Peer-review started: September 14, 2016
First decision: October 21, 2016
Revised: November 2, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: January 26, 2017
To assess the prevalence of depressed heart rate variability (HRV) after an acute myocardial infarction (MI), and to evaluate its prognostic significance in the present era of immediate reperfusion.
Time-domain HRV (obtained from 24-h Holter recordings) was assessed in 326 patients (63.5 ± 12.1 years old; 80% males), two weeks after a complicated MI treated by early reperfusion: 208 ST-elevation myocardial infarction (STEMI) patients (in which reperfusion was successfully obtained within 6 h of symptoms in 94% of cases) and 118 non-ST-elevation myocardial infarction (NSTEMI) patients (percutaneous coronary intervention was performed within 24 h and successful in 73% of cases). Follow-up of the patients was performed via telephone interviews a median of 25 mo after the index event (95%CI of the mean 23.3-28.0). Primary end-point was occurrence of all-cause or cardiac death; secondary end-point was occurrence of major clinical events (MCE, defined as mortality or readmission for new MI, new revascularization, episodes of heart failure or stroke). Possible correlations between HRV parameters (mainly the standard deviation of all normal RR intervals, SDNN), clinical features (age, sex, type of MI, history of diabetes, left ventricle ejection fraction), angiographic characteristics (number of coronary arteries with critical stenoses, success and completeness of revascularization) and long-term outcomes were analysed.
Markedly depressed HRV parameters were present in a relatively small percentage of patients: SDNN < 70 ms was found in 16% and SDNN < 50 ms in 4% of cases. No significant differences were present between STEMI and NSTEMI cases as regards to their distribution among quartiles of SDNN (χ2 =1.536, P = 0.674). Female sex and history of diabetes maintained a significant correlation with lower values of SDNN at multivariate Cox regression analysis (respectively: P = 0.008 and P = 0.008), while no correlation was found between depressed SDNN and history of previous MI (P = 0.999) or number of diseased coronary arteries (P = 0.428) or unsuccessful percutaneous coronary intervention (PCI) (P = 0.691). Patients with left ventricle ejection fraction (LVEF) < 40% presented more often SDNN values in the lowest quartile (P < 0.001). After > 2 years from infarction, a total of 10 patients (3.1%) were lost to follow-up. Overall incidence of MCE at follow-up was similar between STEMI and NSTEMI (P = 0.141), although all-cause and cardiac mortality were higher among NSTEMI cases (respectively: 14% vs 2%, P = 0.001; and 10% vs 1.5%, P = 0.001). The Kaplan-Meier survival curves for all-cause mortality and for cardiac deaths did not reveal significant differences between patients with SDNN in the lowest quartile and other quartiles of SDNN (respectively: P = 0.137 and P = 0.527). Also the MCE-free survival curves were similar between the group of patients with SDNN in the lowest quartile vs the patients of the other SDNN quartiles (P = 0.540), with no difference for STEMI (P = 0.180) or NSTEMI patients (P = 0.541). By the contrary, events-free survival was worse if patients presented with LVEF < 40% (P = 0.001).
In our group of patients with a recent complicated MI, abnormal autonomic parameters have been found with a prevalence that was similar for STEMI and NSTEMI cases, and substantially unchanged in comparison to what reported in the pre-primary-PCI era. Long-term outcomes did not correlate with level of depression of HRV parameters recorded in the subacute phase of the disease, both in STEMI and in NSTEMI patients. These results support lack of prognostic significance of traditional HRV parameters when immediate coronary reperfusion is utilised.
Core tip: Depressed heart rate variability (HRV) is usually considered a negative long-term prognostic factor after an acute myocardial infarction (MI). Anyway, most of the supporting research was conducted before the era of immediate reperfusion by percutaneous coronary intervention. In our study, in MI patients treated by early reperfusion abnormal values of HRV are present in a low percentage of cases. Low HRV does not correlate with long term-prognosis, both in ST-elevation and non-ST-elevation MI patients. Abnormal HRV seems to have lost prognostic significance in the present era of primary percutaneous revascularization.
- Citation: Compostella L, Lakusic N, Compostella C, Truong LVS, Iliceto S, Bellotto F. Does heart rate variability correlate with long-term prognosis in myocardial infarction patients treated by early revascularization? World J Cardiol 2017; 9(1): 27-38
- URL: https://www.wjgnet.com/1949-8462/full/v9/i1/27.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i1.27
The first clinical evidence that one measure of heart rate variability (HRV), the standard deviation of all normal RR intervals (SDNN), was a powerful predictor of cardiac mortality after an acute myocardial infarction (AMI) was given by the wide longitudinal study by Kleiger et al[1] in 1987. Since then, marked abnormalities of various parameters of HRV, indicating profound derangement of the cardiac autonomic system, have been often described after AMI and have been confirmed to be reliable predictors of poor long-term prognosis[2,3]. The large multicentre ATRAMI study conducted in 1998 demonstrated that 15% of AMI patients observed during the first 4 week of the acute event, presented a SDNN < 70 ms, and that among the group with depressed SDNN long-term mortality was 5 times higher than in the patients with better preserved HRV parameters[4]. These results confirmed the findings of previous studies, such as the GISSI-2 study that used similar evaluation parameters[5]. Other studies used various and different methods to assess HRV (time and frequency domain measures, discriminant analysis, fractal and other non-linear HRV analysis, with both short-term and long-term evaluations) and observed AMI patients at variable periods of time after the index event; they reported a wide range of prevalence of reduced HRV parameters (ranging from 7% to 34%)[6,7], and different incidence of long-term mortality (ranging from 0% to 45%)[8-10]. Despite this, the cited studies mainly confirmed that abnormal HRV holds a negative predictive value on both short and long-term prognosis, with HRV parameters holding high specificity, but poor sensitivity[11].
A limitation of the reported studies is that the majority of them did not provide data of how patients with ST-elevation myocardial infarction (STEMI) behave separately from non-ST-elevation myocardial infarction (NSTEMI) patients. Furthermore, the majority of studies on HRV in patients with a recent AMI have been conducted in an era prior to that of immediate reperfusion by percutaneous coronary intervention (PCI). Among 21 papers analyzed in the recent review by Brateanu et al[10], patients had been treated by primary PCI only in 5 studies and in widely variable percentages (ranging from 18% up to 95% of cases)[6,12]; so, little information is currently available on prevalence and prognostic significance of depressed HRV in present day AMI patients.
Aims of this study were to assess the prevalence of severely decreased HRV in patients during the subacute phase of a STEMI treated by primary PCI, and to evaluate if HRV maintains a prognostic value in the current era of immediate percutaneous reperfusion, comparing results with those of NSTEMI cases.
We retrospectively reviewed the clinical files of 326 consecutive patients which were part of a larger study on the effects of cardiac rehabilitation (CR) after AMI. All had suffered a complicated AMI (208 STEMI, 118 NSTEMI) and had been admitted to our CR unit for a period of residential, exercise-based rehabilitation, a median of 13.5 d (95%CI of the mean 15.2-17.3) after the index event. All patients had undergone coronary angiography on initial admission to the Intensive Coronary Care Unit, within 24 h from beginning of AMI symptoms: 194 (94%) STEMI underwent successful PCI of the culprit coronary artery within 6 h of symptoms[13], while only a small percentage of them (14 cases, 6%) could not be revascularized due to unfavourable coronary anatomy and had to be placed on medical therapy; NSTEMI patients underwent coronary angiography within 24 h[14], with successful PCI in 86 cases (73%) and medical therapy in the remaining 32 cases. Of the primary-PCI STEMI patients, 67 (32%) had also been immediately treated on non-culprit coronary lesions; 42 (36%) NSTEMI patients were similarly also immediately treated on non-culprit coronary lesions. Eight (4%) STEMI and 4 (3%) NSTEMI patients received further elective percutaneous revascularization during the initial stay in the Cardiology Department. Overall, at the time of transferral to the CR unit, 121 (58%) STEMI patients and 54 (46%) NSTEMI patients had a complete revascularization, while 87 STEMI and 64 NSTEMI patients were still incompletely revascularized.
Patients were selected for referral to our CR program if they suffered a complicated AMI (cardiogenic shock or pulmonary edema, episode of cardiac arrest, complex ventricular arrhythmias), or if they had incomplete revascularization (because of unfavorable coronary anatomy or technical failure)[15]. Low risk patients were referred as out-patients to a CR program in a different centre and excluded from this study.
Echo- or cardiac MRI- documented intracavitary thrombosis, extreme thinning or intra-myocardial bleeding and/or suspected rupture of the ventricular wall were other exclusion criteria from referral to the CR program.
The following clinical variables were recorded for each patient: Age, gender, body mass index, cardiovascular risk factors, site of infarction, culprit coronary artery vessel, number of diseased coronary artery vessels (defined as presence of diameter stenosis > 50%), history of previous PCI or coronary or valvular surgery, presence of ancillary diseases (renal failure, thyroid dysfunction, known diabetes or abnormal glucose metabolism, pulmonary diseases, history or presence of neoplastic diseases, carotid and peripheral vascular disease) and previous and concurrent drug therapy. During their hospitalisation in CR, all patients without previous diagnosis of diabetes underwent an oral glucose tolerance test to identify subclinical abnormal glucose metabolism. Left ventricular ejection fraction (LVEF) was measured before discharge by 2-D echocardiography, following the Simpson method.
On the day of admittance to CR, all patients underwent 24-h ECG Holter recording, using 3-channel digital recorders (Lifecard CF, Del Mar Reynolds, Irvine, CA, United States), monitoring chest leads CM5, CM3 and modified aVF. Recordings were analyzed using a commercial Holter device system (Del Mar-Reynolds Impresario Holter Analysis System, vers. 2.8.0024; Time-domain HRV Analyzer, vers. 1.0.8.4, CENTUM and Del Mar Reynolds Medical Inc., Irvine, CA, United States; sampling rate of 128 Hz).
After cleansing of arrhythmias and artefacts, the usual time domain HRV variables were assessed including: Standard deviation of all normal-to-normal (NN) intervals (SDNN), standard deviation of all 5-min mean NN intervals (SDANN), root mean square of successive differences (RMSSD), and mean of the standard deviations of all RR intervals of all 5-min segments in the 24 h (SDNN-i). For the purposes of this study, the main variable that was considered in the correlations with other clinical parameters was the SDNN, as it is usually considered a measure of total variance in heart rate; it is also the variable most widely used in previous studies[10] and more strongly associated with mortality compared to other variables[1]. SDNN parameters were analyzed for the entire 24 h period; analysis of day and night hours was also done separately. “Day” was defined as the time period between 06:00 and 22:59 and “night” as the period between 23:00 and 05:59.
Patients with atrial fibrillation, or rhythm disturbances that could interfere with accurate HRV analysis (e.g., frequent ectopic beats, rhythm induced by pacemaker) were excluded from the study, as were patients with inadequate/inaccurate recordings.
The primary outcome measure was the occurrence of cardiac death; the secondary end-point was occurrence of major clinical events (MCE), defined as death (all-cause mortality, cardiac mortality) or readmission for a new AMI, new revascularization, episodes of heart failure or stroke. At the time of follow-up, the clinical status of the patients was assessed by telephonic interviews, performed either by a doctor or a trained team nurse. In case of clinical events, detailed information was obtained from the patient or his/her relatives. Outcomes were analyzed by intention to treat.
Continuous variables were expressed as a mean ± standard deviation (SD) and compared using an unpaired t test; otherwise, variables were expressed with median and interquartile range (IQR) and compared using a Wilcoxon-Mann-Withney test. Categorical variables were expressed as frequencies and percentages and were compared between groups by a χ2 test. The relationships between continuous variables were evaluated by Pearson’s correlation coefficient. A Cox regression multivariate analysis was also performed to determine the influence of different factors on HRV parameters, including in the multivariable model only variables with a P value ≤ 0.1 at univariate analysis. HRV variables were initially analyzed as continuous variables; subsequently, HRV variables that showed a significant association with other factors at multivariate analysis were dichotomized and analyzed according to the lowest quartile value. Kaplan-Meier estimates of the distribution of times from baseline to death were computed, and Mantel-Cox Log-Rank analysis was performed to compare the survival curves between the groups. All reported probability values are two-tailed and the significance level was set at 0.05. Statistical analyses were performed using SPSS 18 software package (SPSS Inc, Chicago, IL, United States).
During CR hospitalization, all participants had been fully informed on the procedures they were undergoing; a written consent was obtained from all patients before performance of the medical procedures. The routine diagnostic examinations and follow-up protocol for CR were applied; no special tests or treatments were performed. The research was conducted in accordance with the ethical guidelines of the 1975 Declaration of Helsinki. This study is part of a larger follow-up study on patients admitted to CR; approval of the Provincial Ethics Committee (Provincial Health Directorate, Belluno, Italy) was obtained for the main research.
Main patients’ characteristics are presented in Table 1, together with the medical therapy prescribed at the time of discharge from hospital. Patients with NSTEMI were older than those with STEMI, and presented more often history of hypertension, previous MI and coronary revascularization procedures, and clinical signs of metabolic syndrome. Patients with NSTEMI had greater number of critical coronary stenoses, revascularization was more often incomplete, and such patients presented more often with symptoms of heart failure on initial admission to the coronary care unit.
| All patients (n = 326) | STEMI (n = 208) | NSTEMI (n = 118) | P1 | P2 | |
| Age, yr | 63.5 ± 12.1 | 61.3 ± 12.5 | 67.4 ± 10.4 | < 0.001 | |
| Male, n (%) | 261 (80) | 171 (82) | 90 (76) | 0.197 | |
| History and cardiovascular risk factors | |||||
| Known diabetes, n (%) | 82 (25) | 45 (22) | 37 (31) | 0.055 | |
| Abnormal glucose metabolism, n (%) | 106 (32) | 68 (33) | 38 (32) | 0.733 | |
| Hypertension, n (%) | 238 (73) | 134 (64) | 104 (88) | < 0.001 | |
| Smoking habit, n (%) | 106 (32) | 81 (39) | 25 (21) | 0.001 | |
| Family history, n (%) | 179 (55) | 111 (53) | 68 (58) | 0.367 | |
| Previous CABG, n (%) | 26 (8) | 7 (3) | 19 (16) | < 0.001 | |
| Previous PCI, n (%) | 43 (13) | 13 (6) | 30 (25) | < 0.001 | |
| Previous AMI, n (%) | 60 (18) | 21 (10) | 39 (33) | < 0.001 | |
| Previous stroke, n (%) | 11 (3) | 5 (2) | 6 (5) | 0.193 | |
| Total cholesterol (under treatment), mg/dL | 124.3 ± 26.0 | 123.4 ± 26.3 | 125.8 ± 25.5 | 0.424 | |
| Metabolic syndrome, n (%) | 204 (62) | 124 (60) | 80 (68) | 0.011 | |
| BMI | 27.2 ± 4.3 | 26.9 ± 3.7 | 27.9 ± 5.2 | 0.090 | |
| AMI characteristics | |||||
| Anterior, n (%) | 171 (52) | 138 (66) | 33 (28) | < 0.001 | |
| Inferior, n (%) | 86 (26) | 66 (32) | 20 (17) | 0.003 | |
| Other, n (%) | 69 (21) | 4 (2) | 65 (55) | < 0.001 | |
| Coronary vessels with critical lesions, n | 2.05 ± 0.85 | 1.94 ± 0.84 | 2.25 ± 0.85 | 0.002 | |
| 1-vessel disease, n (%) | 105 (32) | 75 (36) | 30 (26) | 0.014 | |
| 2-vessels disease, n (%) | 97 (30) | 68 (33) | 29 (25) | ||
| 3-vessels disease, n (%) | 124 (38) | 66 (31) | 58 (49) | ||
| Coronary arteries treated by PCI, n | 1.29 ± 0.82 | 1.34 ± 0.72 | 1.20 ± 0.97 | 0.141 | |
| Incomplete revascularization, n (%) | 151 (46) | 87 (42) | 64 (54) | 0.031 | |
| Left ventricle ejection fraction, % | 47.2 ± 10.3 | 47.8 ± 9.2 | 46.4 ± 12.0 | 0.222 | |
| Patients with LVEF < 40%, n (%) | 85 (26) | 43 (21) | 41 (35) | 0.006 | |
| Patient with heart failure at initial admission, n (%) | 37 (11) | 15 (7) | 22 (19) | 0.002 | |
| Time before Holter, d | 16.2 ± 9.6 | 15.6 ± 9.5 | 17.4 ± 9.8 | 0.117 | |
| Therapy at time of discharge from hospital (number of cases, %) | |||||
| Aspirin | 314 (96) | 202 (97) | 112 (95) | 0.469 | |
| Clopidogrel | 302 (93) | 192 (92) | 110 (93) | 0.458 | |
| Warfarin | 38 (12) | 22 (11) | 16 (14) | 0.399 | |
| β-blocker | 290 (89) | 189 (91) | 101 (86) | 0.198 | |
| Ca-antagonist | 38 (12) | 18 (9) | 20 (17) | 0.022 | |
| ACE-inhibitor | 264 (81) | 180 (86) | 84 (71) | 0.001 | |
| AT-II-antagonist | 43 (13) | 16 (8) | 27 (23) | < 0.001 | |
| Statin | 314 (96) | 201 (97) | 113 (96) | 0.893 | |
| Diuretic(s) | 140 (43) | 75 (36) | 65 (55) | 0.001 | |
| HRV parameters | |||||
| Mean heart rate, bpm | 68.1 ± 10.0 | 69.1 ± 10.1 | 66.2 ± 9.7 | 0.016 | |
| pNN50 | 10.1 ± 12.0 | 9.5 ± 10.5 | 11.1 ± 14.3 | 0.245 | |
| Triangular Index | 18.6 ± 29.9 | 19.6 ± 44.4 | 17.0 ± 26.6 | 0.567 | |
| SDNN, ms | 105.7 ± 39.1 | 105.7 ± 39.2 | 105.7 ± 39.2 | 0.990 | |
| SDNN day, ms | 95.8 ± 35.4 | 95.2 ± 33.9 | 96.8 ± 37.9 | 0.688 | |
| SDNN night, ms | 103.5 ± 41.0 | 102.8 ± 41.6 | 104.8 ± 40.1 | 0.671 | |
| RMSSD, ms | 45.0 ± 37.7 | 42.2 ± 30.6 | 49.9 ± 47.4 | 0.079 | |
| SDANN, ms | 92.1 ± 33.1 | 93.5 ± 35.1 | 89.7 ± 29.4 | 0.331 | |
| SDNN-i, ms | 41.4 ± 22.5 | 40.7 ± 18.9 | 42.6 ± 27.7 | 0.444 |
In the same Table 1, time-domain HRV parameters are also reported. In spite of the above described clinical differences, all main HRV parameters did not show significant differences between STEMI and NSTEMI patients, except for mean heart rate that was lower in NSTEMI cases.
In a total of 52 patients (16% of the whole group; 16% of STEMI and 15% of NSTEMI: χ2 = 0.067, P = 0.796), SDNN was < 70 ms, and in 13 (4% of the whole group; STEMI 5.3%, NSTEMI 1.7%: χ2 = 2.539, P = 0.111) it was < 50 ms. When subdivided into 4 quartiles according to the value of SDNN, the 81 patients in the lowest quartile presented a mean SDNN of 63.7 ± 11.8 ms (vs a mean of 119.4 ± 35.0 ms of the other quartiles; P < 0.001). Patients with STEMI or NSTEMI were equally distributed between quartiles of SDNN (χ2 = 1.536, P = 0.674).
On average, SDNN was higher during night hours than during day-time (P < 0.001), although in 109 patients the day-night variation was insignificant or negative; patients with STEMI or NSTEMI behaved in the same way as regards day vs night SDNN.
Female patients presented on average lower values of SDNN than male patients (97.1 ± 42.2 ms vs 107.9 ± 38.1 ms; P = 0.046), and in 24 out of 65 cases they presented SDNN values in the lower quartile (females 37% vs males 17%; χ2 = 6.723, P = 0.010).
SDNN values in the lower quartile were present more frequently in patients older than 65 years (χ2 = 4.478, P = 0.034), as well as in patients with known diabetes (χ2 = 10.859, P = 0.001) but not in patients with abnormal glucose metabolism detected during the rehabilitation period (χ2 = 0.762, P = 0.383). Patients with known diabetes presented also absence of the day/night variation of SDNN (SDNN day: 85.2 ± 32.2 ms in diabetic patients vs 99.5 ± 35.7 ms in non-diabetics, P = 0.002; SDNN night: 85.3 ± 31.0 ms in diabetic patients vs 109.8 ± 42.2 ms in non-diabetic patients, P < 0.001; SDNN day vs night: P = 0.975 in diabetic vs P < 0.001 in non-diabetic patients).
Patients with history of previous MI, or previous CABG or previous PCI were equally distributed among quartiles of SDNN (respectively: χ2 = 0.017, P = 0.999; χ2 = 1.306, P = 0.728; χ2 = 1.729, P = 0.631).
No correlation was found between number of diseased coronary arteries and quartiles of SDNN (whole group: ρ -0.044, P = 0.428; STEMI: ρ 0.001, P = 0.985; NSTEMI: ρ -0.120, P = 0.199). Patients with complete or incomplete coronary revascularization did not differ as regards distribution among quartile of SDNN (χ2 = 0.059, P = 0.807).
Similarly, no correlation was found between quartile of SDNN and successful vs unsuccessful primary PCI (whole group: χ2 = 0.158, P = 0.691; STEMI: χ2 = 0.031, P = 0.861; NSTEMI: χ2 = 0.684, P = 0.408). Overall, patients with unsuccessful primary PCI presented markedly reduced variation of SDNN values between day and night (SDNN day 94.7 ± 39.3 ms; SDNN night 102.9 ± 37.9 ms; P = 0.092); by the contrary, such variations persisted in patients with successful primary PCI (SDNN day 96.2 ± 34.6 ms; SDNN night 103.9 ± 41.5 ms; P < 0.001).
In the whole group of patients, a linear correlation was found between LVEF and the values of some HRV parameters (SDNN: ρ 0.168, P = 0.002; SDANN: ρ 0.225, P < 0.001), but not with other HRV parameters such as RMSSD, SDNN Index, Triangular Index and pNN50. Patients with lower LVEF (< 40%) presented more often values of SDNN in the lowest quartile (χ2 = 12.668; P < 0.001). Mean heart rate during the 24 h of Holter recording was lower in patients with higher LVEF (ρ -0.310, P < 0.001).
The 37 patients that presented symptoms of heart failure during the acute phase of AMI showed SDNN values in the lower quartile more often than the remaining patients (χ2 = 7.884; P = 0.005), with SDNN < 70 ms in 32% of cases (vs 14% of the patients without initial symptoms of heart failure; χ2 = 8.457; P = 0.004).
At multivariate Cox regression analysis, a significant correlation with the lowest quartile of SDNN was maintained only by female sex, history of diabetes mellitus and LVEF < 40% (respectively: β = -0.143, P = 0.008; β = 0.146, P = 0.008; β = -0.179, P =0.001).
Ten (3.1%) patients were lost to follow up, which occurred a median of 25.0 mo after the index event (95%CI of the mean: 23.3-28.0).
Of the 316 patients which could be interviewed, MCE occurred in 56 (17.2%) of which 20 deaths (6.3%; 14 cardiac deaths), 9 cases of new non fatal AMI (3.0%), 5 patients with stroke (1.6%) and 17 cases of successful elective revascularization (5.4%: 4 CABG, 13 elective PCI); 21 (6.6%) patients had one or more hospital readmissions for heart failure.
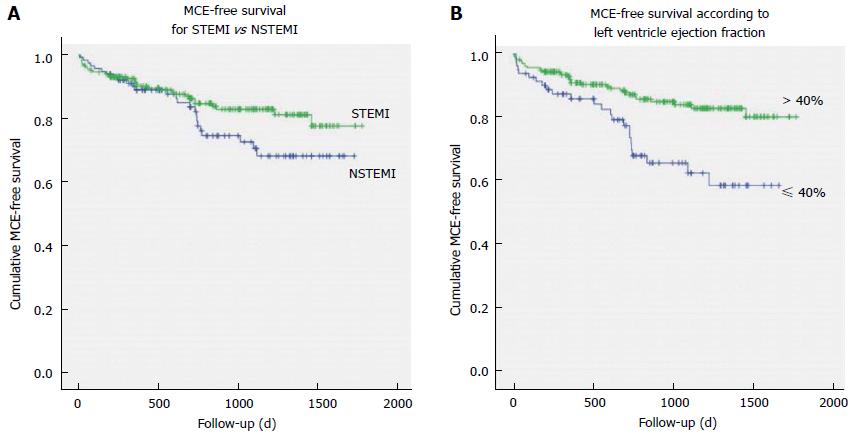
No significant difference in overall incidence of MCE at follow-up was evident between cases with STEMI vs NSTEMI (χ2 = 2.166, P = 0.141), although all-cause mortality was higher among NSTEMI patients (14% vs 2%; χ2 = 17.863, P < 0.001) as well as cardiac mortality (10% vs 1.5%; χ2 = 11.471, P = 0.001). Only after one and a half year of follow-up, the Kaplan-Meier MCE-free survival curves begin to diverge, with NSTEMI patients presenting worse outcomes (Mantel-Cox Log-Rank: χ2 = 5.525, P = 0.019); Figure 1A.
Patients with a 3-vessel disease and those who received an incomplete revascularization had a greater incidence of MCE during the period of follow-up (respectively: χ2 = 14.369, P = 0.006; and χ2 = 6.987, P = 0.008). A significant correlation was evident between all-cause deaths or cardiovascular deaths and number of diseased vessels (respectively: χ2 = 19.218, P = 0.001; and χ2 = 13.077, P = 0.011). Incomplete revascularization showed a correlation with all-cause deaths (χ2 = 4.732, P = 0.030) but not with cardiac deaths (χ2 = 1.859, P = 0.173) at follow-up.
The 234 patients that maintained a better preserved myocardial function, as suggested by LVEF > 40%, had lower number of MCE (32) and cardiovascular deaths (3) in the follow-up, than patients with more compromised LVEF, that suffered 24 MCEs and 11 cardiovascular deaths among 82 patients (for MCE: χ2 = 10.126, P = 0.001, and OR = 0.383, 95%CI: 0.209-0.701; for cardiovascular deaths: χ2 = 21.110, P < 0.001, and OR = 0.084, 95%CI: 0.023-0.309).
At multivariate Cox regression analysis, the variables that showed predictive value for MCE were presence of a three-vessel disease (β = 0.062, P = 0.013), elective PCI (β = 0.250, P = 0.021), known diabetes mellitus (β = 0.124, P = 0.013) and LVEF < 40% (β = -0.114, P = 0.017), while no significant correlation was found with age, sex, number of vessels treated by PCI (β = -0.032, P = 0.229), history of incomplete revascularization, STEMI vs NSTEMI (β = -0.002, P = 0.970), site of infarction or presence of signs of heart failure during initial admission.
In Figure 1B, the Kaplan-Meier events-free survival curves are presented for patients stratified according to LVEF ≤ 40% vs LVEF > 40%; the Mantel-Cox Log-Rank demonstrated statistically significant differences between the curves (χ2 = 10.896, P = 0.001).
Even in the subgroup in the lower quartile of SDNN, no difference was found in incidence of MCE (14/76 cases) in comparison with other subgroups (42/240 cases), (χ2 = 0.034, P = 0.855); this finding was similar for STEMI and NSTEMI patients (respectively: 10 MCE among 52 STEMI patients with lower quartile of SDNN vs 21/150 of the other quartiles, χ2 = 0.813, P = 0.367; and 4 MCE among 24 NSTEMI patients with lower quartile of SDNN vs 21/90 of the other quartiles, χ2 = 0.492, P = 0.483).
Patients with negative day-night variations of SDNN presented long-term events similar to patients with positive SDNN day-night variations (χ2 = 2.107, P = 0.147).
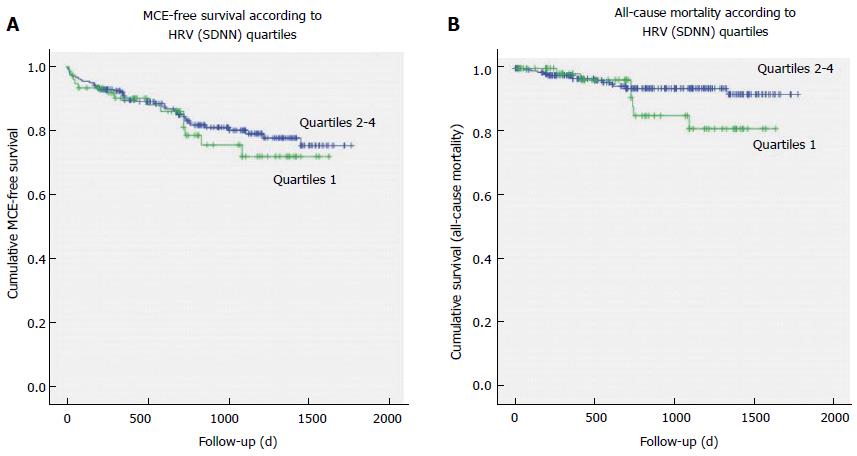
The Kaplan-Meier MCE-free survival curves were similar between the group of patients with SDNN in the lowest quartile vs the patients of the other SDNN quartiles (Log-Rank χ2 = 0.376, P = 0.540; Figure 2A), with no difference for STEMI (Log-Rank χ2 = 1.801, P = 0.180) or NSTEMI patients (Log-Rank χ2 = 0.373, P = 0.541). In particular, no correlation was found between quartile of SDNN and recurrence of MI during the follow-up period (χ2 = 0.489, P = 0.484).
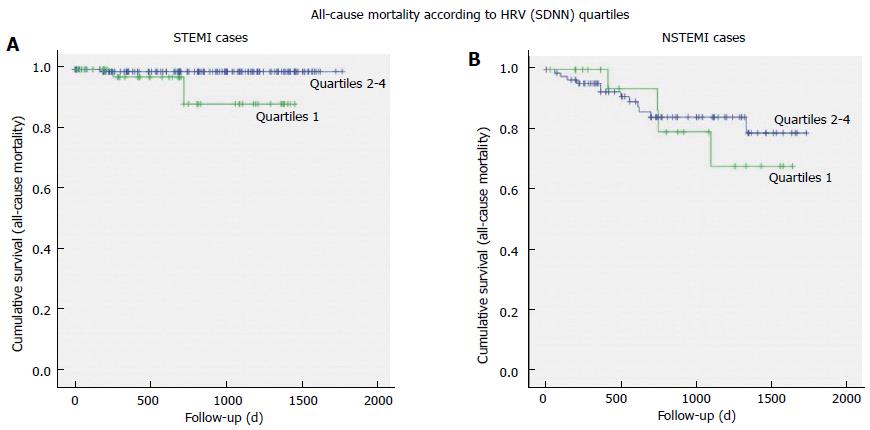
As regards to death for all causes, 7 out of 20 deaths occurred among patients with lowest quartile of SDNN (χ2 = 1.401, P = 0.236); analyzing the whole group of AMI patients, the Kaplan-Meier survival curves for all-cause mortality (Figure 2B) and for cardiac deaths did not evidence significant differences between patients with SDNN in the lowest quartile and other quartiles of SDNN (Mantel-Cox log rank, respectively: χ2 = 2.207, P = 0.137; and χ2 = 0.399, P = 0.527). After separating STEMI from NSTEMI patients, different survival curves have been observed only for all-cause mortality (Figure 3), but not for cardiac mortality: STEMI patients with SDNN in the lowest quartile presented 3 out of 4 all-cause deaths (Mantel-Cox log rank χ2 = 6.591, P = 0.010) and 2 out of 3 cardiac deaths (Log-Rank χ2 = 3.685, P = 0.055), while among NSTEMI patients no difference in the survival curves was observed between patients with SDNN in the lowest quartile vs the other quartiles (all-cause mortality: Log-Rank χ2 = 0.195, P = 0.659; cardiac deaths: Log rank χ2 = 0.040, P = 0.842).
Analysis of the long term outcomes of patients with low values of RMSSD gave similar results as those described for low SDNN values: Kaplan-Meier events free survival did not differ between patients with RSDNN in lowest quartile vs the other quartiles, both for incidence of MCE (χ2 = 0.849, P = 0.357) and all-cause or cardiac mortality (respectively: χ2 = 0.060, P = 0.806; and χ2 = 0.245, P = 0.621).
Patients’ main clinical parameters (age, sex, time from STEMI to CR, site of infarction, number of diseased vessels, fasting glucose, HbA1c, haemoglobin level at admission, LVEF) and HRV values (SDNN, RMSSD, SDNNi, SDANN) have been compared between the 10 cases lost to follow-up and the 314 patients that completed the study. Patients lost to follow-up presented more elevated average values of HbA1c (7.2% ± 2.0% vs 6.4% ± 1.1%; P = 0.022) but not of fasting glucose (109.5 ± 37.9 mg/dL vs 95.2 ± 22.5 mg/dL; P = 0.085) at admission. All the other clinical and HRV parameters were not significantly different in comparison to followed-up patients.
In studied patients with STEMI, treatment by primary PCI did not demonstrate a clear effect in reduction of the prevalence of marked depression of HRV in comparison to what was reported in studies performed in the pre-primary-PCI era: The prevalence of 16% of STEMI patients with SDNN < 70 ms at 2 wk from the acute event is the same as that recorded in the GISSI-2 and the ATRAMI studies, in which patients had been treated conservatively or by thrombolysis[4,5]. It is however much lower than that reported by Wiliński et al[7] in patients treated by primary PCI (21% in patients < 65 years old and 34% in those ≥ 65 years old), as well as in other smaller dimension studies in which patients had also been treated by primary PCI[9,16]. When considering the subgroup of patients with even more depressed HRV (using the cut-off of SDNN < 50 ms, as in the pivotal study by Kleiger et al[1]), the prevalence of this abnormal parameter was lower in our STEMI patients (5% in our study vs 15% in Kleiger’s study), being somehow similar to that observed also by Erdogan et al[6] (7%) in patients treated by immediate revascularization. A number of other studies investigating HRV in the post-acute phase of MI have been conducted in the last 15 years. In such studies the percentage of patients treated by primary PCI varied between 18% and 70%, so that their results about the effects of early revascularization on HRV parameters are not easily comparable between them and with our ones[11,12,17-19].
Even if the percentage of patients with markedly depressed SDNN was not clearly reduced by the immediate reperfusion strategy, the overall derangement of HRV parameters in our cases was limited, in spite of our study population being constituted by patients that suffered a complicated AMI: On average, mean HRV values were only slightly lower than those reported in literature for healthy persons of the same age group[20]. In fact, in previous studies, it has been observed that autonomic function is better preserved in patients treated by primary PCI, in comparison to patients who receive fibrinolysis or are treated conservatively[21]. It must also be added that all our patients were under treatment with ACE-inhibitors and beta-blockers, drugs that may impact on HRV in post-infarction patients[22-25].
Patients with NSTEMI did not show significant differences in the analyzed HRV parameters in comparison to STEMI patients, even though they presented various factors that may have lead to more depressed HRV (older age, greater prevalence of previous AMI, multiple risk factors, heart failure complicating the initial phase of AMI, often a 3-vessel disease and incomplete revascularization). To the best of our knowledge, before the present study no information was available in the literature regarding HRV in NSTEMI patients, when considered separately.
In 13 follow-up studies conducted between 1987 and 1999 where HRV was analyzed in AMI patients not treated with immediate PCI reperfusion, the reported incidence of long-term mortality ranged widely between 3.4% and 45% of study cases; from the data provided in the papers mortality can be estimated on average to be around 10%[1,4,5,8,26-34]. Almost all these studies included both STEMI and NSTEMI patients. More recently, only STEMI patients have been analysed, following treatment by primary PCI: Their long-term mortality rate was reported to be substantially lower than in the pre-primary-PCI era, being possible to calculate it on average around 5% of cases[6,9,16].
In spite of our patients having experienced various kinds of major complications during the initial phase of MI, in STEMI cases both the overall mortality and cardiac mortality at long-term follow-up were rather low, and significantly lower than the long-term mortality of NSTEMI patients. The timely reperfusion strategy, with consequent reduction of infarct size and salvage of more heart muscle, as well as the multiple pharmacological therapy used, may have contributed to the better long-term survival of our STEMI patients[35]; the period of intensive and comprehensive exercise-based cardiac rehabilitation followed by our patients may also have contributed to their better prognosis[36].
Low values of SDNN (lowest quartile) recorded at two weeks from the index event did not possess a predictive value for cardiac mortality in our STEMI patients, or in NSTEMI patients, or in the group of MI patients considered as a whole. In the pivotal study by Kleiger et al[1], the finding of a markedly depressed SDNN was a predictor of long-term mortality more than 5 times higher than in patients with preserved SDNN; identical results have been confirmed in the ATRAMI study[4], and substantially similar outcomes have also been reported in other studies performed in the pre-primary-PCI era[5,29,30], as well as in studies that included low percentage of PCI-treated patients[17].
Among primary-PCI treated patients, Erdogan et al[6] found that SDNN was lower in non-survivors than in survivors after a mean follow-up of 4.3 ± 3 years, but this HRV parameter predicted only 1 in 24 cardiac deaths, indicating that the predictive value of a depressed SDNN is low. Our results indicate that the limited derangement of HRV parameters and the low long-term mortality recorded among our patients do not allow to identify if a markedly depressed HRV (as estimated by low SDNN) could still be considered an indicator of poor survival in patients treated by primary PCI.
In the attempt to identify possible differences in secondary outcomes linked to different levels of HRV derangement among AMI patients treated by primary PCI, we studied the long-term incidence of Major Clinical Events, which is a composite parameter that has recently been used in other studies[9,16]. Other than mortality (cardiovascular and all-cause deaths), it includes also non-fatal events, such as new AMI, new coronary revascularization, episodes of heart failure, episodes of stroke.
Amongst our cases, patients with more depressed SDNN did not show any significant difference of MCE-free outcomes in comparison to patients with preserved parameters, after a median follow-up period of 25 mo (a period that is in range with most of the previous studies)[10]. This is quite a different finding in comparison to other recent small scale studies, that confirmed that abnormal HRV retains some (although low) negative predictive value on long-term prognosis also in primary-PCI treated AMI patients[9,16].
Almost all our patients had been submitted to revascularization of the coronary culprit lesion during the initial phases of their AMI, and a substantial percentage of them had also received revascularization of other critically stenosed coronary arteries, before transferral to CR and recording of 24-h Holter. These facts, together with the pharmacological treatment with beta-blockers and ACE-inhibitors or AT-II-antagonists in use at the time of Holter recording, could have reduced both the autonomic derangement during the subacute phase of the MI and the risk of adverse events in the long-term follow-up.
Criteria of exclusion from this study included presence of atrial fibrillation or flutter, a rhythm induced by the pacemaker, or inadequate Holter recordings. Although such patients may have had significant autonomic dysfunction, HRV could not be measured in them. Consequently it is not known if a depressed HRV could have had any long-term impact on their prognosis.
The degrees of autonomic derangement presented by our patients, that may have been conditioned by the kind of complications suffered during the initial phase of their disease, may possibly not be generalized to the other complicated or uncomplicated STEMI patients.
HRV was performed only in time domain; no analysis was available in the frequency domain. However, it is already known that time-domain HRV indices measured over a 24-h period are well correlated with frequency domain indices in coronary artery disease patients[34]. Among time-domain parameters, SDNN was identified as having the same high predictive value as the frequency-domain LF amplitude in post-AMI patients[16].
In conclusion, in our group of patients with a recent complicated MI, abnormal autonomic parameters (evidenced by low HRV) have been found with a prevalence that was similar for STEMI and NSTEMI cases, and substantially unchanged in comparison to what reported in the pre-primary-PCI era.
Long-term outcomes in PCI-treated STEMI patients were more favorable than in old cohorts of patients. They did not correlate with the level of depression of HRV parameters recorded in the subacute phase of the disease, both in STEMI and in NSTEMI patients. Traditional HRV parameters seem to have lost their prognostic significance in the present era of immediate coronary reperfusion.
After an acute myocardial infarction (AMI), overactivation of the sympathetic component and relative suppression of the parasympathetic component of the autonomic nervous system occur, leading to a marked imbalance of cardiac autonomic regulation. Such sympatho-vagal imbalance is usually considered to be linked to increased short and long-term mortality. Clinically, cardiac autonomic modulation can be evaluated observing the fluctuations of instantaneous heart rate (intervals between normal RR complexes) during variable periods of time (often 24 h). The most simple and widely used parameter for evaluation of heart rate variability (HRV) is the SDNN (standard deviation of all normal RR intervals), that gives an overall estimate of cardiac autonomic control. In the last 30 years, various studies confirmed that depressed values of SDNN indicate poor prognosis after an AMI. Unfortunately, most of these studies on the prognostic value of depressed HRV in post-AMI patients have been conducted before the primary-PCI era: So, their results may not be easily applied to present day patients, in which early reperfusion (that may lead to salvage of cardiac muscle and preservation of better heart function), multiple pharmacological therapies and cardiac rehabilitation may impact on autonomic balance, quality of life and long-term survival.
A few small-scale studies have been conducted so far on AMI patients treated by primary PCI, investigating if depressed HRV maintains a correlation with long-term prognosis. Such studies did not reach clear conclusions. With the present work, the authors aimed at contributing and clarifying if parameters of HRV (in particular SDNN) could still be considered reliable prognostic indicators in primary-PCI-treated AMI patients.
While in the past depressed HRV had been identified as a reliable marker of poor prognosis after an AMI, this paper demonstrates that nowadays in AMI patients treated by early revascularization HRV has lost its prognostic significance. In addition, the paper is the first to report HRV parameters separately for patients with ST-elevation and Non-ST-elevation myocardial infarction.
The evaluation of simple HRV parameters does not seem to hold any more prognostic significance in AMI patients treated by early revascularization. Further studies are needed in these patients to identify reliable long-term prognostic indicators.
HRV refers to the evaluation of the oscillations in the intervals between consecutive heart beats. They are determined by cyclic variations of sympathetic and parasympathetic autonomic influences on cardiac pace-maker cells, modulated by central and peripheral mechanisms (respiratory and vasomotor centres, fluctuations in arterial pressure, humoral factors). After an AMI, HRV usually presents various degrees of depression, linked to a marked increase of the sympathetic activity (probably due to abnormal geometry of the beating heart and consequent distortion of autonomic nervous endings). The most simple method of evaluating HRV is by statistical analysis of the time intervals between consecutive normal RR beats (“time domain” method); the standard deviation of these intervals (SDNN) has been used in our study. SDNN reflects all the cyclic components responsible for variability in the period of recording; for this reason, it has been used in various studies for assessment of risk after AMI.
The authros analysed the potential prognosis of HRV in patients treated with primary PCI.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aronow WS, Sabate M S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Kleiger RE, Miller JP, Bigger JT, Moss AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol. 1987;59:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2735] [Cited by in F6Publishing: 2529] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 2. | Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Eur Heart J. 1996;17:354-381. [PubMed] [Cited in This Article: ] |
| 3. | Buccelletti E, Gilardi E, Scaini E, Galiuto L, Persiani R, Biondi A, Basile F, Silveri NG. Heart rate variability and myocardial infarction: systematic literature review and metanalysis. Eur Rev Med Pharmacol Sci. 2009;13:299-307. [PubMed] [Cited in This Article: ] |
| 4. | La Rovere MT, Bigger JT, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478-484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2192] [Cited by in F6Publishing: 2119] [Article Influence: 81.5] [Reference Citation Analysis (0)] |
| 5. | Zuanetti G, Neilson JM, Latini R, Santoro E, Maggioni AP, Ewing DJ. Prognostic significance of heart rate variability in post-myocardial infarction patients in the fibrinolytic era. The GISSI-2 results. Gruppo Italiano per lo Studio della Sopravvivenza nell’ Infarto Miocardico. Circulation. 1996;94:432-436. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Erdogan A, Coch M, Bilgin M, Parahuleva M, Tillmanns H, Waldecker B, Soydan N. Prognostic value of heart rate variability after acute myocardial infarction in the era of immediate reperfusion. Herzschrittmacherther Elektrophysiol. 2008;19:161-168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Wiliński J, Sondej T, Kusiak A, Wiliński B, Kameczura T, Bacior B, Czarnecka D. Heart rate variability in the course of ST--segment elevation myocardial infarction treated with primary percutaneous transluminal coronary angioplasty in elderly and younger patients. Przegl Lek. 2014;71:61-65. [PubMed] [Cited in This Article: ] |
| 8. | Mäkikallio TH, Høiber S, Køber L, Torp-Pedersen C, Peng CK, Goldberger AL, Huikuri HV. Fractal analysis of heart rate dynamics as a predictor of mortality in patients with depressed left ventricular function after acute myocardial infarction. TRACE Investigators. TRAndolapril Cardiac Evaluation. Am J Cardiol. 1999;83:836-839. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 186] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Larosa C, Sgueglia GA, Sestito A, Infusino F, Niccoli G, Lamendola P, Mariani L, Santangeli P, Lombardo A, Crea F. Predictors of impaired heart rate variability and clinical outcome in patients with acute myocardial infarction treated by primary angioplasty. J Cardiovasc Med (Hagerstown). 2008;9:76-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Brateanu A. Heart rate variability after myocardial infarction: what we know and what we still need to find out. Curr Med Res Opin. 2015;31:1855-1860. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Huikuri HV, Raatikainen MJ, Moerch-Joergensen R, Hartikainen J, Virtanen V, Boland J, Anttonen O, Hoest N, Boersma LV, Platou ES. Prediction of fatal or near-fatal cardiac arrhythmia events in patients with depressed left ventricular function after an acute myocardial infarction. Eur Heart J. 2009;30:689-698. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 180] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 12. | Stein PK, Domitrovich PP, Huikuri HV, Kleiger RE. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol. 2005;16:13-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 200] [Cited by in F6Publishing: 183] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 13. | Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2012;33:2569-2619. [PubMed] [DOI] [Cited in This Article: ] |
| 14. | Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:267-315. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4045] [Cited by in F6Publishing: 4205] [Article Influence: 467.2] [Reference Citation Analysis (0)] |
| 15. | Greco C, Cacciatore G, Gulizia M, Martinelli L, Oliva F, Olivari Z, Seccareccia F, Temporelli PL, Urbinati S. [Selection criteria for referral to cardiac rehabilitation centers]. Monaldi Arch Chest Dis. 2011;76:1-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Coviello I, Pinnacchio G, Laurito M, Stazi A, Battipaglia I, Barone L, Mollo R, Russo G, Villano A, Sestito A. Prognostic role of heart rate variability in patients with ST-segment elevation acute myocardial infarction treated by primary angioplasty. Cardiology. 2013;124:63-70. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Tapanainen JM, Thomsen PE, Køber L, Torp-Pedersen C, Mäkikallio TH, Still AM, Lindgren KS, Huikuri HV. Fractal analysis of heart rate variability and mortality after an acute myocardial infarction. Am J Cardiol. 2002;90:347-352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 172] [Cited by in F6Publishing: 175] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 18. | Mäkikallio TH, Barthel P, Schneider R, Bauer A, Tapanainen JM, Tulppo MP, Schmidt G, Huikuri HV. Prediction of sudden cardiac death after acute myocardial infarction: role of Holter monitoring in the modern treatment era. Eur Heart J. 2005;26:762-769. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Perkiömäki JS, Jokinen V, Tapanainen J, Airaksinen KE, Huikuri HV. Autonomic markers as predictors of nonfatal acute coronary events after myocardial infarction. Ann Noninvasive Electrocardiol. 2008;13:120-129. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Umetani K, Singer DH, McCraty R, Atkinson M. Twenty-four hour time domain heart rate variability and heart rate: relations to age and gender over nine decades. J Am Coll Cardiol. 1998;31:593-601. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 665] [Cited by in F6Publishing: 582] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 21. | Lakusic N, Smalcelj A, Mahovic D, Puljevic D, Lovric-Bencic M. Heart rate variability differences in post-myocardial infarction patients based on initial treatment during acute phase of disease. Int J Cardiol. 2008;126:437-438. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kontopoulos AG, Athyros VG, Papageorgiou AA, Skeberis VM, Basayiannis EC, Boudoulas H. Effect of angiotensin-converting enzyme inhibitors on the power spectrum of heart rate variability in post-myocardial infarction patients. Coron Artery Dis. 1997;8:517-524. [PubMed] [Cited in This Article: ] |
| 23. | Lurje L, Wennerblom B, Tygesen H, Karlsson T, Hjalmarson A. Heart rate variability after acute myocardial infarction in patients treated with atenolol and metoprolol. Int J Cardiol. 1997;60:157-164. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Stein PK, Domitrovich PP, Kleiger RE, Schechtman KB, Rottman JN. Clinical and demographic determinants of heart rate variability in patients post myocardial infarction: insights from the cardiac arrhythmia suppression trial (CAST). Clin Cardiol. 2000;23:187-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Lampert R, Ickovics JR, Viscoli CJ, Horwitz RI, Lee FA. Effects of propranolol on recovery of heart rate variability following acute myocardial infarction and relation to outcome in the Beta-Blocker Heart Attack Trial. Am J Cardiol. 2003;91:137-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 26. | Odemuyiwa O, Malik M, Farrell T, Bashir Y, Poloniecki J, Camm J. Comparison of the predictive characteristics of heart rate variability index and left ventricular ejection fraction for all-cause mortality, arrhythmic events and sudden death after acute myocardial infarction. Am J Cardiol. 1991;68:434-439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 258] [Cited by in F6Publishing: 270] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 27. | Odemuyiwa O, Poloniecki J, Malik M, Farrell T, Xia R, Staunton A, Kulakowski P, Ward D, Camm J. Temporal influences on the prediction of postinfarction mortality by heart rate variability: a comparison with the left ventricular ejection fraction. Br Heart J. 1994;71:521-527. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 28. | Vaishnav S, Stevenson R, Marchant B, Lagi K, Ranjadayalan K, Timmis AD. Relation between heart rate variability early after acute myocardial infarction and long-term mortality. Am J Cardiol. 1994;73:653-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 123] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 29. | Bigger JT, Fleiss JL, Rolnitzky LM, Steinman RC. The ability of several short-term measures of RR variability to predict mortality after myocardial infarction. Circulation. 1993;88:927-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 331] [Cited by in F6Publishing: 341] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 30. | Copie X, Hnatkova K, Staunton A, Fei L, Camm AJ, Malik M. Predictive power of increased heart rate versus depressed left ventricular ejection fraction and heart rate variability for risk stratification after myocardial infarction. Results of a two-year follow-up study. J Am Coll Cardiol. 1996;27:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 166] [Cited by in F6Publishing: 170] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 31. | Fei L, Copie X, Malik M, Camm AJ. Short- and long-term assessment of heart rate variability for risk stratification after acute myocardial infarction. Am J Cardiol. 1996;77:681-684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 32. | Touboul P, Andre-Fouët X, Leizorovicz A, Itti R, Lopez M, Sayegh Y, Milon H, Kirkorian G. Risk stratification after myocardial infarction. A reappraisal in the era of thrombolysis. The Groupe d’Etude du Pronostic de l’Infarctus du Myocarde (GREPI). Eur Heart J. 1997;18:99-107. [PubMed] [Cited in This Article: ] |
| 33. | Voss A, Hnatkova K, Wessel N, Kurths J, Sander A, Schirdewan A, Camm AJ, Malik M. Multiparametric analysis of heart rate variability used for risk stratification among survivors of acute myocardial infarction. Pacing Clin Electrophysiol. 1998;21:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 34. | Bigger JT, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. Am J Cardiol. 1992;69:891-898. [PubMed] [Cited in This Article: ] |
| 35. | Ndrepepa G. Improving myocardial injury, infarct size, and myocardial salvage in the era of primary PCI for STEMI. Coron Artery Dis. 2015;26:341-355. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 36. | Dunlay SM, Pack QR, Thomas RJ, Killian JM, Roger VL. Participation in cardiac rehabilitation, readmissions, and death after acute myocardial infarction. Am J Med. 2014;127:538-546. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 176] [Article Influence: 17.6] [Reference Citation Analysis (0)] |