Published online Oct 26, 2017. doi: 10.4330/wjc.v9.i10.773
Peer-review started: April 16, 2017
First decision: May 9, 2017
Revised: May 23, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: October 26, 2017
Cardiac magnetic resonance (CMR) is a non-invasive, non-ionizing, diagnostic technique that uses magnetic fields, radio waves and field gradients to generate images with high spatial and temporal resolution. After administration of contrast media (e.g., gadolinium chelate), it is also possible to acquire late images, which make possible the identification and quantification of myocardial areas with scar/fibrosis (late gadolinium enhancement, LGE). CMR is currently a useful instrument in clinical cardiovascular practice for the assessment of several pathological conditions, including ischemic and non-ischemic cardiomyopathies and congenital heart disease. In recent years, its field of application has also extended to arrhythmology, both in diagnostic and prognostic evaluation of arrhythmic risk and in therapeutic decision-making. In this review, we discuss the possible useful applications of CMR for the arrhythmologist. It is possible to identify three main fields of application of CMR in this context: (1) arrhythmic and sudden cardiac death risk stratification in different heart diseases; (2) decision-making in cardiac resynchronization therapy device implantation, presence and extent of myocardial fibrosis for left ventricular lead placement and cardiac venous anatomy; and (3) substrate identification for guiding ablation of complex arrhythmias (atrial fibrillation and ventricular tachycardias).
Core tip: Cardiac magnetic resonance (CMR) is a non-ionizing diagnostic technique that generates images with high spatial and temporal resolution. After administration of contrast media (e.g., gadolinium chelate), it is also possible to acquire late images, which make possible the identification and quantification of myocardial areas with scar/fibrosis (late gadolinium enhancement). In recent years, its field of application has extended to arrhythmology, both in diagnostic and prognostic evaluation of arrhythmic risk and in therapeutic decision-making. In this review, we discuss the applications of CMR for the arrhythmologist. It is possible to identify three main fields of application in this context: (1) arrhythmic and sudden cardiac death risk stratification; (2) decision making in cardiac resynchronization therapy device implantation; and (3) substrate identification for guiding ablation of complex arrhythmias.
- Citation: De Maria E, Aldrovandi A, Borghi A, Modonesi L, Cappelli S. Cardiac magnetic resonance imaging: Which information is useful for the arrhythmologist? World J Cardiol 2017; 9(10): 773-786
- URL: https://www.wjgnet.com/1949-8462/full/v9/i10/773.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i10.773
Cardiac magnetic resonance (CMR) is a non-invasive, non-ionizing, diagnostic technique that uses magnetic fields, radio waves and field gradients to generate images with high spatial and temporal resolution and without limitations due to the acoustic window, compared to other imaging techniques[1,2]. It provides a very precise “in vivo” tissue characterization through the different quantity of protons in different chemical environments, identifying the presence of fat, water (oedema), blood, fibrosis and scar[1,2]. In particular, after the administration of contrast media (e.g., gadolinium chelate), it is possible to acquire late images which make possible the identification and quantification of myocardial areas with scar/fibrosis (late gadolinium enhancement, LGE)[1]. First used as a research tool, CMR has become a daily instrument in clinical cardiovascular practice for the assessment of several pathological conditions, including ischemic and non-ischemic cardiomyopathies and congenital heart disease[1].
In recent years, its field of application has also extended to arrhythmology, both in diagnostic and prognostic evaluation of arrhythmic risk and in therapeutic decision-making. It is possible to identify three main fields of application of CMR in arrhythmology: (1) arrhythmic and sudden cardiac death (SCD) risk stratification in different heart diseases; (2) decision-making in cardiac resynchronization therapy (CRT) device implantation [cardiac vein anatomy, scar burden and left ventricular (LV) lead placement]; and (3) substrate identification for guiding ablation of complex arrhythmias [atrial fibrillation and ventricular tachycardias (VTs)].
In this review, we discuss the possible useful applications of CMR that can help the arrhythmologist in the management of patients with this broad spectrum of arrhythmological conditions.
SCD is responsible for 25% of 17 million cardiovascular deaths every year in the world. The great majority of these deaths (> 90%) have an arrhythmic origin, namely, VT degenerating into ventricular fibrillation (VF), primary VF or torsade de pointes[3].
The underlying causes vary in different age groups, with channelopathies and cardiomyopathies prevailing in young people, while degenerative diseases are more common in older people. In general, the main causes are: Acute and chronic coronary heart disease (75%-80%); cardiomyopathies (10%-15%); valvular, inflammatory and infiltrative diseases (5%-10%); and molecular/genetic conditions (< 5%)[3]. Prevention can be made with pharmacological or device therapy. This latter consists in ICD (implantable cardioverter defibrillator) implantation that is recommended in different groups of high risk patients with ischemic or non-ischemic heart diseases. However, risk stratification is sometimes very challenging, expecially in primary prevention. Current approaches have limited sensitivity and specificity in many clinical settings, identifying only a very small portion of future cardiac arrests with sufficient precision to justify ICD therapy[3,4]. Moreover, ICD implantation is not without complications and many patients will not to benefit even if implanted according to guidelines[3,4]. Lately, scientific interest is pointing to a polyparametric approach, using a combination of different risk markers to better dichotomize high and low risk patients[4,5]. In this context, CMR can give its contribution, expecially through the identification and quantification of myocardial areas with scar and fibrosis. Ventricular fibrosis is an important substrate for the genesis of ventricular arrhythmias (VA): Within fibrotic tissue the slow and heterogeneous conduction favors re-entrant circuits, increasing vulnerability to VT and VF[6-8].
A left ventricular ejection fraction (LVEF) of 35% or less is the major determinant of ICD implantation for SCD primary prevention in patients with ischemic or nonischemic LV dysfunction[3]. Even in the recent European Society of Cardiology (ESC) guidelines[3], the only suggested markers of arrhythmic risk to guide ICD implant are LVEF and NYHA functional class (Table 1). However, it is now well-known that ejection fraction alone has limited sensitivity and specificity as a risk marker for SCD, because it is not able to distinguish the risk of sudden death from death caused by heart failure or other non-cardiac diseases. Subsequently, many patients implanted for primary prevention according to current guidelines will have little benefit from their ICD, with a low rate of appropriate ICD therapy (2%-4%/year)[9], while they can suffer from side effects (even > 10%/year overall), in particular inappropriate shocks, lead failure and infections[10,11]. On the other side, many patients who are at risk of SCD are missed when using only LVEF, because the largest part of sudden arrhythmic death patients have only mildly depressed ejection fraction[9,12,13]. Anyway, the substrates of SCD are particularly complex, so it is unlikely for a single test to achieve significantly better predictive accuracy than LVEF. To overcome this limitation, a combination of markers has been proposed[9], for example, combining ejection fraction with different tests that investigate different arrhythmic mechanisms (LGE-CMR, T-wave alternans, programmed ventricular stimulation, evaluation of autonomic tone, etc.).
| Recommendations |
| Class I: ICD therapy is recommended to reduce SCD in patients with symptomatic HF (NYHA class II–III) and LVEF ≤ 35% after ≥ 3 mo of optimal medical therapy who are expected to survive for at least 1 yr with good functional status |
| Level of evidence A: Ischemic etiology (at least 6 wk after myocardial infarction) |
| Level of evidence B: Non-ischemic etiology |
The pathophysiology of VA in structural heart diseases is due - in most cases - to re-entrant circuits. Electrophysiological studies and anatomic mapping have highlighted, in these cases, the presence of extensive areas where the electrical potentials are absent (indicating the absence of viable myocardium, scar and fibrosis) and areas with low-amplitude, fragmented, late potentials compared to healthy myocardium (conduction with high anisotropy and low speed)[6-8]. The classic arrhythmogenic substrate of re-entry arrhythmias is represented by a mix of these areas, with inflammation often acting as a trigger. Thanks to its ability to identify both areas of myocardial scar/fibrosis and inflammation, CMR can provide essential information in this context[8,9].
