanner bore. Even the limited inner bore diameter of the MRI system (60 cm) left enough space to maneuver the bioptome. The handling was acceptable, but required a leaned-forward position of the interventionalist that was less comfortable than when carrying out a fluoroscopy.
When an access into the femoral venous system was simulated, the interventionalist stood in front of the CMR system. Here, the handling was better because it allowed a more upright position during the procedure.
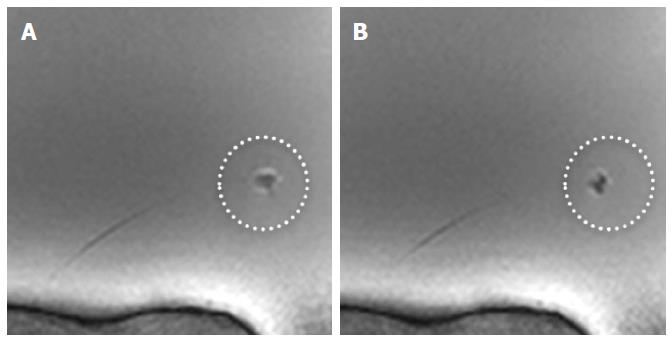
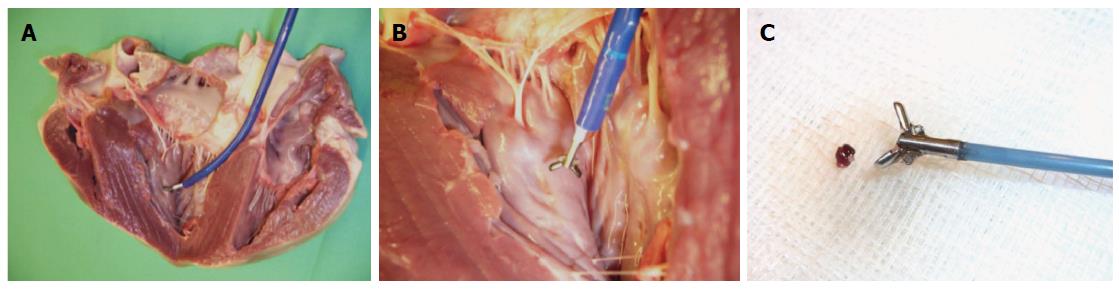
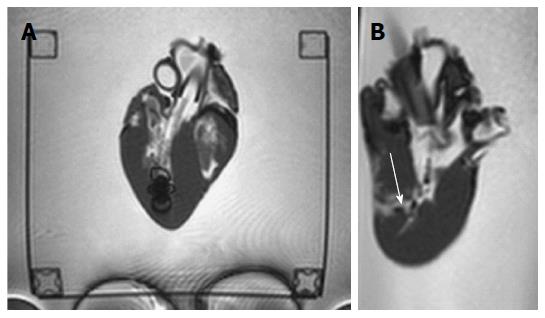
The new MR bioptome retrieved tissue samples of appropriate size and quality (Figure 7). As with a conventional X-ray bioptome, the samples were derived from the endocardium and a visual inspection of the lesion area showed no irregular defects of the tissue. The samples were all taken at the targeted areas (Figure 8) and no rupture or substantial damage to the free lateral wall of the right ventricle was induced. Histological examinations of the specimen were abandoned because of the expected tissue degradation due to the time delay of about 12 h between the slaughter of the animals and the actual experiments.
To our knowledge, this is the first in-vitro study using MR-guided endomyocardial biopsy. The main findings of this study are as follows: (1) The visualization of the anatomical soft-tissue structures inside the heart is superior compared to fluoroscopy and allowed a good localization of the MR bioptome; (2) The biopsies could be performed successfully without any unintended damages to the endomyocardial tissue; and (3) No dangerous heating of the introduced instruments and surrounding environment was observed.
In the study presented here, we could show the feasibility of MRI-guided myocardial biopsies. During the development of the novel device, we were able to reduce the size and the amount of artifacts to a level where no relevant areas in the vicinity of the instrument where obstructed. The visualization by MRI imaging allowed a clear and reliable distinction of the myocardial structures and therefore a safe and precise navigation to the targeted location previously marked with gadolinium injections. This targeted approach can potentially provide a higher sensitivity and specificity of each individual biopsy tissue sample and therefore reduce the number of required biopsy samples as well as the likelihood of complications.
The decision to use a separate bioptome and sheath system, as opposed to an all-in-one design provided substantial advantages. It allowed the maintenance of the position of the sheath while retrieving the individual samples from the myocardium. This could reduce the risk of valvular damage caused by repeated passages for sample acquisitions.
In conclusion, the complete absence of ionizing radiation is an important benefit of MRI based procedures, especially in younger patients with the need of repeated biopsies or multiple interventional procedures, i.e., in cardiac transplant recipients.
Despite the significant advantages of MR-guided compared to fluoroscopically guided endomyocardial biopsies, MRI guidance also provides a number of challenges: (1) An overestimation of the image quality might be caused by the absence of motion artifacts of the static ex-vivo heart models. Further in-vivo experiments are required to evaluate the imaging capabilities when the heart as well as the instrument are constantly shifting and twisting during a cardiac cycle. Additionally, blood flow artifacts around the instrument could further impair the tracking performance; and (2) Since only one spatial slice is scanned, the operator can lose the tip’s location of a passively tracked object when the instrument leaves the actual slice, whereas fluoroscopy with its projected image will always display the instrument as long as it is in the X-ray beam. This issue was successfully compensated by adding multiple passive markers at the distal end of the device. It could be further compensated with an active tracking system.
The aim of this study was to demonstrate the feasibility of magnetic resonance imaging (MRI)-guided endomyocardial biopsy in an in-vitro study. The motivation to use MRI was its superior soft tissue visualization and three-dimensional imaging capabilities when compared to the traditionally used fluoroscopy providing only two-dimensional projection images. Furthermore, the complete absence of ionizing radiation.
As conventional bioptoms comprise multiple metallic components that prevent their use in an MRI environment. Furthermore, the navigation is more complex in MRI as this modality can only visualize objects in the imaging plane, whereas fluoroscopy creates projection images containing all objects in the X-ray beam.
In this study, a novel and fully MRI compatible instrument was developed. It could be successfully evaluated in a cylindrical 1.5T MRI system using bovine heart models. The bioptome was well visible and allowed precise discrimination from the surrounding tissue.
The new instrument allowed us to show the feasibility of MRI-guided interventional procedures, enabling radiation free procedures while benefitting from the widely accepted soft-tissue visualization and characterization capabilities of cardiac MRI.
The article under review represents the authors to present an in-vitro study to explore the feasibility of real-time MRI-guided endomyocardial biopsies. They found that MRI-guided endomyocardial biopsies provide a better than conventional X-ray guided navigation and could therefore improve the specificity and reproducibility of cardiac biopsies in future studies. The issue is interesting.
P- Reviewer: Chung FT, Kilickesmez O S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, Levine GN, Narula J, Starling RC, Towbin J. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Circulation. 2007;116:2216-2233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 540] [Cited by in F6Publishing: 549] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 2. | Executive summary: HFSA 2006 Comprehensive Heart Failure Practice Guideline. J Card Fail. 2006;12:10-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 370] [Cited by in F6Publishing: 391] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 3. | Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K. 2009 focused update incorporated into the ACC/AHA 2005 Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391-e479. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1025] [Cited by in F6Publishing: 1123] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 4. | Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A. [Guidelines for the Diagnosis and Treatment of Chronic Heart Failure: executive summary (update 2005)]. Rev Esp Cardiol. 2005;58:1062-1092. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1348] [Cited by in F6Publishing: 1299] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 5. | Magnani JW, Dec GW. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113:876-890. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 424] [Cited by in F6Publishing: 358] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 6. | Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error. Mayo Clin Proc. 1989;64:1235-1245. [PubMed] [Cited in This Article: ] |
| 7. | Einstein AJ. Effects of radiation exposure from cardiac imaging: how good are the data? J Am Coll Cardiol. 2012;59:553-565. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 162] [Cited by in F6Publishing: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 8. | Dickerson JA, Raman SV, Baker PM, Leier CV. Relationship of cardiac magnetic resonance imaging and myocardial biopsy in the evaluation of nonischemic cardiomyopathy. Congest Heart Fail. 2012;19:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Langman DA, Finn JP, Ennis DB. Abandoned pacemaker leads are a potential risk for patients undergoing MRI. Pacing Clin Electrophysiol. 2011;34:1051-1053. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Mattei E, Triventi M, Calcagnini G, Censi F, Kainz W, Mendoza G, Bassen HI, Bartolini P. Complexity of MRI induced heating on metallic leads: experimental measurements of 374 configurations. Biomed Eng Online. 2008;7:11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 11. | König CW, Trübenbach J, Böhm P, Fritz J, Duda SH, Pereira PL. Magnetic resonance-guided transcortical biopsy of bone marrow lesions using a magnetic resonance imaging-compatible piezoelectric power drill: preliminary experience. Invest Radiol. 2003;38:159-163. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Oxner CR, Vora L, Yim J, Kruper L, Ellenhorn JD. Magnetic resonance imaging-guided breast biopsy in lesions not visualized by mammogram or ultrasound. Am Surg. 2012;78:1087-1090. [PubMed] [Cited in This Article: ] |
| 13. | Fischbach F, Eggemann H, Bunke J, Wonneberger U, Ricke J, Strach K. MR-guided freehand biopsy of breast lesions in a 1.0-T open MR imager with a near-real-time interactive platform: preliminary experience. Radiology. 2012;265:359-370. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Chen AV, Wininger FA, Frey S, Comeau RM, Bagley RS, Tucker RL, Schneider AR, Gay JM. Description and validation of a magnetic resonance imaging-guided stereotactic brain biopsy device in the dog. Vet Radiol Ultrasound. 2012;53:150-156. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Weiss S, Wirtz D, David B, Krueger S, Lips O, Caulfield D, Pedersen SF, Bostock J, Razavi R, Schaeffter T. In vivo evaluation and proof of radiofrequency safety of a novel diagnostic MR-electrophysiology catheter. Magn Reson Med. 2011;65:770-777. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Mattei E, Triventi M, Calcagnini G, Censi F, Kainz W, Bassen HI, Bartolini P. Temperature and SAR measurement errors in the evaluation of metallic linear structures heating during MRI using fluoroptic probes. Phys Med Biol. 2007;52:1633-1646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Neufeld E, Kühn S, Szekely G, Kuster N. Measurement, simulation and uncertainty assessment of implant heating during MRI. Phys Med Biol. 2009;54:4151-4169. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |