Published online Jul 24, 2020. doi: 10.5306/wjco.v11.i7.495
Peer-review started: January 18, 2020
First decision: April 12, 2020
Revised: May 26, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 24, 2020
Oncocytic carcinoma of the thyroid is a rare disease, characterized by a poor prognosis and low response rate to radioiodine therapy. Crizotinib is a specific anaplastic lymphoma kinase (ALK) inhibitor, which was initially developed in non-small cell lung cancer. Other solid tumors harboring a translocation in ALK have been described, such as renal carcinoma, thyroid, colorectal, ovarian cancers, and spitzoid melanoma. The research of ALK rearrangements in thyroid tumor is a promising therapeutic track, and treatments need to be explored.
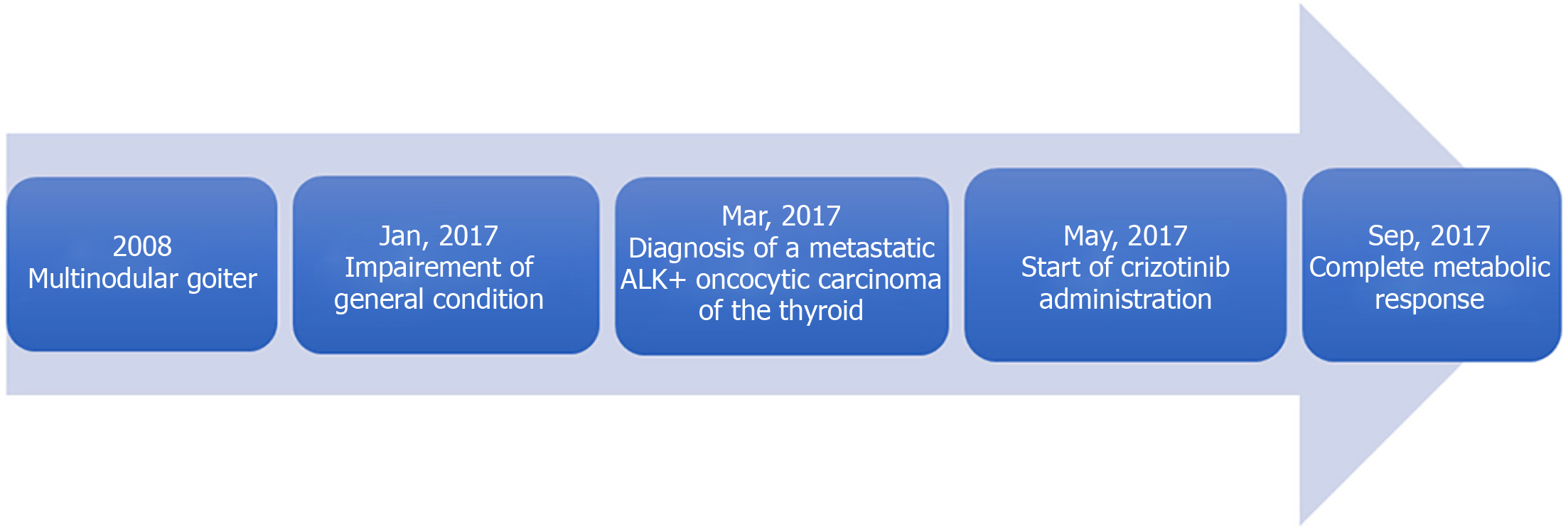
We report the case of a 76-year-old woman with a history of multinodular goiter, who was hospitalized for impairment of her general condition. She was diagnosed with metastatic oncocytic thyroid cancer. Synchrone metastases were found: Multiple mediastinal lymphadenopathies, lytic bone lesions and bilateral mammary lumps. Fluorescence in situ hybridization analysis revealed an ALK rearrangement in 61% of cells. No other mutation was found. A tumor board discussion based on molecular characteristics of the tumor suggested initiating a daily treatment by crizotinib, a specific ALK inhibitor. A positron emission tomography scan performed 4 mo after the initiation of crizotinib showed a complete metabolic response.
This case highlights an unexpected efficacy of crizotinib in an ALK-rearranged thyroid tumor, and the need of further assessments.
Core tip: Oncocytic carcinoma of the thyroid is a rare disease, characterized by a poor prognosis and low response rate to radioiodine therapy. We present here a case of a 76-year-old woman who was diagnosed with metastatic oncocytic thyroid cancer. A rearrangement of the anaplastic lymphoma kinase (ALK) gene within the thyroid tumor was found, and the patient started a daily treatment of crizotinib, a specific ALK inhibitor. After 4 mo, an unexpected complete metabolic response was observed. The efficacy of crizotinib in an ALK-rearranged thyroid tumor is a promising therapeutic track and needs to be explored.
- Citation: de Salins V, Loganadane G, Joly C, Abulizi M, Nourieh M, Boussion H, Belkacemi Y, Tournigand C, Kempf E. Complete response in anaplastic lymphoma kinase–rearranged oncocytic thyroid cancer: A case report and review of literature. World J Clin Oncol 2020; 11(7): 495-503
- URL: https://www.wjgnet.com/2218-4333/full/v11/i7/495.htm
- DOI: https://dx.doi.org/10.5306/wjco.v11.i7.495
Since 1980, the incidence of thyroid cancer has strongly increased, despite a recent decrease in the number of female cases. Each year, around 63000 new cases of thyroid cancer are diagnosed worldwide, leading to 2820 deaths. Thanks to initial diagnoses made at an earlier stage, the overall survival (OS) of patients with thyroid cancer has recently improved. The 5-year OS rate expended from 83% to 95% in the early 2000s[1].
Oncocytic tumors of the thyroid, also known as Hürthle cell tumors, are rare. The frequency of this tumor is actually unknown[2]. Hürthle (oncocytic) cell tumors are neoplasms composed of oncocytic cells. Non-invasive cases are called Hürthle cell adenomas, and cases with capsular or vascular invasion are called Hürthle cell carcinomas[3]. Follicular neoplasms can be composed in part by Hürthle cells and considered as Hürthle cell tumors only if more than 75% of the tumor is composed of Hürthle cells[4]. Oncocytic cells are large, polygonal cells with marked eosinophilic, granular cytoplasm reflective of overly abundant mitochondria[5]. Metastatic dissemination involves both blood and lymph nodes - contrasting with follicular carcinoma. Oncocytic carcinomas are characterized by a poor prognosis when compared to other differentiated thyroid carcinomas[6-8].
Papillary thyroid carcinoma cases with Hürthle cell changes are likely to have higher rates of recurrence (28% vs 11%)[9]. A SEER database cohort study showed that 25-year OS rates reached 82.1% and 89.1% in both populations, respectively[10].
In 1994, the anaplastic lymphoma kinase (ALK) gene was discovered through the identification of its fusion with nucleophosmin-1 (NPM1) in anaplastic large cell lymphomas[11]. In 2007, Hiroyuki Mano and colleagues[12] discovered a novel ALK fusion echinoderm-microtubule-associated protein-like 4 (referred to as EML4) that was found in a small percentage of Japanese lung cancers. ALK can be activated by translocation, as well as by mutation at the ALK locus, most commonly within the kinase domain, as reported in patients with thyroid cancer[13].
Crizotinib is a specific ALK inhibitor, which was initially developed in non-small cell lung cancer (NSCLC). The phase III study PROFILE 1014 compared crizotinib with chemotherapy as a first-line treatment in patients with advanced ALK-positive non-squamous NSCLC. The median progression-free survival was significantly better with crizotinib than chemotherapy [10.9 mo vs 7.0 mo, respectively, with hazard ratio of 0.45 and 95% confidence interval (CI) of 0.35, 0.60]. The objective response rates reached 74% and 45%, respectively. As compared with chemotherapy, crizotinib was associated with a greater reduction in lung cancer-related symptoms and a greater improvement in quality of life[14,15].
Other solid tumors harboring a translocation in ALK have been described, such as renal carcinoma, thyroid, colorectal, ovarian cancers, and spitzoid melanomas[16]. The identification of ALK rearrangements in thyroid tumors is highly promising. It has been reported that administration of crizotinib led to tumor stability in 1 case of advanced aggressive papillary cancer and to a response rate of 90% in 1 case of anaplastic thyroid cancer; both tumor responses lasted more than 6 mo[17,18]. We will present herein the case of a complete tumor response in 1 case of metastatic oncocytic thyroid cancer. Informed written consent was obtained from the patient for publication of this report and any accompanying images.
A 76-year-old woman, former personal assistant, was hospitalized because of an impaired general condition.
The patient suffered from an impaired general condition, associated with a body weight loss of 15 kg over 7 mo.
In 2008, the patient had received a diagnosis of multinodular goiter. She currently suffered from type 2 diabetes, hypertension, and dyslipidemia, and had undergone an appendectomy in 1960. Her daily drug medicine routine was 500 mg metformin three times a day, 120 mg gliclazide, 100 mg sitagliptin, 50 mg fosinopril, 12.5 mg hydrochlorothiazide, 300 mg piascledine in the morning, and 20 mg lercanidipine, 10 mg simvastatin and 2.5 mg bisoprolol at night.
The patient had no medical history other than arterial hypertension. Her family reported no other cases of cancer.
Physical examination found an altered patient with a moderate goiter without clinical signs of compression, and a suspect left sub-clavicular adenopathy. The remainder of the medical examination, including palpation of the breast, showed no anomalies.
A chest-abdomen-pelvis computed tomography (CT) revealed a multinodular thyroid associated with multiple mediastinal lymphadenopathies, lytic bone lesions and bilateral mammary lumps. The positron emission tomography (PET) scan found no FDG uptake in the thyroid while hypermetabolic activity was observed in the left inferior jugular and the supra-clavicular lymphadenopathies. Several bilateral mediastinal hypermetabolic lymphadenopathies and a right upper outer mammary lesion were also hypermetabolic.
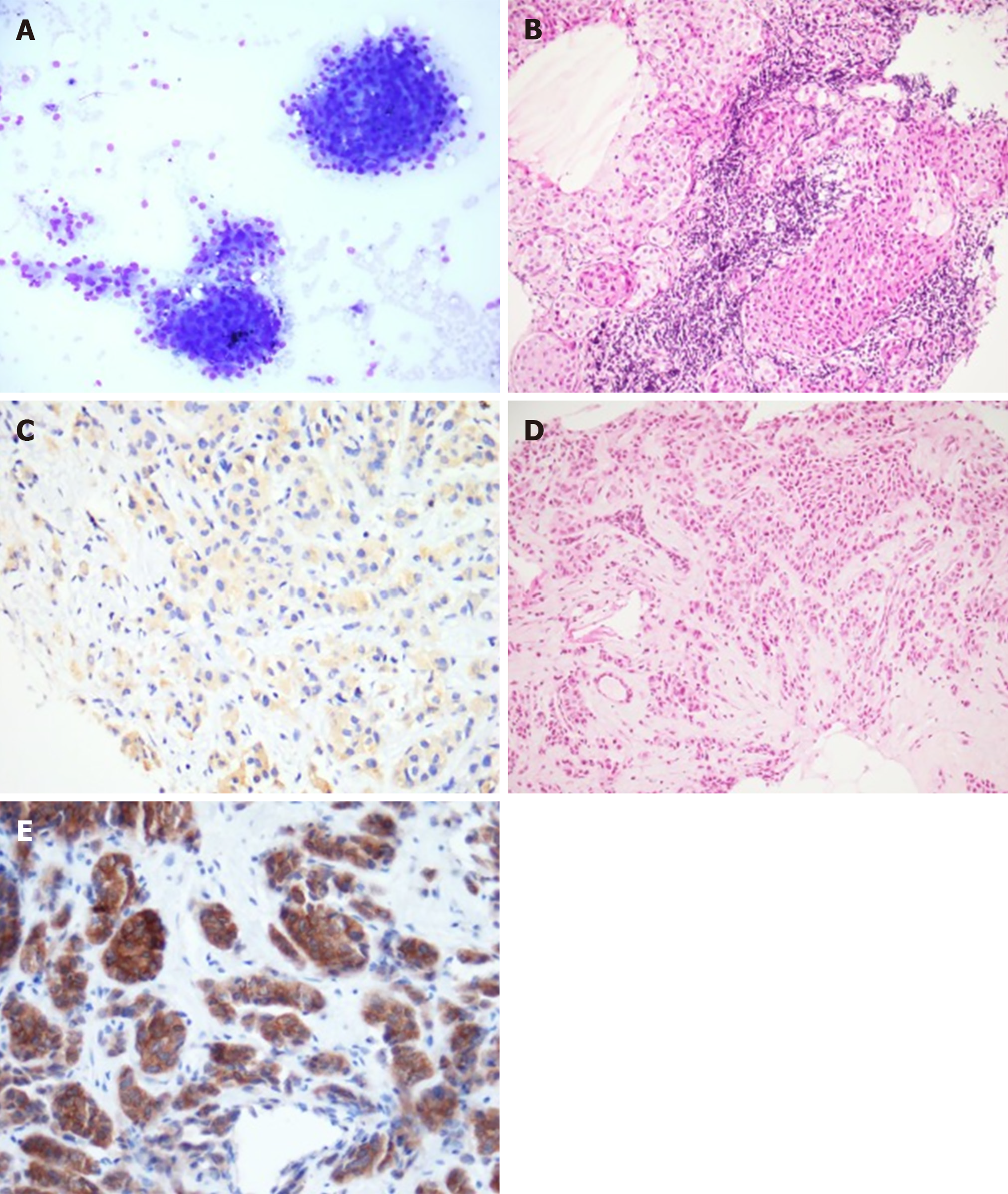
The blood tests showed normal thyroid function and inflammatory anemia. Fine needle aspiration of the right thyroid nodule was contributive and revealed a category V lesion according to The Bethesda System for Reporting Thyroid Cytopathology (suspicious for malignancy), isolated cells, a cluster of large cells with oncocytic features, and scanty colloid. Metastasis from a distant organ malignancy could not be ruled out solely based on this exam. The supra-clavicular biopsy revealed a metastasis from carcinoma with an oncocytic feature, as well as cytokeratin 7 and TTF1 expression in immunohistochemistry. We found moderate cytoplasmic staining (2+) of ALK protein (clone 5A4). A bronchoscopy with a bronchial biopsy showed carcinoma cells that expressed TTF1 within lymphatic vessels. Similar type of cells were observed in bronchoalveolar lavage. The 9 mm and 5 mm right mammary nodules were classified as ACR4 and the biopsies showed an infiltrating carcinoma with oncocytic features, without vascular and perineural invasion. Complementary immunohistochemical assessments showed that tumor cells in the breast biopsy also presented nuclear staining for TTF1 and no staining for estrogen receptor, androgen receptor, GATA3 or HER2. Interestingly, strong cytoplasmic staining (3+) of ALK protein (clone D5F3) was observed (Figure 1). Fluorescence in situ hybridization analysis revealed a rearrangement of ALK in 61% of the cells. We found no mutations in the EGFR, BRAF, RAS, ERBB2, and PIK3CA genes within the lymph node metastasis.
Based on the molecular and anatomical features found, we made a diagnosis of oncocytic carcinoma of the thyroid with nodes, breast and bone metastasis, with a rearrangement of ALK.
A multidisciplinary tumor board discussion, based on molecular characteristics of the tumor, suggested initiating a daily treatment of crizotinib (500 mg per day, taken as 250 mg in the morning and the night). The treatment was well tolerated, and the physical condition improved. No vision disorder, nausea, diarrhea, pneumonitis, hepatotoxicity or skin toxicity was reported. The patient complained of grade 2 constipation. No dose reduction or interruption was required.
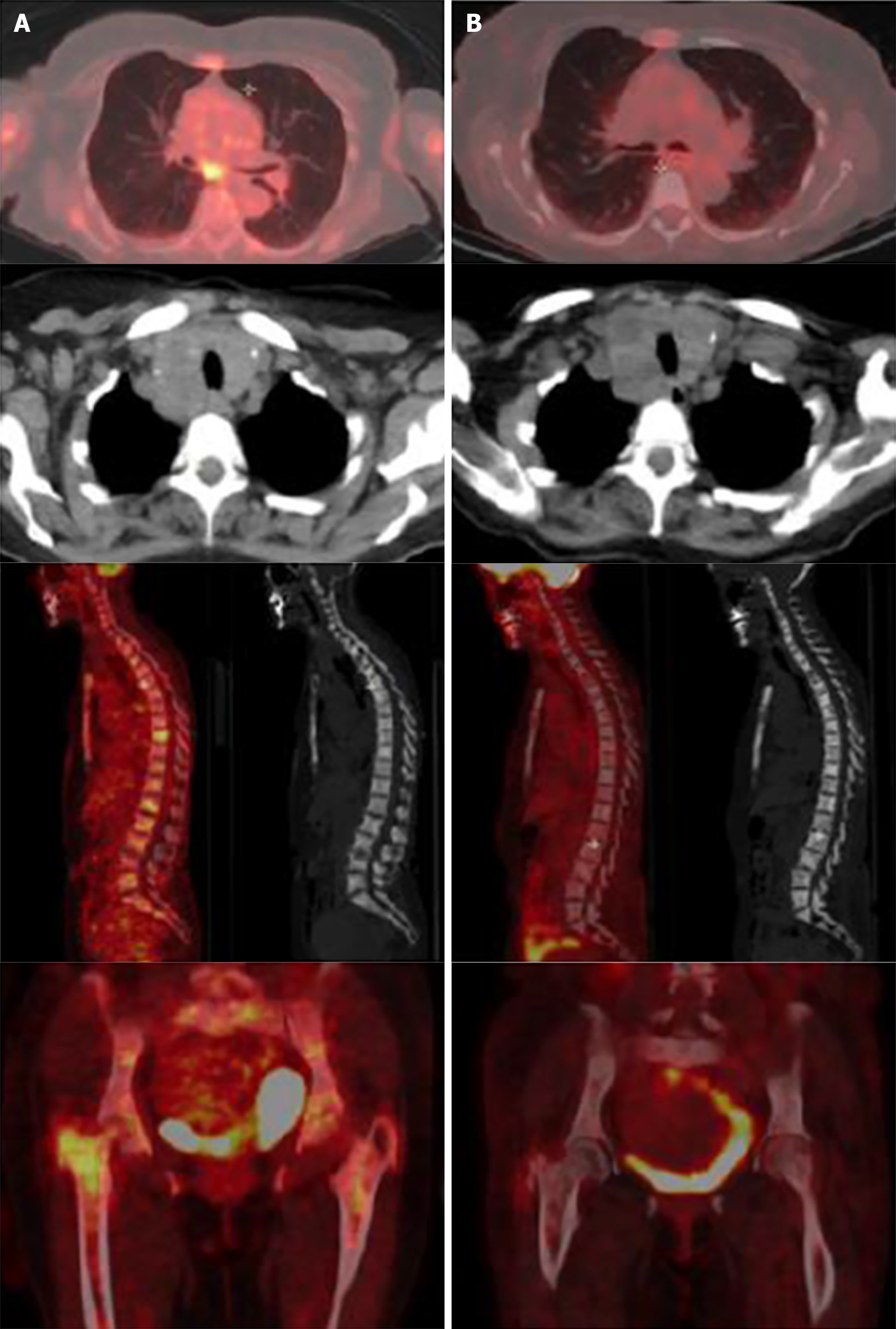
The PET scan performed 4 mo after the initiation of crizotinib showed a complete metabolic response with no thyroid, pulmonary, nodal or visceral hypermetabolic spot but with multiple condensing bone metastases on most of the skeleton without significant hypermetabolism (scar lesions) (Figure 2). The patient presented a complete response according to RECIST criteria.
Two months later, the patient experienced a loss of balance that caused multiple falling episodes and a frontal syndrome with logorrhea and disinhibition. The cerebral scan revealed multiple supra and infra tentorial nodules suggestive of brain metastases for which whole brain radiotherapy (WBRT) has been indicated. Crizotinib was stopped during WBRT delivering 30 Gy in 10 fractions that resulted in a stability of the cerebral lesions. However, the patient’s condition stayed fragile with remaining walking and behavioral disorders, which prevented us from starting a new line of anticancer treatment. The patient died after 3 mo of palliative care.
To the best of our knowledge, we report herein the first complete metabolic response in an ALK-rearranged oncocytic tumor of the thyroid (Figure 3).
This new approach is interesting, as the current recommended treatment for disseminated oncocytic tumors is radioiodine, which is associated with a lower response rate than other thyroid carcinomas[19,20]. Oncocytic carcinoma is a rare type of cancer with a higher postoperative recurrence rate (28% vs 11%, P < 0.0001) and a poorer cause-specific mortality rate than papillary thyroid carcinoma (1.7% vs 4%, P < 0.0005)[16,19]. The cumulative rate of recurrence or death within 5 years after initial diagnosis of a stage III–IV disease reaches 74% in women and 91% in men, respectively[21].
Recently, understanding of the molecular pathogenesis of thyroid cancer leads to increased interest in tyrosine kinase-targeted therapy. Mutations in several oncogenes and tumor suppressor genes have been described, including the BRAF, RAS, PIK3CA, and APC genes[22,23]. In 2014, a patient diagnosed with anaplastic thyroid cancer showing an ALK rearrangement experienced an excellent response rate to crizotinib, reaching up to a 90% reduction in tumor size across all pulmonary lesions[18]. Oncocytic tumors with an ALK rearrangement have not been described extensively in the literature. Our rationale for using crizotinib in this situation was based on the following arguments.
First, disseminated oncocytic thyroid cancers are known to have poor prognoses and are likely less avid to radioactive iodine compared to papillary thyroid cancer[10,20]. The SEER study from 1988 to 2009 identified 3311 patients with oncocytic tumors who were less likely to undergo total thyroidectomy than patients with other thyroid carcinomas (P = 0.028). Absence of surgery and an existence of metastatic disease were factors independently associated with a worse prognosis in this population (hazard ratio of > 3.0, 95%CI of 1.89-6.38)[10]. A study published in 2003 showed that radioactive iodine therapy may confer a survival benefit when it is used for adjuvant ablation therapy but not when residual or metastatic disease is present. Survival benefits for the use of extensive surgery, external beam radiation therapy, or chemotherapy by doxorubicin, cisplatin or the two drugs together could not be demonstrated[24].
Second, we followed the “AcSé crizotinib” phase II study, promoted by the French National Cancer Institute, which encouraged secure access to innovative treatments for 235 patients with malignant disease harboring a molecular target such as ALK, MET or ROS1[25].
Third, crizotinib is a specific ALK inhibitor recommended at that time as a first-line treatment in NSCLC with ALK rearrangement[14]. Finally, this targeted therapy showed unexpected responses in 2 cases of aggressive papillary and anaplastic thyroid cancers, respectively[17,18].
Despite this encouraging response, this case report presents some limitations. On this unique case, there is a lack of evidence to generalize and no possibility to establish cause-effect relationship.
Biology-driven decisions should be enhanced, especially for patients suffering from rare tumors with bad clinical prognosis who could benefit from the screening of oncogenic molecular targets. Further studies are warranted to confirm our findings, with comparison with standard of care treatment.
In this case report, the patient developed secondary cerebral lesions after 7 mo under crizotinib. A study showed that crizotinib was associated with systemic and intracranial disease control in patients with ALK-rearranged NSCLC who were ALK inhibitor-naïve and had asymptomatic brain metastases (systemic disease control: 63%, 95%CI: 54%, 72%, intracranial disease control; 56%, 95%CI: 46%, 66% at 12 wk). However, progression of pre-existing or development of new intracranial lesions while receiving therapy was a common manifestation of acquired resistance to crizotinib. Twenty percent of the patients without baseline brain metastases and whose tumor progressed under crizotinib were finally diagnosed with brain metastases[26,27]. A new approach could be undertaken with alectinib, which recently showed better intracranial responsiveness[28], superior efficacy and lower toxicity[29,30] compared to crizotinib (central nervous system response rate for alectinib of 81% with 95%CI: 58-95 and for crizotinib of 50% with 95%CI: 28-72) in primary treatment of ALK-positive NSCLCs.
This case reports unexpected and dramatic efficacy of crizotinib in a thyroid tumor with an ALK rearrangement. A comparison of crizotinib with standard radio iodine as a first therapeutic option in this indication still needs to be assessed. More studies are required to confirm clinical treatments for this rare and aggressive disease.
We thank Mrs. Fabienne Vaillant for her help in editing the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Oncology
Country/Territory of origin: France
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Koizumi T, Yung TS S-Editor: Dou Y L-Editor: A E-Editor: Li X
| 1. | Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9-29. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8789] [Cited by in F6Publishing: 9418] [Article Influence: 941.8] [Reference Citation Analysis (0)] |
| 2. | Kakudo K, Bychkov A, Bai Y, Li Y, Liu Z, Jung CK. The new 4th edition World Health Organization classification for thyroid tumors, Asian perspectives. Pathol Int. 2018;68:641-664. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 3. | Erickson LA, Jin L, Goellner JR, Lohse C, Pankratz VS, Zukerberg LR, Thompson GB, van Heerden JA, Grant CS, Lloyd RV. Pathologic features, proliferative activity, and cyclin D1 expression in Hurthle cell neoplasms of the thyroid. Mod Pathol. 2000;13:186-192. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Hundahl SA, Fleming ID, Fremgen AM, Menck HR. A National Cancer Data Base report on 53,856 cases of thyroid carcinoma treated in the U.S., 1985-1995 [see commetns]. Cancer. 1998;83:2638-2648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 5. | Carcangiu ML, Bianchi S, Savino D, Voynick IM, Rosai J. Follicular Hurthle cell tumors of the thyroid gland. Cancer. 1991;68:1944-1953. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 6. | Yutan E, Clark OH. Hürthle cell carcinoma. Curr Treat Options Oncol. 2001;2:331-335. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Sugino K, Kameyama K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Uruno T, Akaishi J, Suzuki A, Masaki C, Ito K. Does Hürthle cell carcinoma of the thyroid have a poorer prognosis than ordinary follicular thyroid carcinoma? Ann Surg Oncol. 2013;20:2944-2950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Kim WG, Kim TY, Kim TH, Jang HW, Jo YS, Park YJ, Kim SW, Kim WB, Shong M, Park DJ, Chung JH, Shong YK, Cho BY. Follicular and Hurthle cell carcinoma of the thyroid in iodine-sufficient area: retrospective analysis of Korean multicenter data. Korean J Intern Med. 2014;29:325-333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Hong JH, Yi HS, Yi S, Kim HW, Lee J, Kim KS. Implications of oncocytic change in papillary thyroid cancer. Clin Endocrinol (Oxf). 2016;85:797-804. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Goffredo P, Roman SA, Sosa JA. Hurthle cell carcinoma: a population-level analysis of 3311 patients. Cancer. 2013;119:504-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Mano H. ALKoma: a cancer subtype with a shared target. Cancer Discov. 2012;2:495-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 135] [Cited by in F6Publishing: 142] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 12. | Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, Watanabe H, Kurashina K, Hatanaka H, Bando M, Ohno S, Ishikawa Y, Aburatani H, Niki T, Sohara Y, Sugiyama Y, Mano H. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448:561-566. [PubMed] [Cited in This Article: ] |
| 13. | Hallberg B, Palmer RH. Mechanistic insight into ALK receptor tyrosine kinase in human cancer biology. Nat Rev Cancer. 2013;13:685-700. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 425] [Cited by in F6Publishing: 433] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 14. | Solomon BJ, Mok T, Kim DW, Wu YL, Nakagawa K, Mekhail T, Felip E, Cappuzzo F, Paolini J, Usari T, Iyer S, Reisman A, Wilner KD, Tursi J, Blackhall F; PROFILE 1014 Investigators. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371:2167-2177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2160] [Cited by in F6Publishing: 2314] [Article Influence: 231.4] [Reference Citation Analysis (0)] |
| 15. | Shaw AT, Kim DW, Nakagawa K, Seto T, Crinó L, Ahn MJ, De Pas T, Besse B, Solomon BJ, Blackhall F, Wu YL, Thomas M, O'Byrne KJ, Moro-Sibilot D, Camidge DR, Mok T, Hirsh V, Riely GJ, Iyer S, Tassell V, Polli A, Wilner KD, Jänne PA. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368:2385-2394. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2534] [Cited by in F6Publishing: 2584] [Article Influence: 234.9] [Reference Citation Analysis (0)] |
| 16. | Kruczynski A, Delsol G, Laurent C, Brousset P, Lamant L. Anaplastic lymphoma kinase as a therapeutic target. Expert Opin Ther Targets. 2012;16:1127-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Demeure MJ, Aziz M, Rosenberg R, Gurley SD, Bussey KJ, Carpten JD. Whole-genome sequencing of an aggressive BRAF wild-type papillary thyroid cancer identified EML4-ALK translocation as a therapeutic target. World J Surg. 2014;38:1296-1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Godbert Y, Henriques de Figueiredo B, Bonichon F, Chibon F, Hostein I, Pérot G, Dupin C, Daubech A, Belleannée G, Gros A, Italiano A, Soubeyran I. Remarkable Response to Crizotinib in Woman With Anaplastic Lymphoma Kinase-Rearranged Anaplastic Thyroid Carcinoma. J Clin Oncol. 2015;33:e84-e87. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 19. | Herrera MF, Hay ID, Wu PS, Goellner JR, Ryan JJ, Ebersold JR, Bergstralh EJ, Grant CS. Hürthle cell (oxyphilic) papillary thyroid carcinoma: a variant with more aggressive biologic behavior. World J Surg. 1992;16:669-74; discussion 774-5. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 20. | Jillard CL, Youngwirth L, Scheri RP, Roman S, Sosa JA. Radioactive Iodine Treatment Is Associated with Improved Survival for Patients with Hürthle Cell Carcinoma. Thyroid. 2016;26:959-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Chindris AM, Casler JD, Bernet VJ, Rivera M, Thomas C, Kachergus JM, Necela BM, Hay ID, Westphal SA, Grant CS, Thompson GB, Schlinkert RT, Thompson EA, Smallridge RC. Clinical and molecular features of Hürthle cell carcinoma of the thyroid. J Clin Endocrinol Metab. 2015;100:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 22. | Kloos RT, Ringel MD, Knopp MV, Hall NC, King M, Stevens R, Liang J, Wakely PE, Vasko VV, Saji M, Rittenberry J, Wei L, Arbogast D, Collamore M, Wright JJ, Grever M, Shah MH. Phase II trial of sorafenib in metastatic thyroid cancer. J Clin Oncol. 2009;27:1675-1684. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 396] [Cited by in F6Publishing: 385] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 23. | Pennell NA, Daniels GH, Haddad RI, Ross DS, Evans T, Wirth LJ, Fidias PH, Temel JS, Gurubhagavatula S, Heist RS, Clark JR, Lynch TJ. A phase II study of gefitinib in patients with advanced thyroid cancer. Thyroid. 2008;18:317-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 24. | Lopez-Penabad L, Chiu AC, Hoff AO, Schultz P, Gaztambide S, Ordoñez NG, Sherman SI. Prognostic factors in patients with Hürthle cell neoplasms of the thyroid. Cancer. 2003;97:1186-1194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 25. | Vassal G, Cozic N, Ferretti G, Taieb S, Houot R, Brugieres L, Aparicio T, Blay JY, Bieche I, Lantuejoul S, Mahier - Ait Oukhatar C, Hoog Labouret N, Moro-Sibilot D. Biomarker-driven access to crizotinib in ALK, MET, or ROS1 positive (+) malignancies in adults and children: The French National AcSé Program. J Clin Oncol. 2018;36, 2504–2504. [DOI] [Cited in This Article: ] [Cited by in Crossref: 2] [Cited by in F6Publishing: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Allouche M. ALK is a novel dependence receptor: potential implications in development and cancer. Cell Cycle. 2007;6:1533-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Costa DB, Shaw AT, Ou SH, Solomon BJ, Riely GJ, Ahn MJ, Zhou C, Shreeve SM, Selaru P, Polli A, Schnell P, Wilner KD, Wiltshire R, Camidge DR, Crinò L. Clinical Experience With Crizotinib in Patients With Advanced ALK-Rearranged Non-Small-Cell Lung Cancer and Brain Metastases. J Clin Oncol. 2015;33:1881-1888. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 411] [Cited by in F6Publishing: 483] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 28. | Paik J, Dhillon S. Alectinib: A Review in Advanced, ALK-Positive NSCLC. Drugs. 2018;78:1247-1257. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 29. | Peters S, Camidge DR, Shaw AT, Gadgeel S, Ahn JS, Kim DW, Ou SI, Pérol M, Dziadziuszko R, Rosell R, Zeaiter A, Mitry E, Golding S, Balas B, Noe J, Morcos PN, Mok T; ALEX Trial Investigators. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:829-838. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1385] [Cited by in F6Publishing: 1509] [Article Influence: 215.6] [Reference Citation Analysis (0)] |
| 30. | Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, Hotta K, Watanabe S, Goto K, Satouchi M, Kozuki T, Shukuya T, Nakagawa K, Mitsudomi T, Yamamoto N, Asakawa T, Asabe R, Tanaka T, Tamura T. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390:29-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 555] [Cited by in F6Publishing: 621] [Article Influence: 88.7] [Reference Citation Analysis (0)] |