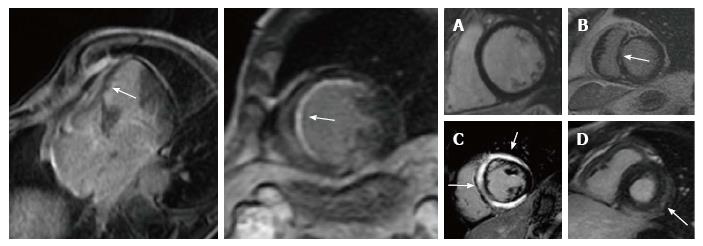
Myocardial fibrosis can be evaluated with the LGE imaging technique. Gadolinium-based contrast agents are washed out by viable myocytes and accumulate in extracellular spaces, such as areas of fibrotic tissue, where cardiomyocytes have been replaced by collagen, or in areas of acutely damaged myocardium[7,8]. The LGE imaging techniques have been validated by histology in several studies with animal models[14]. To date, due to the high spatial resolution (approximately 2 mm), it is the most accurate method to detect myocardial fibrosis and to precisely identify its location and extension, distinguishing in particular endocardial, epicardial or transmural involvement. The pattern of LGE distribution is particularly useful in the differential diagnosis between ischemic and non-ischemic fibrosis[15-17]. Virtually all patients with ischemic cardiomyopathy have LGE, presenting with a subendocardial or transmural distribution in myocardial segments following a coronary artery territory[16]; the most common pattern consists of core dense fibrosis within a heterogeneous peri-infarct (gray) zone, indicating the presence of both viable and nonviable myocardium[9]. On the other side, in non-ischemic dilated cardiomyopathy fibrosis is present only in about 30%-40% of cases and it shows a “midwall” pattern, mostly located in the interventricular septum[17] (Figure 1). From an etiological and therapeutical point of view, this is a very important issue: non-ischemic cardiomyopathy on the basis of a traditional definition (clinical history, ECG, echocardiogram and coronary angiography) may be reclassified as ischemic cardiomyopathy thanks to CMR in about 20% of cases[15].
Numerous studies have demonstrated that LGE is a powerful predictor of VA events both in ischemic and non-ischemic cardiomyopathy patients, with moderately to severely depressed LVEF[18-21]. An overview of 19 studies, all with an arrhythmic endpoint, for a total of 2692 patients, indicated that the presence and extension of myocardial fibrosis, documented by LGE, predicted VA both in ischemic and non-ischemic diseases, even in patients with only mildly depressed LVEF[9,18,19]. Furthermore, CMR increased the negative predictive value for SCD prediction to 95%[9,20-22].
Taking into account only non-ischemic dilated cardiomyopathy, the cut-off for risk definition was the presence or absence of fibrosis and its midwall location. These markers were successfully used to dichotomize patients at high vs low risk of ventricular arrhythmic events[23-32]. The largest prospective study in non-ischemic cardiomyopathy by Gulati et al[26] included 472 patients followed for > 5 years. In this paper midwall fibrosis was an independent risk factor for ventricular tachyarrhythmias [hazard ratio (HR) = 4.61], while combining ventricular fibrosis with LVEF significantly improved risk reclassification for the arrhythmic endpoint. A recent meta-analysis of 29 studies including 2948 patients with idiopathic dilated cardiomyopathy[32,33] confirmed that the presence of ventricular fibrosis, identified by LGE, was an important risk factor for arrhythmic endpoints (SCD, VT, VF and ICD therapies): Clinical events occurred in 21% of LGE positive vs 4.7% of LGE negative patients, with an annual event rate of 6.9% and 1.6%, respectively.
In ischemic dilated cardiomyopathy, the issue is more complex: The majority of studies evaluating total LGE or “gray zone” (peri-infarct area) reported a statistically significant dose-response effect for arrhythmic risk, with larger and more heterogeneous scar associated with the higher risk of VA during follow-up[34-39]. Currently, there is not a definite cut-off value of fibrosis/scar extent to adequately differentiate patients at high vs low risk of arrhythmic events, especially in ischemic etiology[18]. The presence of a large amount of ventricular fibrosis/scar has been generally used as a marker of higher risk. However, a great variety of analysis methods and diagnostic thresholds exists[34-39]: Standardization of LGE-CMR should be a target to reach before spreading practical use of this technique for arrhythmic risk stratification. Moreover, no randomized study has been concluded so far: The DETERMINE study[40] was planned to demonstrate the role of LGE-CMR in decision-making for ICD implantation in patients with ischemic cardiomyopathy, but it was prematurely terminated due to a low rate of patient enrollment.
Even with the above limitations, a polyparametric approach, using a combination of different risk markers (including LGE-CMR), could help to refine risk stratification in at least two subsets of patients who are not adequately assessed by current guidelines[9].
The first group is represented by patients with LVEF less than 35% and high risk of death due to heart failure or non-cardiac causes. In this setting, the absence of LGE-CMR (non-ischemic etiology) or a small extension of fibrosis/scar (ischemic etiology), expecially if coupled with negative T-wave alternans test, identifies patients with a relatively low risk of sudden arrhythmic death (about 1%/year) for whom ICD implantation should be critically considered because they will hardly have a benefit[9,21,22,32].
The second group includes patients with LVEF of 35%-50% and high risk of SCD defined by: (1) presence or high burden of fibrosis on LGE-CMR; (2) VT/VF inducibility by programmed ventricular stimulation in post-infarction etiology; and (3) lamin A/C pathological mutation associated with familial sudden death in idiopathic cardiomyopathy. For these patients, even if the current guidelines do not recommend the use of ICD, such a therapy could be critically evaluated, discussed and offered case by case[9,37,38].
Finally, a small portion of patients without LGE at CMR will suffer from sudden death, especially in non-ischemic disease. LGE imaging is not suited to detect diffuse fibrosis that may be present in idiopathic dilated cardiomyopathy. New imaging techniques, such as T1 mapping, are able to detect and quantify diffuse fibrosis by means of extracellular volume fraction and preliminary data show that this pattern is associated with worse outcome in non-ischemic patients[41].
Currently, neither American nor European guidelines support CMR as a first-line tool for risk stratification in dilated cardiomyopathies, so further studies are needed to define its role in this context.
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiomyopathy and cause of SCD in young people, including competitive athletes. It is caused by mutations in genes encoding cardiac sarcomere proteins and has a prevalence of 1:500 in the general population[42]. HCM is defined by the presence of unexplained LV hypertrophy (wall thickness ≥ 15 mm), associated with non-dilated ventricular chambers, in the absence of other cardiac or systemic diseases that might cause hypertrophy[42,43]. Hypertrophied myocytes are arranged in a chaotic architecture with increased extracellular matrix[42]. The myocardium may also present ischemic areas, caused by microvasculature obstruction, with replacement fibrosis and scar[42-44]. This modified cardiac structure predisposes to the risk of malignant VA such as VT and VF[42,43].
SCD represents the most feared complication, occurring in about 5% of patients[43,44]. In patients with HCM, at high risk for SCD, ICD reduces mortality rate to 0.5% per year[44]. A primary prevention risk model has been proposed to identify high risk patients and guide ICD implant[43,44], based on: (1) family history of premature HCM-related SCD, in close or multiple relatives; (2) unexplained non-reflex syncope, particularly if recent and in young patients; (3) nonsustained VTs on ambulatory ECG, particularly if multiple, repetitive or prolonged; (4) hypotensive or attenuated blood pressure in response to exercise; and (5) extreme hypertrophy (wall thickness ≥ 30 mm). Although current risk factor model is effective, not all high-risk patients are identified and the absence of conventional risk factors does not eliminate the risk of SCD.
In this context, CMR is increasingly considered an important tool, in particular for the evaluation of fibrotic areas (LGE-CMR) and wall thickness[44]. Moreover, it allows more precise characterization of the phenotype, which helps to differentiate HCM from other causes of LV hypertrophy.
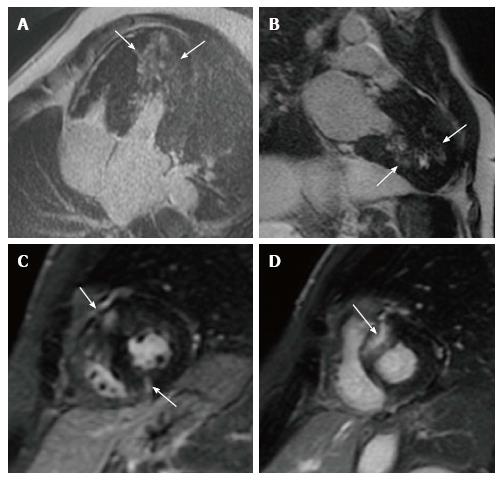
Approximately 50%-60% of HCM patients demonstrate LGE-CMR which, when present, occupies on average 10% of the LV myocardial mass. LGE can be observed in any location or distribution, although most frequently in the ventricular septum and free wall (> 30% of patients), with mid-myocardial distribution, and less often involving the apex and the right ventricular insertion into ventricular septum[42] (Figure 2). Moreover, patients with LGE have greater maximal LV wall thickness and LV mass index than patients without[43]. A large number of studies demonstrated that the presence of LGE-CMR identifies areas of myocardial fibrosis where life-threatening VA can originate and is associated with a significant higher risk of SCD, even in patients without conventional risk factors[45-50]. LGE extension, expressed as a percentage of myocardial mass, correlates with the risk of developing life-threatening VA, in particular if LGE exceeds 15% of LV mass[50]. On the contrary, patients without LGE have a low arrhythmic risk and can be reassured.
CMR also enables the identification of other high-risk subsets of patients such as those with massive LV hypertrophy and apical aneurysms (the latter being a subgroup at increased risk for VA and thromboembolic stroke)[51,52]. Notably apical HCM may be underlooked by echocardiography, while CMR can precisely visualize apical segments and detect hypertrophy and aneurisms.
Current schemes for SCD risk determination, such as American algorithm[51] and ESC risk calculator[53], are not completely effective and precise in risk evaluation. CMR, instead, has shown to improve stratification, providing additional information in patients for whom the current markers underestimate the risk (for example, young asymptomatic patients without conventional risk factors but with LGE) and in patients for whom decision-making about ICD implantation is difficult and ambiguous (for example, patients with a single risk factor and at intermediated risk), and potentially acting as an “arbitrator”. Anyway, at the moment, neither American nor European guidelines support CMR as a first-line tool for risk stratification in HCM[51,53].
Arrhythmogenic cardiomyopathy (AC) is a group of heart muscle disease clinically characterized by life-threatening VA and pathologically by a progressive dystrophy of the ventricular myocardium with fibro-fatty replacement[54,55]. AC affects mostly young males < 40 years old. Its estimated prevalence ranges between 1:2000 and 1:5000, therefore it is considered among rare diseases[54,55]. It is mostly caused by autosomal dominant genetic mutations (with incomplete penetrance and variable expressivity) of desmosomal proteins like desmoplakin and plakoglobin[56,57]. The desmosomal complex, situated in the cardiac intercalated disk, is responsible for tissue strength and stability, binding cells to one another. Consequently, a defective desmosomal complex can cause cell loss with fibro-fatty tissue replacement[58-60]. In its most common form the disease affects the right ventricle, but in a minority of patients it may affect both ventricles or only the left ventricle, thus supporting the use of the more general term AC[54,55]. Clinical manifestations differ in the different phases of the disease, from asymptomatic patients to patients with heart failure and VA or SCD[56-60]. AC is a major cause of sudden death in young and athletes, with VT and VF occurring at any stage[54-61].
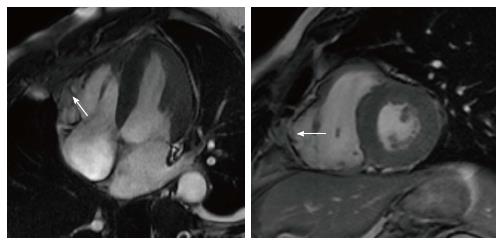
Considering the most frequent variant, arryhtmogenic right ventricular cardiomyopathy (ARVC), the diagnosis is based on a score obtained from the assessment of several parameters combined into major and minor criteria[56-59], as there is no single gold standard diagnostic test. CMR has an important role for a comprehensive and precise assessment of right ventricular volumes, function and kinesis[56-59]. Typically, in ARVC myocardial disarray involves the entire ventricular wall, in particular the subtricuspid region and the right ventricle outflow tract (“triangle of dysplasia”), leading to aneurysm formation. In these regions wall motion abnormalities (akinesia or dyskinesia) and aneurysms can be detected by CMR, representing one of the criteria for diagnosis (Figure 3). The usefulness of CMR to detect fatty replacement or fibrosis is limited because the right ventricle has a thin wall and the differentiation between normal epicardial and intramyocardial infiltration is challenging. Therefore, to date, tissue characterization by CMR is not considered in the diagnostic work-up for ARVC[54-59]. Arrhythmic risk stratification in ARVC is based on multiparametric evaluation mainly based on clinical variables; patients at higher risk indicated for ICD implantation are those resuscitated after cardiac arrest, those with sustained and unstable monomorphic VT or exercise-induced unexplained syncope[60,61]. The role of CMR for risk stratification in ARVC is marginal, although significant: the extension of the disease to the left ventricle, identified by LGE-CMR, seems to to associated with a worse arrhythmic outcome and must be looked for[60-62].
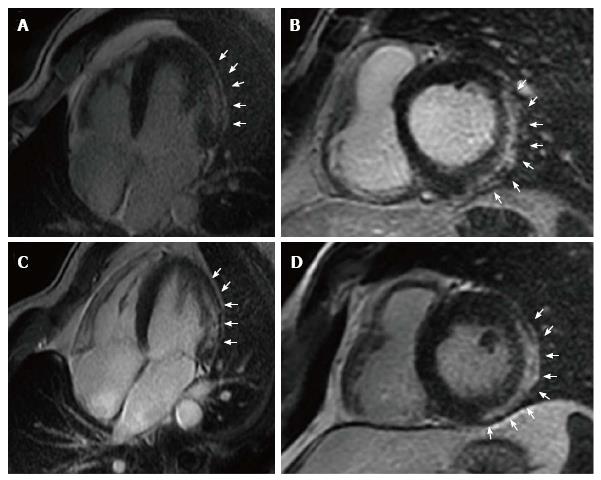
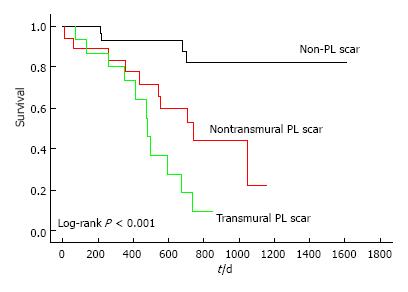
On the other side, and even more rare, left-dominant arrhythmogenic cardiomyopathy (LDAC) is characterized by epicardial or midmyocardial fibrotic or fibro-fatty replacement in postero-infero-lateral LV wall (“isolated nonischemic scar”) associated with life-threatening VA exceeding the degree of LV dysfunction (LVEF is often normal)[63-65]. LDAC is increasingly recognized as a cause of SCD in young athletes[66-68]. ECG often shows T-wave inversion in infero-lateral leads and low-voltage QRS complexes; VTs have right bundle branch block configuration and are often exercise-induced. In genetic familiar forms, usually autosomal-dominant, gene mutation mostly concerns components of cardiac desmosomes. In non-familiar forms LDAC phenotype can be the result of myocarditis leading to disruption of desmosomal architecture[63-65]. LGE-CMR plays a major diagnostic role because subepicardial/midmyocardial scar location is usually missed by echocardiography (Figure 4). Risk stratification is not well defined: By extrapolation from ARVC, ICD is indicated in patients who survived VF, with poorly tolerated sustained VT, or exercise-induced syncope. LGE-CMR also helps in risk stratification because a “stria” pattern in postero-lateral LV wall has been recently associated with a higher arrhythmic risk compared to the “benign” junctional “spotty” pattern, in a population of young athletes[68].
Sarcoidosis is an idiopathic non-caseating granulomatous disease that affects several organs, mostly the lungs, but also the heart, skin, liver, spleen, eye, and lymph nodes. Sarcoidosis occurs worldwide, being more frequent in African-American and Northern Europeans, especially women. Disease prevalence ranges between 4.7 and 64 in 100000[69]. Cardiac involvement is clinically evident in approximately 5% of patients, in form of: (1) conduction abnormalities; (2) VA including unexpected SCD; and (3) heart failure with reduced LVEF. Moreover, about 25% of patients with systemic sarcoidosis have asymptomatic cardiac involvement. At CMR cardiac sarcoidosis can appear as LGE in a patchy pattern or in longitudinal striae in the midwall or subepicardium, usually located in basal septum or LV wall. Delayed enhancement represents focal scarring, while inflammation areas can be detected with T2-weighted and STIR sequences[70,71]. CMR is also useful for differential diagnosis with ARVC that sometimes can resemble cardiac sarcoidosis. A recent consensus document[69] provided guidance for diagnosis and management of this disease, with a particular focus on arrhythmias. There are few data to help with SCD risk stratification[71]; in general, evidence from major randomized ICD trials of dilated cardiomyopathy is applicable, both in primary and secondary prevention. The presence of inflammation in both ventricles may increase ventricular arrhythmic risk; indeed, patients with implanted ICD have more frequent therapies from their devices, compared to other non-ischemic cardiomyopathies[69,70]. Current consensus recommendations[69] consider the use of CMR and the presence/absence of LGE (combined with electrophysiological study) to guide decision-making about ICD implant.
Amyloidosis is a disease characterized by protein misfold, aggregating into fibrils, and depositing extracellularly with disruption of organ architecture and function. There are two main types which affect the heart: Light chain (AL) amyloidosis and transthyretin cardiac amyloidosis (ATTR), both associated with the risk of VA and SCD[72,73]. Systemic amyloidosis occurs in more than 10 per million person-years in the United States population, with about 2000 new cases of AL amyloidosis occurring each year, approximately half of whom with significant cardiac involvement. The median age at presentation is 55-60 years, especially affecting women. The gold standard for diagnosis is endomyocardial biopsy, but CMR is increasingly used because it provides an accurate tissue characterization without the invasiveness of biopsy. At CMR the most frequent finding is a global subendocardial and circumferential LGE that is specific for cardiac amyloidosis. CMR findings (in particular LGE) have also been associated with prognosis and arrhythmic risk stratification, with the potential for guiding decision about ICD implant[73].
Left ventricular non-compaction (LVNC) is a relatively rare congenital disease, caused by an embryogenesis arrest, in which LV seems to be spongy. Ventricular wall anatomy is characterized by prominent LV trabeculae, a thin compacted layer, and deep intertrabecular recesses[74]. Clinical symptoms are related to neuromuscular disorders, heart failure with reduced LVEF, ventricular arrhythmic events and systemic thromboembolism. CMR can accurately identify this pathology, delineating hypertrabeculations of the apex and the LV lateral wall with subendocardial, midwall or transmural LGE[75]. CMR also helps to differentiate true LVNC from normal variants of increased trabeculations that can be found expecially in young athletes. There are also recent data about the role of CMR for risk stratification. In a recent prospective multicenter study[75], 113 patients underwent CMR, looking for diagnostic criterion of noncompacted/compacted ratio > 2.3 in end-diastole and LGE assessment. At a mean follow-up of 48 ± 24 mo the degree of LV trabeculation had no prognostic impact on the primary outcome (a composite of thromboembolic events, heart failure hospitalizations, VA and cardiac death) above LV dilation and dysfunction. LGE-CMR, instead, showed a significant correlation with life-threatening VA events and SCD.
Myocarditis is a group of heart-specific immune diseases classified by clinical and histopathological manifestations. Myocarditis may resolve spontaneously, recur or become chronic, leading about 30%-40% of biopsy-proven cases to dilated cardiomyopathy (DCM), death or heart transplantation. In the 2013 ESC myocarditis Task Force report[76], the disease was defined histologically as an inflammatory disease of the myocardium diagnosed on endomyocardial biopsy (EMB). Although EMB remains the diagnostic gold standard for diagnosis, it is not widely used. Traditionally, when the diagnosis is only based upon the histological Dallas criteria, myocarditis results to be a relatively rare disease. However, the use of highly sensitive immunohistochemical and molecular tools applied to EMB and of CMR suggests that there is a substantial clinical underestimation of its frequency and of its role in DCM[77,78]. CMR sequences have important diagnostic and prognostic value. T2-weighted CMR sequences detect edema or water, and T1-weighted sequences detect inflammation or fibrosis. LGE imaging can help in distinguishing nonischemic patterns of myocyte damage and fibrosis from ischemic injury, and T2-weighted and early gadolinium enhancement imaging detect other inflammatory features of edema, capillary leakage and hyperemia[78,79]. LGE has been associated with a higher (3.7%/year) risk of a composite of cardiovascular adverse events and its extent also predicted a composite endpoint of cardiac death, heart failure hospitalization, VT, and sudden death[80].
Anderson-Fabry disease is a X-linked disorder due to a deficiency of the alpha-galactosidase enzyme that causes an inability to catabolize glycosphingolipids, leading to their accumulation in several organs, including the heart[81]. The storage of lipids causes an increase of the ventricular wall thickness that simulates HCM and leads to heart failure[81,82]. Diagnosis can be made with CMR showing LGE within the basal infero-lateral wall but tipically sparing the endocardium, related to myocardial collagen scarring that represents the substrate for re-entry mechanism and SCD. Patients who have significant fibrosis on MRI and those with nonsustained VT are at higher risk for arrhythmic complications and may be considered for ICD[82,83].
CRT is a well-established therapy in patients with heart failure with reduced LVEF (< 35%) and a wide QRS (> 120 ms), usually with left bundle branch block[84]. In this setting, compared to optimal medical therapy, CRT reduces all-cause mortality and heart failure hospitalization, both in ischemic and non-ischemic cardiomyopathy, with larger benefit in non-ischemic etiology[84,85]. However, about 30%-40% of patients implanted according to current guidelines[86] do not show any benefit from CRT or even get worse[87]. This is hardly acceptable considering costs and risk of the procedure. There are several reasons explaining suboptimal CRT response: (1) patient’s characteristics (absence of ventricular dyssynchrony, too advanced heart disease to get a benefit, severe right ventricular dysfunction, untreated arrhythmias, severe medical co-pathologies, etc.); (2) suboptimal LV lead position at implant; (3) suboptimal CRT device programming during subsequent course[87,88].
The LV pacing site is an important determinant of a good outcome after CRT[88]. According to current guidelines, LV lead should be placed in non-apical posterolateral region to pace the latest activated areas[86]. Intuitively, deploying the LV lead over the latest electrical or (preferably) mechanical activated segments is likely to maximize the effects of CRT. However, recent evidence suggests that there is a large interindividual variability as concerns the latest activated areas and, subsequently, optimal LV pacing site[89-91]. Indeed, the latest mechanical activation is localized in posterolateral regions in 85%-90% of patients with non-ischemic dilated cardiomyopathy, but only in 10% of those with ischemic etiology[15].
Moreover, scar in proximity of LV pacing stimulus interferes with resynchronization, leading to QRS fragmentation and prolongation, and this is true both in ischemic and non-ischemic etiologies[92,93]. Chalil[94] showed that pacing over scar was associated with a higher risk of cardiac mortality or heart failure hospitalizations compared with pacing viable myocardium (Figure 5). In a study of 559 patients undergoing CRT, Leyva[95] found that LV lead positions over scar was associated with poorer CRT response, higher risk of cardiovascular death, heart failure hospitalizations and SCD at follow-up.
In this context, a multimodality imaging approach[96-98] is emerging with a dedicated “CRT team”[99-102], composed of electrophysiologists, cardiac imaging specialists and radiologists working together to identify the target areas (the most delayed and viable region) for LV pacing, by using CMR, myocardial perfusion imaging and newer echocardiographic techniques (such as longitudinal myocardial strain). Recent studies applying this method have demonstrated better clinical outcomes with the LV lead positioned at the latest mechanically activated region and away from myocardial scar[99-102]. In a study by Bertini et al[102], 100 patients with ischemic and non-ischemic dilated cardiomyopathy were enrolled: Group 1 with 50 consecutive patients scheduled for CRT and prospectively included, and group 2 (control) including 50 patients with a CRT device implanted according to standard clinical practice. In group 1, patients underwent two-dimensional speckle-tracking assessment of longitudinal myocardial strain and CMR imaging to identify the target area for LV lead. A positive response to CRT was defined as a ≥ 15% reduction of LV end-systolic volume at 6-mo follow-up. The result was that 78% of patients in group 1 were classified as responders to CRT compared to only 56% in group 2 (P = 0.019). The “CRT team” identified as target for LV pacing the lateral area in 60% of patients, but notably, in 16% of patients, the target was far from the lateral area, in the anterior or posterior regions. The patients with concordant position showed the highest positive response (93.1%) to CRT. These encouraging results need further validation in future larger multicenter trials with longer follow-up.
Placement of the LV lead is restricted by variable cardiac venous anatomy. Retrograde cardiac venography via the coronary sinus, at the time of implantation, is the gold-standard approach to imaging the coronary veins. It has been suggested that coronary vein imaging before CRT implantation could be useful and, in this respect, coronary venography is feasible with both CMR and computed tomography (CT)[15,96]. However, this approach has major limitations because it is technically challenging and can miss little veins that are beyond the spatial resolution of CMR and CT, but anyway these veins could be suitable for implantation. In addition, neither CMR nor CT provides adequate imaging of Thebesian and Vieussens valves or vein stenoses[15,96].
Catheter ablation is a well-established therapy for patients with scar-related sustained monomorphic VT, usually seen after myocardial infarction, and for atrial fibrillation (AF), the most common cardiac arrhythmia. Anyway, these arrhythmias are the most complex and challenging for the electrophysiologist[103].
For a successful ablation, the correct identification of underlying arrhythmogenic substrates is critical. With the use of standard electroanatomic mapping techniques, substrates are identified only indirectly, with local voltage amplitudes as a surrogate of the state of surrounding myocardium[103]. This approach, in addition to being time-consuming, lacks sensitivity for deep scar and lacks specificity when there is poor catheter contact or thinner myocardium[103]. Therefore, improved strategies to define arrhythmogenic scar substrates would be welcome. In this context, CMR could give an important contribution due to its ability to characterize cardiac anatomy and function without exposing the patient to additional radiation[7,41]. As validated histopathologically, CMR can visualize fibrosis and scar by delayed imaging of gadolinium contrast agents that accumulate in the extracellular matrix and have slower washout from scar than from normal myocardium[7,8,14]. Thanks to newer mapping technologies, CMR images can be merged with electrograms acquired from the conventional electrophysiologic study, thus creating an anatomic roadmap to guide ablation procedure[103].
Myocardial scar, the most common substrate for reentrant VA, can be easily displayed by LGE-CMR, allowing to shorten the procedure time devoted to substrate identification and enabling ablation of hemodynamically unstable VT (when conventional electrophysiologic and point-by-point voltage mapping is impossible)[104]. Moreover, a better understanding of the physiologic conduction characteristics associated with various anatomic scar substrates may improve patient selection for ablation, avoiding the procedure when scar burden is too high and complex, with few chances of success[105].
In the setting of AF ablation, LGE-CMR could be useful for patient selection, guidance of ablation procedure and post-ablation follow-up. Importantly, atrial LGE-CMR may allow improved patient selection so that unnecessary procedures are avoided in cases with little chance of procedural success[106]: Extensive left atrial LGE (> 35%) has been associated with a high rate (96%) of AF recurrence after catheter ablation[107]. Moreover, when procedure is planned in patients with a high burden of LGE, a more extensive ablation strategy could be pursued in addition to isolation of the pulmonary veins[108]. During the follow-up period, CMR can be useful to assess ablation success, for example, in terms of complete/incomplete isolation of pulmonary veins.
The main limitations for such approach are the added costs and expertise required for adequate image acquisition and analyses, the need for dedicated software, as well as inadequate spatial resolution in the atria. Moreover, CMR can create potential problems in patients already implanted with a cardiac device (pacemaker, ICD and CRT). Even when the device is “MRI safe” and CMR is technically feasible, lead artifacts can significantly alter image integrity and its clinical utility.
Hopefully, with improving techniques, accurate pre-procedural identification of the arrhythmogenic substrate by CMR may become in the near future an important adjunct for patient selection, procedural planning and post-procedural evaluation.
Cardiac MRI is revolutionizing the approach to the arrhythmologic patients both in diagnostic and therapeutic work-up. It provides information that other diagnostic imaging techniques do not allow to obtain, without radiation exposure, facilitating the initial evaluation and, once established a diagnosis, the choice of the most appropriate treatment. Current limitations are: (1) the paucity of randomized studies evaluating the outcome of patients treated with a CMR-based approach; (2) CMR is time-consuming, expensive, and requires experienced personnel for image acquisition and analysis; and (3) CMR still has inadequate spatial resolution in the left atrium and right ventricle, limiting its routine use for most arrhythmias arising from these chambers.
Lastly, a mention has to be made to nephrogenic systemic fibrosis that is a devastating (albeit extremely rare) potential complication in patients exposed to gadolinium-based contrast agents. This complication occurs almost exclusively in patients with moderate to severe kidney disease, particularly those on dialysis with incidences, in this latter group, ranging from 2.5% to 5%[109].
Based on the current literature and waiting for more data from future studies, it is foreseeable that CMR use in daily arrhythmologic practice will be increasingly implemented.
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cheng TH, Lai S S- Editor: Ji FF L- Editor: Wang TQ E- Editor: Lu YJ
| 1. | Saeed M, Van TA, Krug R, Hetts SW, Wilson MW. Cardiac MR imaging: current status and future direction. Cardiovasc Diagn Ther. 2015;5:290-310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 42] [Reference Citation Analysis (0)] |
| 2. | Kumar A, Bagur R. Cardiac magnetic resonance in clinical cardiology. World J Cardiol. 2015;7:6-9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Priori SG, Blomström-Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G. 2015 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC)Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Europace. 2015;17:1601-1687. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 4. | Wellens HJ, Schwartz PJ, Lindemans FW, Buxton AE, Goldberger JJ, Hohnloser SH, Huikuri HV, Kääb S, La Rovere MT, Malik M. Risk stratification for sudden cardiac death: current status and challenges for the future. Eur Heart J. 2014;35:1642-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 309] [Cited by in F6Publishing: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 5. | Goldberger JJ, Basu A, Boineau R, Buxton AE, Cain ME, Canty JM, Chen PS, Chugh SS, Costantini O, Exner DV. Risk stratification for sudden cardiac death: a plan for the future. Circulation. 2014;129:516-526. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 108] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Wu TJ, Ong JJ, Hwang C, Lee JJ, Fishbein MC, Czer L, Trento A, Blanche C, Kass RM, Mandel WJ. Characteristics of wave fronts during ventricular fibrillation in human hearts with dilated cardiomyopathy: role of increased fibrosis in the generation of reentry. J Am Coll Cardiol. 1998;32:187-196. [PubMed] [Cited in This Article: ] |
| 7. | Ipek EG, Nazarian S. Cardiac magnetic resonance for prediction of arrhythmogenic areas. Trends Cardiovasc Med. 2015;25:635-642. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Franco A, Javidi S, Ruehm SG. Delayed Myocardial Enhancement in Cardiac Magnetic Resonance Imaging. J Radiol Case Rep. 2015;9:6-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Disertori M, Gulizia MM, Casolo G, Delise P, Di Lenarda A, Di Tano G, Lunati M, Mestroni L, Salerno-Uriarte J, Tavazzi L. Improving the appropriateness of sudden arrhythmic death primary prevention by implantable cardioverter-defibrillator therapy in patients with low left ventricular ejection fraction. Point of view. J Cardiovasc Med (Hagerstown). 2016;17:245-255. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | De Maria E, Diemberger I, Vassallo PL, Pastore M, Giannotti F, Ronconi C, Romandini A, Biffi M, Martignani C, Ziacchi M. Prevention of infections in cardiovascular implantable electronic devices beyond the antibiotic agent. J Cardiovasc Med (Hagerstown). 2014;15:554-564. [PubMed] [Cited in This Article: ] |
| 11. | De Maria E, Borghi A, Bonetti L, Fontana PL, Cappelli S. Externalized conductors and insulation failure in Biotronik defibrillator leads: History repeating or a false alarm? World J Clin Cases. 2017;5:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 5] [Cited by in F6Publishing: 7] [Article Influence: 1.0] [Reference Citation Analysis (2)] |
| 12. | Gorgels AP, Gijsbers C, de Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest--the relevance of heart failure. The Maastricht Circulatory Arrest Registry. Eur Heart J. 2003;24:1204-1209. [PubMed] [Cited in This Article: ] |
| 13. | Wellens HJ, Gorgels AP, de Munter H. Sudden death in the community. J Cardiovasc Electrophysiol. 2003;14:S104-S107. [PubMed] [Cited in This Article: ] |
| 14. | Wong TC, Piehler K, Meier CG, Testa SM, Klock AM, Aneizi AA, Shakesprere J, Kellman P, Shroff SG, Schwartzman DS. Association between extracellular matrix expansion quantified by cardiovascular magnetic resonance and short-term mortality. Circulation. 2012;126:1206-1216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 356] [Cited by in F6Publishing: 381] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 15. | Leyva F. The Role of Cardiovascular Magnetic Resonance in Cardiac Resynchronization Therapy. Card Electrophysiol Clin. 2015;7:619-633. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Schelbert EB, Cao JJ, Sigurdsson S, Aspelund T, Kellman P, Aletras AH, Dyke CK, Thorgeirsson G, Eiriksdottir G, Launer LJ. Prevalence and prognosis of unrecognized myocardial infarction determined by cardiac magnetic resonance in older adults. JAMA. 2012;308:890-896. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 211] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 17. | McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108:54-59. [PubMed] [Cited in This Article: ] |
| 18. | Klem I, Weinsaft JW, Bahnson TD, Hegland D, Kim HW, Hayes B, Parker MA, Judd RM, Kim RJ. Assessment of myocardial scarring improves risk stratification in patients evaluated for cardiac defibrillator implantation. J Am Coll Cardiol. 2012;60:408-420. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 19. | Disertori M, Quintarelli S, Mazzola S, Favalli V, Narula N, Arbustini E. The need to modify patient selection to improve the benefits of implantable cardioverter-defibrillator for primary prevention of sudden death in non-ischaemic dilated cardiomyopathy. Europace. 2013;15:1693-1701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Merchant FM, Zheng H, Bigger T, Steinman R, Ikeda T, Pedretti RF, Salerno-Uriarte JA, Klersy C, Chan PS, Bartone C. A combined anatomic and electrophysiologic substrate based approach for sudden cardiac death risk stratification. Am Heart J. 2013;166:744-752. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Disertori M, Masè M, Ravelli F. Myocardial fibrosis predicts ventricular tachyarrhythmias. Trends Cardiovasc Med. 2017;27:363-372. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 22. | Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. Eur J Heart Fail. 2013;15:1019-1027. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 77] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, Sheppard MN, Poole-Wilson PA, Pennell DJ. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977-1985. [PubMed] [Cited in This Article: ] |
| 24. | Iles L, Pfluger H, Lefkovits L, Butler MJ, Kistler PM, Kaye DM, Taylor AJ. Myocardial fibrosis predicts appropriate device therapy in patients with implantable cardioverter-defibrillators for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2011;57:821-828. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 238] [Cited by in F6Publishing: 239] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 25. | Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2014;7:250-258. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 232] [Cited by in F6Publishing: 254] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 26. | Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, Morarji K, Brown TD, Ismail NA, Dweck MR. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309:896-908. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 775] [Cited by in F6Publishing: 805] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 27. | Neilan TG, Coelho-Filho OR, Danik SB, Shah RV, Dodson JA, Verdini DJ, Tokuda M, Daly CA, Tedrow UB, Stevenson WG. CMR quantification of myocardial scar provides additive prognostic information in nonischemic cardiomyopathy. JACC Cardiovasc Imaging. 2013;6:944-954. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 151] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 28. | Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, Vettor G, Tona F, Tarantini G, Cacciavillani L. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance on arrhythmic outcome and sudden cardiac death in nonischemic dilated cardiomyopathy. Heart Rhythm. 2014;11:856-863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 123] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Masci PG, Doulaptsis C, Bertella E, Del Torto A, Symons R, Pontone G, Barison A, Droogné W, Andreini D, Lorenzoni V. Incremental prognostic value of myocardial fibrosis in patients with non-ischemic cardiomyopathy without congestive heart failure. Circ Heart Fail. 2014;7:448-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 30. | Chimura M, Kiuchi K, Okajima K, Shimane A, Sawada T, Onishi T, Yamada S, Taniguchi Y, Yasaka Y, Kawai H. Distribution of ventricular fibrosis associated with life threatening ventricular tachyarrhythmias in patients with nonishcemic dilated cardiomyopathy. J Cardiovasc Electrophysiol. 2015;26:1239-1246. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Piers SR, Everaerts K, van der Geest RJ, Hazebroek MR, Siebelink HM, Pison LA, Schalij MJ, Bekkers SC, Heymans S, Zeppenfeld K. Myocardial scar predicts monomorphic ventricular tachycardia but not polymorphic ventricular tachycardia or ventricular fibrillation in nonischemic dilated cardiomyopathy. Heart Rhythm. 2015;12:2106-2114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 32. | Di Marco A, Anguera I, Schmitt M, Klem I, Neilan TG, White JA, Sramko M, Masci PG, Barison A, Mckenna P. Late Gadolinium Enhancement and the Risk for Ventricular Arrhythmias or Sudden Death in Dilated Cardiomyopathy: Systematic Review and Meta-Analysis. JACC Heart Fail. 2017;5:28-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 225] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 33. | Arbustini E, Disertori M, Narula J. Primary Prevention of Sudden Arrhythmic Death in Dilated Cardiomyopathy: Current Guidelines and Risk Stratification. JACC Heart Fail. 2017;5:39-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 34. | Bello D, Fieno DS, Kim RJ, Pereles FS, Passman R, Song G, Kadish AH, Goldberger JJ. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104-1108. [PubMed] [Cited in This Article: ] |
| 35. | Roes SD, Borleffs CJ, van der Geest RJ, Westenberg JJ, Marsan NA, Kaandorp TA, Reiber JH, Zeppenfeld K, Lamb HJ, de Roos A. Infarct tissue heterogeneity assessed with contrast-enhanced MRI predicts spontaneous ventricular arrhythmia in patients with ischemic cardiomyopathy and implantable cardioverter-defibrillator. Circ Cardiovasc Imaging. 2009;2:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 355] [Cited by in F6Publishing: 340] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 36. | Scott PA, Morgan JM, Carroll N, Murday DC, Roberts PR, Peebles CR, Harden SP, Curzen NP. The extent of left ventricular scar quantified by late gadolinium enhancement MRI is associated with spontaneous ventricular arrhythmias in patients with coronary artery disease and implantable cardioverter-defibrillators. Circ Arrhythm Electrophysiol. 2011;4:324-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 37. | Alexandre J, Saloux E, Dugué AE, Lebon A, Lemaitre A, Roule V, Labombarda F, Provost N, Gomes S, Scanu P. Scar extent evaluated by late gadolinium enhancement CMR: a powerful predictor of long term appropriate ICD therapy in patients with coronary artery disease. J Cardiovasc Magn Reson. 2013;15:12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Demirel F, Adiyaman A, Timmer JR, Dambrink JH, Kok M, Boeve WJ, Elvan A. Myocardial scar characteristics based on cardiac magnetic resonance imaging is associated with ventricular tachyarrhythmia in patients with ischemic cardiomyopathy. Int J Cardiol. 2014;177:392-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Zeidan-Shwiri T, Yang Y, Lashevsky I, Kadmon E, Kagal D, Dick A, Laish Farkash A, Paul G, Gao D, Shurrab M. Magnetic resonance estimates of the extent and heterogeneity of scar tissue in ICD patients with ischemic cardiomyopathy predict ventricular arrhythmia. Heart Rhythm. 2015;12:802-808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Kadish AH, Bello D, Finn JP, Bonow RO, Schaechter A, Subacius H, Albert C, Daubert JP, Fonseca CG, Goldberger JJ. Rationale and design for the Defibrillators to Reduce Risk by Magnetic Resonance Imaging Evaluation (DETERMINE) trial. J Cardiovasc Electrophysiol. 2009;20:982-987. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 41. | Bucciarelli-Ducci C, Baritussi A, Auricchio A. Cardiac MRI Anatomy and Function as a Substrate for Arrhythmias. Europace. 2016;18:iv130-iv135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 42. | Maron BJ, Ommen SR, Semsarian C, Spirito P, Olivotto I, Maron MS. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J Am Coll Cardiol. 2014;64:83-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 428] [Cited by in F6Publishing: 448] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 43. | Maron BJ, Maron MS. Contemporary strategies for risk stratification and prevention of sudden death with the implantable defibrillator in hypertrophic cardiomyopathy. Heart Rhythm. 2016;13:1155-1165. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 44. | Maron BJ. Historical perspectives on the implantable cardioverter-defibrillator and prevention of sudden death in hypertrophic cardiomyopathy. Card Electrophysiol Clin. 2015;7:165-171. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Rowin EJ, Maron MS. The Role of Cardiac MRI in the Diagnosis and Risk Stratification of Hypertrophic Cardiomyopathy. Arrhythm Electrophysiol Rev. 2016;5:197-202. [PubMed] [DOI] [Cited in This Article: ] |
| 46. | Kamal MU, Riaz IB, Janardhanan R. Cardiovascular magnetic resonance imaging in hypertrophic cardiomyopathy: Current state of the art. Cardiol J. 2016;23:250-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 47. | Maron MS. The role of cardiovascular magnetic resonance in sudden death risk stratification in hypertrophic cardiomyopathy. Card Electrophysiol Clin. 2015;7:187-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 48. | Maron MS, Maron BJ. Clinical Impact of Contemporary Cardiovascular Magnetic Resonance Imaging in Hypertrophic Cardiomyopathy. Circulation. 2015;132:292-298. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 49. | Weng Z, Yao J, Chan RH, He J, Yang X, Zhou Y, He Y. Prognostic Value of LGE-CMR in HCM: A Meta-Analysis. JACC Cardiovasc Imaging. 2016;9:1392-1402. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 236] [Cited by in F6Publishing: 277] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 50. | Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484-495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 592] [Cited by in F6Publishing: 669] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 51. | Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2761-2796. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 575] [Cited by in F6Publishing: 589] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 52. | Rowin EJ, Maron BJ, Haas TS, Garberich RF, Wang W, Link MS, Maron MS. Hypertrophic Cardiomyopathy With Left Ventricular Apical Aneurysm: Implications for Risk Stratification and Management. J Am Coll Cardiol. 2017;69:761-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 175] [Cited by in F6Publishing: 216] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 53. | Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733-2779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2292] [Cited by in F6Publishing: 2797] [Article Influence: 279.7] [Reference Citation Analysis (0)] |
| 54. | Pilichou K, Thiene G, Bauce B, Rigato I, Lazzarini E, Migliore F, Perazzolo Marra M, Rizzo S, Zorzi A, Daliento L. Arrhythmogenic cardiomyopathy. Orphanet J Rare Dis. 2016;11:33. [PubMed] [Cited in This Article: ] |
| 55. | Akdis D, Brunckhorst C, Duru F, Saguner AM. Arrhythmogenic Cardiomyopathy: Electrical and Structural Phenotypes. Arrhythm Electrophysiol Rev. 2016;5:90-101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 56. | Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227-3237. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 72] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Corrado D, Link MS, Calkins H. Arrhythmogenic Right Ventricular Cardiomyopathy. N Engl J Med. 2017;376:61-72. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 385] [Cited by in F6Publishing: 394] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 58. | Orgeron GM, Calkins H. Advances in the Diagnosis and Management of Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Curr Cardiol Rep. 2016;18:53. [PubMed] [Cited in This Article: ] |
| 59. | Haugaa KH, Haland TF, Leren IS, Saberniak J, Edvardsen T. Arrhythmogenic right ventricular cardiomyopathy, clinical manifestations, and diagnosis. Europace. 2016;18:965-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 60. | Zorzi A, Rigato I, Bauce B, Pilichou K, Basso C, Thiene G, Iliceto S, Corrado D. Arrhythmogenic Right Ventricular Cardiomyopathy: Risk Stratification and Indications for Defibrillator Therapy. Curr Cardiol Rep. 2016;18:57. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 61. | Brun F, Groeneweg JA, Gear K, Sinagra G, van der Heijden J, Mestroni L, Hauer RN, Borgstrom M, Marcus FI, Hughes T. Risk Stratification in Arrhythmic Right Ventricular Cardiomyopathy Without Implantable Cardioverter-Defibrillators. JACC Clin Electrophysiol. 2016;2:558-564. [PubMed] [Cited in This Article: ] |
| 62. | te Riele AS, Marcus FI, James CA, Murray BA, Tichnell C, Zimmerman SL, Kamel IR, Crosson J, Cramer MJ, Velthuis BK. The Value of Cardiac Magnetic Resonance Imaging in Evaluation of Pediatric Patients for Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. J Am Coll Cardiol. 2015;66:873-874. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | De Pasquale CG, Heddle WF. Left sided arrhythmogenic ventricular dysplasia in siblings. Heart. 2001;86:128-130. [PubMed] [Cited in This Article: ] |
| 64. | Sen-Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left-dominant arrhythmogenic cardiomyopathy: an under-recognized clinical entity. J Am Coll Cardiol. 2008;52:2175-2187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 475] [Cited by in F6Publishing: 456] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 65. | di Gioia CR, Giordano C, Cerbelli B, Pisano A, Perli E, De Dominicis E, Poscolieri B, Palmieri V, Ciallella C, Zeppilli P. Nonischemic left ventricular scar and cardiac sudden death in the young. Hum Pathol. 2016;58:78-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 66. | Busardò FP, Cappato R, D’Ovidio C, Frati P, Riezzo I, Fineschi V. Fatal left-dominant arrhythmogenic cardiomyopathy involving a 25-year old professional football player: could it have been prevented? Int J Cardiol. 2014;174:423-425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 67. | d’Amati G, De Caterina R, Basso C. Sudden cardiac death in an Italian competitive athlete: Pre-participation screening and cardiovascular emergency care are both essential. Int J Cardiol. 2016;206:84-86. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 68. | Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B. Nonischemic Left Ventricular Scar as a Substrate of Life-Threatening Ventricular Arrhythmias and Sudden Cardiac Death in Competitive Athletes. Circ Arrhythm Electrophysiol. 2016;9:pii: e004229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 181] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 69. | Birnie DH, Sauer WH, Judson MA. Consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart. 2016;102:411-414. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 70. | Greulich S, Kitterer D, Latus J, Aguor E, Steubing H, Kaesemann P, Patrascu A, Greiser A, Groeninger S, Mayr A. Comprehensive Cardiovascular Magnetic Resonance Assessment in Patients With Sarcoidosis and Preserved Left Ventricular Ejection Fraction. Circ Cardiovasc Imaging. 2016;9:pii: e005022. [PubMed] [Cited in This Article: ] |
| 71. | Ekström K, Lehtonen J, Hänninen H, Kandolin R, Kivistö S, Kupari M. Magnetic Resonance Imaging as a Predictor of Survival Free of Life-Threatening Arrhythmias and Transplantation in Cardiac Sarcoidosis. J Am Heart Assoc. 2016;5:pii: e003040. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 72. | White JA, Fine NM. Recent Advances in Cardiovascular Imaging Relevant to the Management of Patients with Suspected Cardiac Amyloidosis. Curr Cardiol Rep. 2016;18:77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Fontana M, Pica S, Reant P, Abdel-Gadir A, Treibel TA, Banypersad SM, Maestrini V, Barcella W, Rosmini S, Bulluck H. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. 2015;132:1570-1579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 342] [Cited by in F6Publishing: 375] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 74. | Arbustini E, Favalli V, Narula N, Serio A, Grasso M. Left Ventricular Noncompaction: A Distinct Genetic Cardiomyopathy? J Am Coll Cardiol. 2016;68:949-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 75. | Andreini D, Pontone G, Bogaert J, Roghi A, Barison A, Schwitter J, Mushtaq S, Vovas G, Sormani P, Aquaro GD. Long-Term Prognostic Value of Cardiac Magnetic Resonance in Left Ventricle Noncompaction: A Prospective Multicenter Study. J Am Coll Cardiol. 2016;68:2166-2181. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 91] [Cited by in F6Publishing: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 76. | Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB, Fu M, Heliö T, Heymans S, Jahns R. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636-2648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1714] [Cited by in F6Publishing: 1968] [Article Influence: 178.9] [Reference Citation Analysis (0)] |
| 77. | Caforio AL, Marcolongo R, Basso C, Iliceto S. Clinical presentation and diagnosis of myocarditis. Heart. 2015;101:1332-1344. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 57] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 78. | Heymans S, Eriksson U, Lehtonen J, Cooper LT. The Quest for New Approaches in Myocarditis and Inflammatory Cardiomyopathy. J Am Coll Cardiol. 2016;68:2348-2364. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 202] [Cited by in F6Publishing: 213] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 79. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1600] [Cited by in F6Publishing: 1627] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 80. | Schumm J, Greulich S, Wagner A, Grün S, Ong P, Bentz K, Klingel K, Kandolf R, Bruder O, Schneider S. Cardiovascular magnetic resonance risk stratification in patients with clinically suspected myocarditis. J Cardiovasc Magn Reson. 2014;16:14. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 115] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 81. | Nagueh SF. Anderson-Fabry disease and other lysosomal storage disorders. Circulation. 2014;130:1081-1090. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 82. | Acharya D, Doppalapudi H, Tallaj JA. Arrhythmias in Fabry cardiomyopathy. Card Electrophysiol Clin. 2015;7:283-291. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 83. | Krämer J, Niemann M, Störk S, Frantz S, Beer M, Ertl G, Wanner C, Weidemann F. Relation of burden of myocardial fibrosis to malignant ventricular arrhythmias and outcomes in Fabry disease. Am J Cardiol. 2014;114:895-900. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 84. | Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845-1853. [PubMed] [Cited in This Article: ] |
| 85. | Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, Foster E, Greenberg H, Higgins SL. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329-1338. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2231] [Cited by in F6Publishing: 2182] [Article Influence: 145.5] [Reference Citation Analysis (0)] |
| 86. | Brignole M, Auricchio A, Baron-Esquivias G, Bordachar P, Boriani G, Breithardt OA, Cleland J, Deharo JC, Delgado V, Elliott PM. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy: the Task Force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J. 2013;34:2281-2329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1437] [Cited by in F6Publishing: 1435] [Article Influence: 130.5] [Reference Citation Analysis (0)] |
| 87. | Mullens W, Grimm RA, Verga T, Dresing T, Starling RC, Wilkoff BL, Tang WH. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765-773. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 88. | Ypenburg C, van Bommel RJ, Delgado V, Mollema SA, Bleeker GB, Boersma E, Schalij MJ, Bax JJ. Optimal left ventricular lead position predicts reverse remodeling and survival after cardiac resynchronization therapy. J Am Coll Cardiol. 2008;52:1402-1409. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 316] [Cited by in F6Publishing: 323] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 89. | Derval N, Steendijk P, Gula LJ, Deplagne A, Laborderie J, Sacher F, Knecht S, Wright M, Nault I, Ploux S. Optimizing hemodynamics in heart failure patients by systematic screening of left ventricular pacing sites: the lateral left ventricular wall and the coronary sinus are rarely the best sites. J Am Coll Cardiol. 2010;55:566-575. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 205] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 90. | Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70-78. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 218] [Cited by in F6Publishing: 203] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 91. | Shanks M, Delgado V, Bax JJ. Cardiac Resynchronization Therapy in Non-Ischemic Cardiomyopathy. J Atr Fibrillation. 2016;8:1362. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 3] [Reference Citation Analysis (0)] |
| 92. | Ypenburg C, Schalij MJ, Bleeker GB, Steendijk P, Boersma E, Dibbets-Schneider P, Stokkel MP, van der Wall EE, Bax JJ. Impact of viability and scar tissue on response to cardiac resynchronization therapy in ischaemic heart failure patients. Eur Heart J. 2007;28:33-41. [PubMed] [Cited in This Article: ] |
| 93. | Leyva F, Foley PW, Chalil S, Ratib K, Smith RE, Prinzen F, Auricchio A. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 94. | Chalil S, Stegemann B, Muhyaldeen SA, Khadjooi K, Foley PW, Smith RE, Leyva F. Effect of posterolateral left ventricular scar on mortality and morbidity following cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2007;30:1201-1209. [PubMed] [Cited in This Article: ] |
| 95. | Leyva F, Taylor RJ, Foley PW, Umar F, Mulligan LJ, Patel K, Stegemann B, Haddad T, Smith RE, Prasad SK. Left ventricular midwall fibrosis as a predictor of mortality and morbidity after cardiac resynchronization therapy in patients with nonischemic cardiomyopathy. J Am Coll Cardiol. 2012;60:1659-1667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 138] [Cited by in F6Publishing: 147] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 96. | Leyva F. The Role of Cardiovascular Magnetic Resonance in Cardiac Resynchronization Therapy. Heart Fail Clin. 2017;13:63-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 97. | Nguyên UC, Mafi-Rad M, Aben JP, Smulders MW, Engels EB, van Stipdonk AM, Luermans JG, Bekkers SC, Prinzen FW, Vernooy K. A novel approach for left ventricular lead placement in cardiac resynchronization therapy: Intraprocedural integration of coronary venous electroanatomic mapping with delayed enhancement cardiac magnetic resonance imaging. Heart Rhythm. 2017;14:110-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 98. | Carità P, Corrado E, Pontone G, Curnis A, Bontempi L, Novo G, Guglielmo M, Ciaramitaro G, Assennato P, Novo S. Non-responders to cardiac resynchronization therapy: Insights from multimodality imaging and electrocardiography. A brief review. Int J Cardiol. 2016;225:402-407. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 99. | Saba S, Marek J, Schwartzman D, Jain S, Adelstein E, White P, Oyenuga OA, Onishi T, Soman P, Gorcsan J. Echocardiography-guided left ventricular lead placement for cardiac resynchronization therapy: results of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region trial. Circ Heart Fail. 2013;6:427-434. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 274] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 100. | Khan FZ, Virdee MS, Palmer CR, Pugh PJ, O’Halloran D, Elsik M, Read PA, Begley D, Fynn SP, Dutka DP. Targeted left ventricular lead placement to guide cardiac resynchronization therapy: the TARGET study: a randomized, controlled trial. J Am Coll Cardiol. 2012;59:1509-1518. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 490] [Cited by in F6Publishing: 515] [Article Influence: 42.9] [Reference Citation Analysis (0)] |
| 101. | Sommer A, Kronborg MB, Nørgaard BL, Poulsen SH, Bouchelouche K, Böttcher M, Jensen HK, Jensen JM, Kristensen J, Gerdes C. Multimodality imaging-guided left ventricular lead placement in cardiac resynchronization therapy: a randomized controlled trial. Eur J Heart Fail. 2016;18:1365-1374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 92] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 102. | Bertini M, Mele D, Malagù M, Fiorencis A, Toselli T, Casadei F, Cannizzaro T, Fragale C, Fucili A, Campagnolo E. Cardiac resynchronization therapy guided by multimodality cardiac imaging. Eur J Heart Fail. 2016;18:1375-1382. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 103. | Romero J, Natale A, Di Biase L. Cardiac magnetic resonance imaging and electrophysiology “the beauty is in the eye of the beholder”. Trends Cardiovasc Med. 2015;25:643-645. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 104. | Estner HL, Zviman MM, Herzka D, Miller F, Castro V, Nazarian S, Ashikaga H, Dori Y, Berger RD, Calkins H. The critical isthmus sites of ischemic ventricular tachycardia are in zones of tissue heterogeneity, visualized by magnetic resonance imaging. Heart Rhythm. 2011;8:1942-1949. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 105. | Desjardins B, Crawford T, Good E, Oral H, Chugh A, Pelosi F, Morady F, Bogun F. Infarct architecture and characteristics on delayed enhanced magnetic resonance imaging and electroanatomic mapping in patients with postinfarction ventricular arrhythmia. Heart Rhythm. 2009;6:644-651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 118] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 106. | Peters DC, Wylie JV, Hauser TH, Kissinger KV, Botnar RM, Essebag V, Josephson ME, Manning WJ. Detection of pulmonary vein and left atrial scar after catheter ablation with three-dimensional navigator-gated delayed enhancement MR imaging: initial experience. Radiology. 2007;243:690-695. [PubMed] [Cited in This Article: ] |
| 107. | Spragg DD, Khurram I, Zimmerman SL, Yarmohammadi H, Barcelon B, Needleman M, Edwards D, Marine JE, Calkins H, Nazarian S. Initial experience with magnetic resonance imaging of atrial scar and co-registration with electroanatomic voltage mapping during atrial fibrillation: success and limitations. Heart Rhythm. 2012;9:2003-2009. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 128] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 108. | McGann C, Akoum N, Patel A, Kholmovski E, Revelo P, Damal K, Wilson B, Cates J, Harrison A, Ranjan R. Atrial fibrillation ablation outcome is predicted by left atrial remodeling on MRI. Circ Arrhythm Electrophysiol. 2014;7:23-30. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 245] [Cited by in F6Publishing: 277] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 109. | Kribben A, Witzke O, Hillen U, Barkhausen J, Daul AE, Erbel R. Nephrogenic systemic fibrosis: pathogenesis, diagnosis, and therapy. J Am Coll Cardiol. 2009;53:1621-1628. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |