Introduction
Recent studies report that 13% of celiac disease (CD) patients have a body mass index (BMI) of 30 or above [4]. Celiac disease is a rare but potentially fatal cause of severe malnutrition in patients undergoing bariatric surgery. A preoperatory diagnosis could change the treatment strategy or surgical technique.
Materials and Methods
In June 2011, a thirty six year old female patient with class III obesity (BMI: 48,4 kg/m2) and a normal pre-surgical esophagogastroduodenoscopy (EGD) underwent gastric by-pass surgery. Four months after surgery, in spite of continuous treatment with poli-vitamins, iron supplements and adequate compliance to the diet, the patient referred significant hair loss.
A year later, she developed severe post-prandial pain, nausea, vomiting, chronic diarrhea with 5 to 10 liquid bowel movements per day, nocturnal diarrhea, and asthenia. She had lost 70 kg in 14 months reaching a BMI of 21 kg/m2. The patient was admitted at our institution afebrile with normal blood pressure and heart rate.
Investigations
Initial laboratory results showed WBC 6,06 x109/L, Hemoglobin 6,51 mmol/L, Platelets 191 x10 9/L, Glucose 4,11 mmol/L, Urea 8,92 mmol/L, Total Cholesterol 2,09 mmol/L, Creatinine 48,62 μmol/L, ALT 58 U/L, AST 111 U/L, Albumin 1,7 gr/dL, Na 139 mmol/L, K 4,2 mmol/L, Prothrombin 64%, TSH 3,48 mlU/ml and cholecalciferol and vitamin B12 deficit. Parasitologic fecal test, fecal leukocytes and cultures were negative but stool steatocrit was 6,6% (< 4%).
The patient received empirical treatment for bacterial overgrowth with antibiotics, probiotics, bismuth subsalicylate and loperamide without response. Abdominal Doppler ultrasound for vascular lesions was normal and CT scan with mesenteric angiotomography informed anatomical findings according to surgical records with mild ascites and mucosal edema.
An EGD was performed with findings according to surgical history; gastric remnant, gastrojejunal anastomosis and alimentary limb presented no evident lesions. Biopsies from jejunal folds informed increased intraepithelial lymphocytes, mucosal atrophy with moderate villous atrophy and crypt hyperplasia. (FIGURE 1 and FIGURE 2)
Five days later, the following results were received: Total IgA: 340 mg/dL, anti transglutaminase IgA: 117,2 U/ml (<30), antiendomise IgA: positive.
Treatment
As soon as the diagnosis of CD was made, the patient was started on gluten-free enteral nutrition due to gastric intolerance and three days later oral intake was resumed. All clinical symptoms were resolved and the patient was discharged on the seventh day.
Outcome and Follow-up
The patient remained symptom free and regained 4,6 kg thirty days after discharge.
Discussion
CD is a systemic immune-mediated disorder triggered by dietary gluten in genetically susceptible persons. CD affects 0.6 to 1.0% of the population worldwide [1,2,3] and has classically been related to malabsorption and weight loss. Recent studies show that the majority of CD sufferers do not display classic symptoms and are more likely to be overweight or obese than underweight at the time of presentation.[4,5]
Obesity is a chronic disease that is increasing in prevalence in the United States and worldwide. In general, greater body mass index (BMI) is associated with increased rate of death from all causes and from cardiovascular disease. Estimates for the annual number of excess deaths attributable to obesity in the United States are variable and range from 111,909 to 365,000 [6] Dickey and col. (2006) [5] and Tucker and col. (2012) [4] report a median BMI of 24,6 and 23,6 kg/m2 with 39% and 31% of overweight, and 13% of obesity in both populations at the initial assessment of CD patients. Prevalence of obesity was higher in women. They also noted that the percentage of patients who are overweight or obese at first referral increased every year.
Furse and Mee (2005) [7] describe atypical presentations of CD in four obese women with a BMI over 40 kg/m2. Semenaro (1986) [8] postulates that obese CD patients are probably able to compensate for proximal malabsorption by using intact absorptive mechanisms more distally. Fat absorption remains static and so do the ability to maintain energy intake, and as the surface area of the small bowel increases with age, children develop the ability to ingest adequate compensatory energy. Those whose energy intake is excessive will become obese.
Cuenca-Abente and col. (2012) report five patients being diagnosed with CD based on endoscopic findings during pre-surgical evaluation for bariatric surgery [9] and bibliographic investigation offered three cases where the diagnosis of CD was performed after a jejunoileal by-pass [10,11,12], one of them with fatal outcome due to hepatorenal failure.
The case we present shows the development of CD with severe malnutrition and clinical deterioration in a patient who had undergone recent gastric by-pass surgery. After jejunal by-pass surgery, adaptation of the small intestine occurs over a period of months and is evidenced by ileal villi hypertrophy. CD may interfere with this adaptation and exacerbate malabsorption leading to malnutrition. CD alters natural absorptive mechanisms and any surgical procedure affecting or interfering with absorption should be avoided.
Should a similar clinical scenario be presented, with the diagnosis of CD after a gastric by-pass surgery, it is imperative to start adequate nutritional support with gluten free diet and reposition of minerals and vitamins. Although the literature is scarce, if the patient remains unresponsive, steroid treatment could be an option (1) and one could consider a return to normal anatomy surgery or a reverse jejunoileal by-pass surgery as last resource alternatives (10).
Italian physicians De´ Angelis, Carra and Vincenzi (2012) propose serologic screening test for patients undergoing bariatric surgery [11] based on the excellent sensitivity (94%) and specificity (97%) of serum IgA antitransglutaminase antibodies [1-3, 11]. Cuenca-Abente and col. (2012) recommend only a careful evaluation of the duodenum, serological test and biopsies on those patients with abnormal endoscopic appearance. [9] The latter group prefers sleeve gastrectomy over gastric by-pass in obese patients with CD. Three patients were operated after being placed on a gluten free diet. None developed complications or classic symptoms of CD, serological marker levels normalized and all three achieved standard weight loss on follow-up.
In conclusion, obesity is a pandemic disease and CD is a condition that can affect morbidly obese patients. Serological screening test for patients enrolling a bariatric surgery program could be beneficial because a preoperatory diagnosis could help make better decisions both in the treatment strategy and surgical technique.
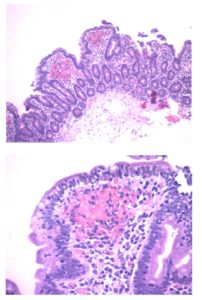
Figure 1 and 2: Biopsies from jejunal folds (HE stain, x10 and x40): Increased intraepithelial lymphocytes, mucosal atrophy with moderate villous atrophy and crypt hyperplasia.
No funding was received
We manifest no conflict of interest
Ethics: The patient has given her informed consent and the study protocol has been approved by the Hospital´s Ethics committee.
References
1. Green, P. H. R. and Cellier, C. (2007) “Celiac Disease,” N Engl J Med, 357: 1731-43.
Google Scholar
2. Fasano , A., Catassi, C. (2012)” Celiac disease,” N Engl J Med, 367: 2419-26.
Publisher – Google Scholar
3. Harris, L. A. , Park, J. Y. and col. (2012) “Celiac disease: clinical, endoscopic, and histopathologic review,” GIE, 76( )3: 625-40.
Google Scholar
4. Tucker, E. , Rostami, K and col. (2012) “ Patients with coeliac disease are increasingly overweight or obese on presentation,” J Gastrointestin Dis, 21: 11-15.
Google Scholar
5. Dickey, W. , Kearney , N. (2006) “Overweight in celiac disease:prevalence, clinical characteristics, and effect of a gluten-free diet,” Am J Gastroenterol, 101: 2356-59.
Publisher – Google Scholar
6. Flegal, K. M. , Graubard, B. I. and col. (2005) “Excess deaths associated with underweight, overweight, and obesity,” JAMA, 293(15):1861-67.
Google Scholar
7. Furse, R. M. and Mee, A. S. (2005) “Atypical presentation of coeliac disease”, BMJ, 330: 773-774.
Publisher – Google Scholar
8. Cuenca-Abente, F. , Nachman, F. and Bai, J. C. (2012) “Diagnosis of celiac disease during pre-operative work-up for bariatric surgery, “Acta Gastroenterol Latinoam, 42: 321-324.
Google Scholar
9. Logan, R. F. , Ferguson, A. (1982) “ Jejunal villous atrophy with morbid obesity:death after jejunoileal bypass,” Gut, 23: 999-1004.
Publisher – Google Scholar
10. De´ Angelis, N. , Carra, M. C. and Vincenzi, F. (2012) “Gluten-Free Diet in Obese Patients with Celiac Disease: An Enemy of the Bariatric Surgeon?, “ Obes Surg, 22: 995-996.
Publisher – Google Scholar
11. Owen, D.A. , Thorlakson, T. K. and Walli, J. E. , (1980) “Celiac disease in a patient with morbid obesity,” Arch Intern Med, 140: 1380-81.
Publisher – Google Scholar
12. Semenaro, L. A. , Barwick, K. W. and Gryboski, J. D. (1986) “ Obesity in celiac sprue,” J Clin Gastroenterol, 8: 177-80.
Publisher – Google Scholar



