Published online Oct 24, 2021. doi: 10.5306/wjco.v12.i10.845
Peer-review started: March 1, 2021
First decision: July 6, 2021
Revised: July 21, 2021
Accepted: September 16, 2021
Article in press: September 16, 2021
Published online: October 24, 2021
Cancer is the second leading cause of death worldwide and epidemiological projections predict growing cancer mortality rates in the next decades. Cancer has a close relationship with the immune system and, although Th17 cells are known to play roles in the immune response against microorganisms and in autoimmunity, studies have emphasized their roles in cancer pathogenesis. The Th17 immune response profile is involved in several types of cancer including urogenital, respiratory, gastrointestinal, and skin cancers. This type of immune response exerts pro and antitumor functions through several mechanisms, depending on the context of each tumor, including the protumor angiogenesis and exhaustion of T cells and the antitumor recruitment of T cells and neutrophils to the tumor microenvironment. Among other factors, the paradoxical behavior of Th17 cells in this setting has been attributed to its plasticity potential, which makes possible their conversion into other types of T cells such as Th17/Treg and Th17/Th1 cells. Interleukin (IL)-17 stands out among Th17-related cytokines since it modulates pathways and interacts with other cell profiles in the tumor microenvironment, which allow Th17 cells to prevail in tumors. Moreover, the IL-17 is able to mediate pro and antitumor processes that influence the development and progression of various cancers, being associated with variable clinical outcomes. The understanding of the relationship between the Th17 immune response and cancer as well as the singularities of carcinogenic processes in each type of tumor is crucial for the identification of new therapeutic targets.
Core Tip: Cancer is still an important cause of death worldwide. Its development and progression are intimately related to the host immune response. In that context, the Th17 profile plays crucial roles in the pathogenesis of several cancers, promoting antitumor and protumor mechanisms. This study reviews the interactions occurring between Th17 responses and cancer.
- Citation: Marques HS, de Brito BB, da Silva FAF, Santos MLC, de Souza JCB, Correia TML, Lopes LW, Neres NSM, Dórea RSDM, Dantas ACS, Morbeck LLB, Lima IS, de Almeida AA, Dias MRJ, de Melo FF. Relationship between Th17 immune response and cancer. World J Clin Oncol 2021; 12(10): 845-867
- URL: https://www.wjgnet.com/2218-4333/full/v12/i10/845.htm
- DOI: https://dx.doi.org/10.5306/wjco.v12.i10.845
Cancer represents the second leading cause of death worldwide and has been responsible for 8.97 million deaths from 2000 to 2016 according to the World Health Organization (WHO). The five most lethal malignancy types are lung, liver, gastric, breast, and colon cancers. Four out of the five aforementioned cancers are among the 20 main causes of death in the world and epidemiological studies predict a tendency of increasing mortality rates associated with these diseases in the next 40 years[1]. The knowledge on the relationship between cancer and the immune system was limited for a long time due to the tumor capacity of evading immune response through various mechanisms[2]. However, current evidence has emphasized that CD4+ T cells are intimately associated with processes that unleash cancer as well as with the responses against the malignant cells through the detection of foreign antigens. These cells interact with each other and with other immune system components by releasing cytokines that are able to potentialize or suppress mechanisms in the tumor microenvironment, playing protumor and antitumor roles in that context[3].
Th17 cells is a type of T cell and its main related cytokine is the interleukin (IL)-17, which plays roles in the expression of other cytokines, including IL-6, IL-21, IL-22, interferon (IFN)-γ, and IL-10[4]. The Th17 cells are associated mainly with the immune response against bacteria and fungi, and also perform remarkable contributions in the promotion of inflammation and autoimmunity[5-7]. Moreover, the Th17 profile plays controversial roles in the tumor immunity and can be associated both to unfavorable and favorable outcomes[8]. The duality of Th17 cells in the setting of tumor immunity is potentialized by their plasticity, since they are able to switch to a Th1 phenotype, performing antitumor activities, and they are also capable of expressing a Treg phenotype, which can exert pro and antitumor activities, depending on the context of the immune response[9,10]. The Th17 cells have a peculiar relationship with the tumor microenvironment and use various molecular processes such as the induction and recruitment of further Th17 cells to maintain tumor infiltration[11-14]. Moreover, these cells play important roles in the growing and development of cancerous cells[15]. In that context, the mechanisms performed by Th17 cells that favor or impair tumor progression are complex and depend mainly on the type of cancer. These cells induce some mechanisms with a high carcinogenic potential such as angiogenesis, whereas they can also promote the recruitment of immune system cells to the tumor microenvironment and the activation of effector CD8+ T cells, promoting antitumor activities[16].
The understanding of the mechanisms developed by Th17 cells to modulate the tumor microenvironment and to interact with other cells is a way to identify potential therapeutic targets for various types of cancer that are associated with cellular activities related to that immune profile. Those cells interact in interesting manners with various interleukins and with cancer stem cells (CSCs)[17]. In addition, the relationship between Th17 cells and CSCs can be considered as bidirectional, with mutual modulation mechanisms[18-20]. This paper aims to review the role played by the Th17 response in various types of cancer, describing its presence in the tumor microenvironment and comparing the repercussions related to this immune profile in cancers, and to approach potential therapeutic targets associated with the immune system mechanisms related to Th17 cells.
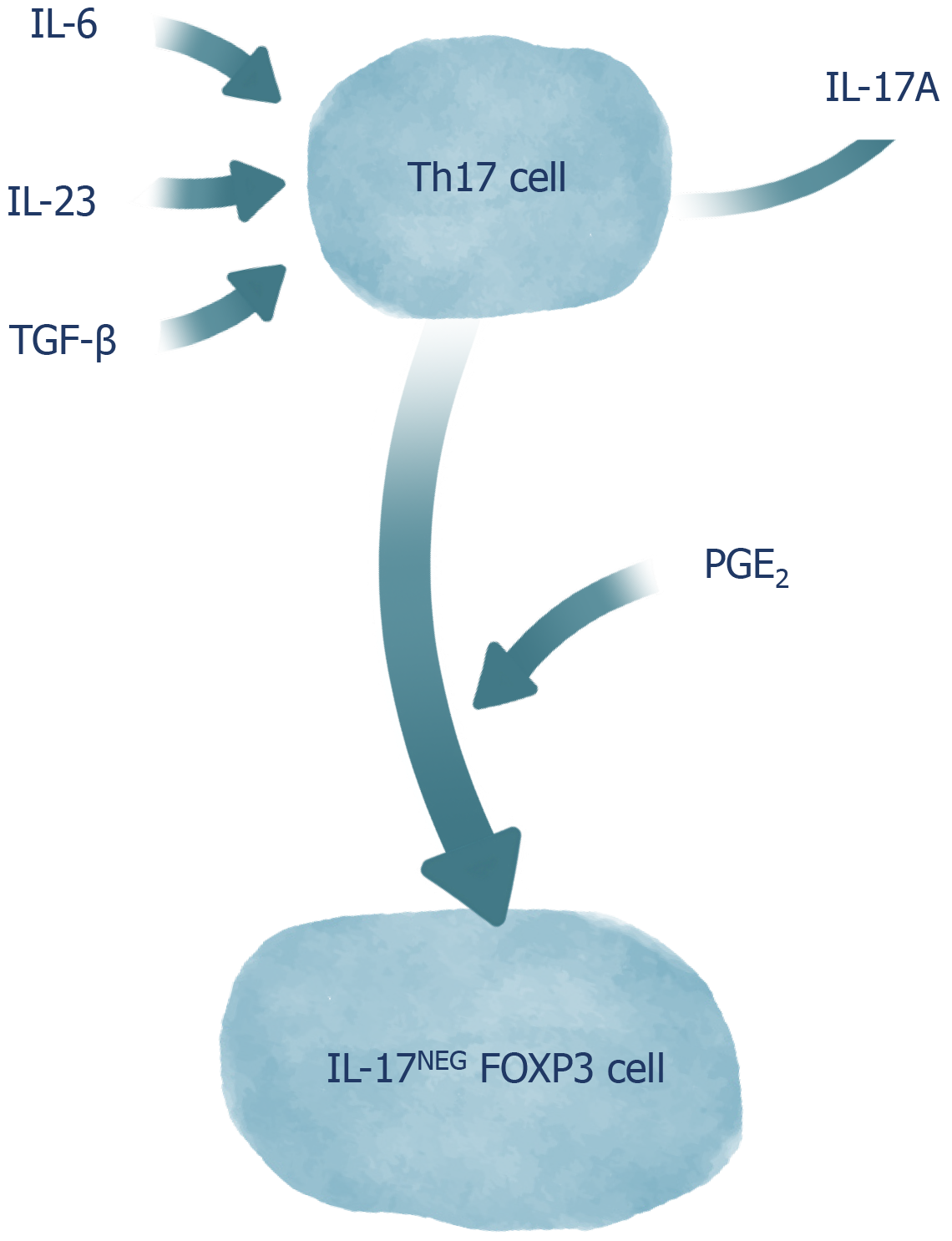
CD4+ T cells can play proinflammatory and regulating roles in the immune response. The classic differentiation of CD4+ T cells into two sets of cells with patterns of cytokine secretion and distinct functions, known as Th1 cells and Th2 cells, has changed with the discovery of a new set of cells known as Th17 cells[21]. Th17 cells express the RORγt (Retinoic-acid-receptor-related orphan nuclear receptor gamma), which is a molecular determinant for its polarization through IL-17A expression[22,23]. In rodents, these cells seem to have the same precursor as Foxp3+ Treg cells, since naive CD4+ T cells stimulated only by transforming growth factor (TGF)-β, convert into Treg cells. On the other hand, TGF-β and IL-6 together induce the emergence of Th17 cells. A study using splenocytes from mice found that an environment containing TGF-β predisposed the emergence of ex-Th17 Foxp3 cells, and simultaneous TGF-β and IL-6 stimuli led to enhanced production of IL-17Foxp3++Neg cells. In addition, that study showed that TGF-β, IL-6, and IL-23 together induced an increase in IL-17A release by Th17 cells[24]. IL-23, in its turn, plays the role of maintaining and expanding these cells. In humans, a relationship between Th17 cells and Th1 cells is evident. Naive CD4+ T cells in the presence of IL-23 and IL-1β positively regulate RORγt, T-bet, IL-23R, and IL-12R. When the two aforementioned cytokines are expressed, IL-17 is produced alone or in combination with INF-γ. TGF-β inhibits the development of both Th1 cells and Th2 cells, and is not essential for the development or inhibition of Th17 cells; therefore, it indirectly favors the expansion of the latter[25,26]. The main function of Th17 cells is to contribute to the immune response against extracellular pathogens during infectious processes, but they are also suggested to play an important role in the pathogenesis of autoimmune and inflammatory diseases, as well as in acute graft-versus-host disease[5-7]. Th17 cells activate neutrophils, stimulate the emergence of CXCL chemokines and MUC5AC, the production of MUC5B mucins by bronchial epithelial cells, the expression of beta defensin-2 and CCL20 by lung epithelial cells, and contribute to the migration and activation of macrophages[7].
The IL-17 family is made up of six different cytokines: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E (IL-25), and IL-17F. IL-17 cytokines have proinflammatory properties, are expressed in various parts of the body, and signals by interacting with their transmembrane receptors. Five receptors from the IL-17 family have been identified: IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE. In addition to Th17 Lymphocytes, CD8+ T cells, macrophages, and γδ T cells produce IL-17. These cells also express IL-23R and secrete IL-21 and IL-22. Among all IL-17 cytokines, IL-17F has the highest degree of conservation with the main cytokine in the family, IL-17A[27]. The IL-17 induces neutrophil recruitment and production of proinflammatory mediators, such as IL-1, IL-8, metalloproteinases 1 and 13, and prostaglandin E2[28,29].
Th17 cells can also produce IL-22. This cytokine is a member of the IL-10 family, and the IL-22R and IL-10R2 receptors have been identified as heterodimeric receptors mediating IL-22 signaling. Whereas IL-10R is ubiquitously expressed, IL-22R is restricted to cells harbored in tissues[30]. IL-22 targets epithelial and non-hematopoietic stromal cells and can promote cell proliferation, playing a role in tissue regeneration. In addition, it regulates host defenses on barrier surfaces. However, IL-22 has also been associated with the development of several diseases involving inflammatory mechanisms[31].
The inflammatory effects produced by IL-17 include stimulus for secretion of IL-6 by human fibroblasts and increased expression of the intercellular adhesion molecule-1[32]. In addition, Th17 cells that co-produce IL-21 regulate B cell responses, induce differentiation of plasma cells, and lead to the formation of antibodies[33]. In that context, the role of Th17 cells as key promoters of inflammation in various pathophysiological contexts, including cancer, has been investigated[8]. Of note, Th17 cells have already been identified in various types of human tumors[8], such as melanoma, ovarian cancer, colorectal cancer, and lung cancer[34,35].
Despite the advances in the knowledge about the roles of Th17 cells in complex biological contexts, many interactions involving these cells remain unknown mainly due to their plasticity[35]. The differentiation of T cells is no longer considered as linear and irreversible, since evidence has shown that populations of differentiated CD4+ T cells can alter the spectrum of cytokines produced and, thus, the outcomes they promote[36]. The plasticity of Th17 cells stands out among the other T cells due to its high level of complexity, involving cytokine-dependent and -independent events, and due to the variability of functional phenotypes that can be adopted by the cells[37]. Th17 cells can play roles that are often heterogeneous, depending on the environmental conditions they are found in. Phenotypes similar to Th1 cells are expressed by Th17 cells in inflammatory environments, with a remarkable production of IFN-γ, contributing as an immunological support against fungal and extracellular bacterial infections, but also for intestinal inflammation observed in colitis, when the activity of these cells is not properly controlled[38-40]. The transfer of antigen-specific Th17 cells to a host has shown that Th17 cells can assume the phenotype of a Th2 cell in infections by Nippostrongilus brasiliensis[41]. This Th17/Th2 conversion has also been identified in the peripheral blood of patients with asthma, whose Th17/Th2 cells secreted cytokines from both profiles (Il-17, IL-22, IL-4, and IL-5), which evidences the pathogenic potential of these cells in the induction of intense inflammatory infiltrates[42]. In addition to their pro-inflammatory role, Th17 cells are also able to adopt a phenotype similar to that of Treg cells. In fact, both the conversion of Treg cells into Th17 cells and the conversion of Th17 cells into Treg cells are described by previous studies, possibly because both cell types share differentiation characteristics, given the participation of TGF-β in inducing the differentiation of both Treg and Th17 cells[43]. Although TGF-β, IL-6, and IL-23 alone tend to promote IL-17A release by Th17 cells, the presence of Prostalglandins E2 (PGE2) makes the stimuli with these cytokines result in the conversion of Th17 cells into regulatory IL-17AnegFoxp3 cells, which demonstrates the importance of the PGE2 in the transdifferentiation of Th17 cells along with TGF-β[24]. Although it presents a certain stability, the aforementioned process has been shown to be bidirectional and, in the presence of Th17-polarizing cytokines, Th17-derived Treg cells can reassume their original Th17 cells phenotype (Figure 1)[9]. Moreover, in response to IL-27, Th17 cells acquire a phenotype similar to that observed in TR1 cells via activation of the Blimp-1 factor, which results in the secretion of cytokines such as IL-10[44].
Although advances have been achieved in the study of Th17 responses, the knowledge regarding its roles in the immune system is still limited. Moreover, the tumor microenvironment has been broadly studied because it is directly associated with cancer development and progression. The understanding on how the immune system cells behave in the aforementioned microenvironment and how this environment is influenced by those cells make possible a broader comprehension about cancer and the therapeutic possibilities against this disease[45]. The participation of Th17 cells in the different types of cancer is paradoxical, since they can play antitumor and protumor roles[8]. It is believed that the Th17 cells prevail in the tumor microenvironment through some mechanisms, including: (1) The induction of T cells involving the TGF-β1 and IL-6. Signal transducer and activator of transcription 3 (STAT3)[11]; (2) Recruitment of Th17 cells dependent on various chemokines including CCL20, CCL17, CCL22, MIF, RANTES, and MCP1[12-15]; (3) Conversion from other cell types[15]; and (4) Th17 cell polarization through cytokines such as IL-1β and IL-13 that are produced in the tumor environment by specific myeloid cells[46]. In addition, the tumor microenvironment has antigens and metabolites that are able to suppress CD4+ T cells, which start producing co-inhibitory and less effective molecules[47]. However, the Th17 cells from the tumor microenvironment seem to have an enhanced resistance to dysfunctionality because they present less exhaustion markers than other T cells as well as more CCR7, Lef1, and TCF7 markers[48,49].
An important aspect regarding Th17 cells in cancer settings is the aforementioned plasticity potential through their transformation into Treg cells, which occurs with TCR engagement leading to the expression of Foxp3 and subsequent imunossupressive roles by those cells. Of note, IL17+Foxp3+ T cells have been associated with the emergence of CSCs and with the inhibition of tumor-specific T CD8+ cells in colorectal cancer. A study showed that IL17+Foxp3+ T cells induce the expression of markers such as CD133, CD44s, CD166, EpCAM, and ALDH1 in bone marrow-derived mononuclear cells and promoted their conversion into cancer-initiating cells[50,51].
Conversely, the production of IL-6 in mice with melanoma led to the conversion of Treg cells into Th17 cells, which resulted in the promotion of the activation of CD8+ T cells and reduction in tumor growth[52]. A recent study on CpG (ODNs)/CpG 1826 oligodeoxynucleotides demonstrated the potential of these cells in the inhibition of Treg cells and in the stimulation of Th17 cells, extending survival among mice with leukemia[53]. Another study observed that the Treg/Th17 cell ratio was higher among patients with oral squamous cell carcinoma (OSCC) than in controls, suggesting that these cells are involved in the progression of OSCC and have the potential to be used as a prognostic indicator[54]. Considering the importance of the Th17/Treg axis in cancer-related immune response and inflammation, a study assessed the metabolic features involved in that setting. The researchers found that Th17 cells are more dependent on the synthesis of fatty acids than Treg cells, which primarily perform the oxidation of fatty acids to keep their energetic homeostasis. This metabolic differences can make the manipulation of the Th17/Treg axis possible for new therapeutic alternatives against neoplasms[55].
Interestingly, the plasticity of Th17 cells has also been observed with their conversion into Th1 Lymphocytes, exerting antitumor effects[10,56]. It is not well known if that transformation occurs inside the tumor microenvironment or if Th17/Th1 cells are recruited to the tumor microenvironment[57,58]. Muranski et al[48] reported that the Th17 cells polarization leads to the production of Th1 cells-related molecules such as INF-γ and T-bet, which are associated with remarkable antitumor activities[48].
The mechanisms taking place in tumor-related Th17 responses are various and depend on the type of cancer. A study observed that the transference of Th17 cells implied in the recruitment of CD4+ T cells, CD8+ T cells, and DCs for the tumor microenvironment. Additionally, when in contact with tumor antigens, Th17 cells acquired dendritic cell MCHI-peptide complexes and, through MHCI-TCR interaction and IL-2 release, there was an activation of CD8+ T cells, reinforcing the role of Th17 cells in tumor immunity[16]. Studies have verified that IL-17 may indirectly potentialize the functions of cytotoxic T lymphocytes by stimulating the expression of IL-6 and IL-12, leading to antitumor effects[59]. The Il-17 acts in the recruitment and expansion of neutrophils that are essential to the destruction of tumor cells as well as contributes to the expression of various proinflammatory molecules including IL-1β, IL-6, tumor necrosis factor (TNF)-α, PGE2, CXCL1, CXCL5, CXCL8, and GMCSF[60-65]. On the other hand, Wang et al[54] demonstrated that Th17 cells play protumor roles since IL-17 induces the expression of IL-6, which is responsible for activating oncogenic signal transducers and STAT3. The STAT3 aids in the promotion of tumor growth through the regulation of pro-angiogenic genes[66]. Differently from the IL-17 dualism, the IL-22 has shown to be a protumor interleukin that, through STAT3, is involved in the development of tumor cells[67-69].
The Th17 cytokines, especially IL-17, play important roles in the promotion of tumor angiogenesis[70]. Studies have described that the IL-17 induces the expression of vascular endothelial growth factor (VEGF), and that cytokine seems to be associated with a higher tumor vascular density[71]. In addition, the Il-17 induced the expression of angionenic chemokines including CXCL-1, CXCL-5, CXCL-6, and CXCL-8, leading to an increased angiogenic potential among immunocompromised rats with non-small cell lung cancer (NSCLC)[72]. Another study described that IL-17 is involved in the activation of angiogenic genes through the IL-6/STAT3 pathway[73]. The IL-22 has also been positively correlated with angiogenesis. A study suggested that it is involved in the proliferation, survival, and migration of endothelial cells. Moreover, that investigation described that IL-22 leads to vascular growth in animals[74]. Finally, a systematic review highlighted that, although there is a paradox in the behavior of the Th17 response in the different types of cancer, the expression of Th17 cells is often associated with better prognosis whereas the IL-17 is related to cancer progression[75].
Studies have described the IL-27 potential as a Th17 response inhibitor. This process occurs through the downregulation of RORγt[17]. The IL-27 is involved in the suppression of protumor cytokines such as IL-23 and IL-17[76]. A recent study described the existence of an inverse relationship between IL-27 and IL-17 as well as between IL-27 and IL-6. In patients with gastric cancer, studies have observed high concentrations of Th17 cells-related cytokines including IL-1β, IL-6, IL-17A, IL-23, and TGF-β and it is believed that this phenomenon occurs due to the low or null IL-27 Levels, since IL-27 inhibits RORγt and IL-6. This relationship has been observed in other cancers as well[77]. Additionally, another study observed that the IL-27 performed an antitumor activity through the IL-17 inhibition via RORγt among patients with small-cell lung cancer[78]. Of note, these findings suggest a therapeutic potential of the IL-27 through the inhibition of protumor Th17 cells-related mechanisms.
Interestingly, the cancerous cells inside individual tumors frequently exist in various phenotypic states. CSCs are a subpopulation of cells present in several types of cancer and that have self-renewal ability and tumorigenicity when transplanted to an animal host. Evidence on CSCs have aimed at developing a promising approach for the improvement of antitumor therapies. The CSCs are closely related to Th17 cells-related cytokines and other components in the tumor microenvironment, and they play crucial roles in the tumor progression and metastasis[79,80]. These cells can promote the differentiation of CD4+ T cells into Th17 cells through the release of soluble mediators and cell-to-cell contact[18,19]. Moreover, a study observed that the interaction between stem cells and CD4+ T cells can lead to the transformation of remaining Th17 cells through the activity of STAT3[81]. On the other hand, the IL-17 has shown to participate in the maintenance of CSCs through the action of the IL-17A receptor and the capacity of activating these cells in their quiescent state, configuring a more aggressive protumor behavior for the CSCs[20]. The recurrence of tumors after a primary treatment is still very frequent, and studies have supported that a successfull cancer therapy might be related to the elimination of CSCs[82]. In that context, the understanding of the relationship between these cells and the Th17 response in the various types of tumor can lead to new therapeutic possibilities[18].
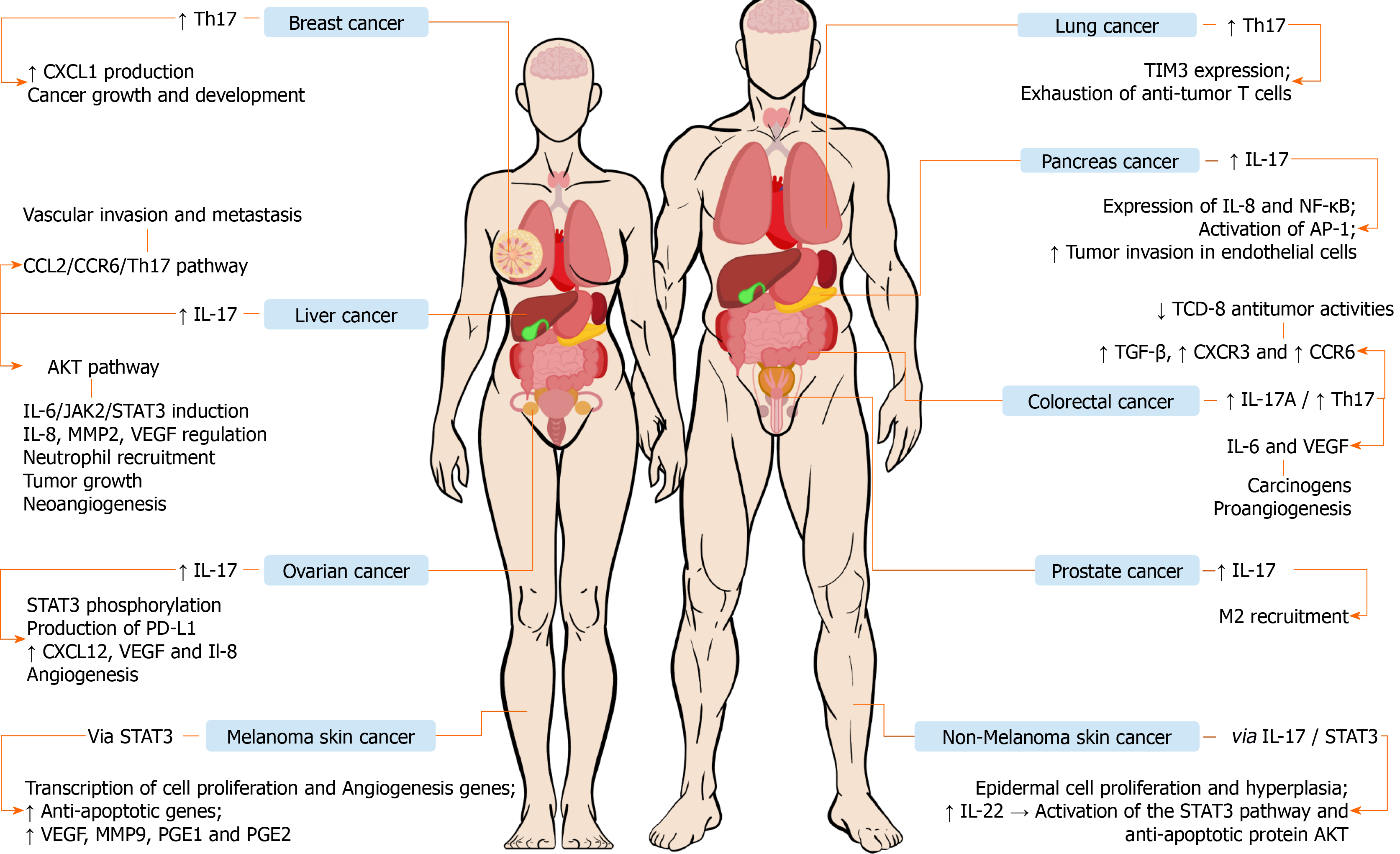
Ovarian cancer: Ovarian cancer ranks fifth in cancer deaths among women[83]. This cancer usually has a late and advanced diagnosis causing resistance to treatment[84]. Studies have emphasized the involvement of the immune system in the process of development and progression of ovarian cancer, including the interactions of immune cells in the ovarian tumor microenvironment[85,86]. The expression of IL-23 is increased and positively correlated with IL-17 in immunohistochemical analyses of ovarian cancers[87]. Studies showed that IL-17 and IL-6 induce STAT3 phosphory
Another study reported that there is an inverse relationship between Treg and Th17 cells observed in ovarian cancer and this difference leads to an antitumor response, which significantly influences survival, and the presence of these polyfunctional Th17 cells is statistically related to more favorable clinical outcomes[96]. In contrast, the significant presence of regulatory T cells is associated with worse survival[97]. Studies suggest that the responses of T cells directed to ovarian cancer may be related to the expansion of Th17 cells and may neutralize the suppression of these cells through the activity of Treg cells[97,98]. Moreover, the decrease in Th17 Lymphocytes and the increase in Treg cells was related to an increased level of TGF-β and this, in turn, is associated with metastatic processes resulting in poorer clinical prognosis[99,100]. Additionally, in vivo studies observed that a high Treg cells/Th17 cells ratio seems to predispose tumor progression because this ratio has shown to be significantly higher in epithelial ovarian cancer and peritoneal metastasis than in benign ovarian tumors and benign peritoneum[101]. It is believed that the presence of tumor-associated macrophages may induce this imbalance[102]. M2 macrophages have been shown to modulate the tumor microenvironment and to promote a relative deviation to the Treg immune profile through the release of exosomes carrying miRNAs that are often overexpressed in some types of cancer, such as ovarian cancer[103,104].
Th17 cells in ovary cancer secrete IL-21, which, along with TGF-β, can interfere in the differentiation of T cells into Th17 Lymphocytes and stimulate the transformation of Th17 cells into Treg cells. In addition, there is a regulation in the distribution of Th17 and Treg cells via CCR6 chemokine receptors to direct Th17 cells to specific sites[105,106]. In this sense, some therapeutic methods have been developed aiming at increasing the patients' survival as well as at reducing the recurrence rates. Among these methods, stand out immunotherapy approaches based on therapeutic vaccines that stimulate the expansion of specific T cells in patients with ovarian cancer, using the alpha folate receptor (Fra) as the vaccine target antigen, since this receptor is overexpressed in patients with high-grade serous ovarian cancer[97,107]. The vaccine induces stimulation of Fra-specific INF-γ+ and IL-17+ T cells[97]. Furthermore, a study showed that the Th17 profile and IFN-γ induce the production of CXCL9 and CXCL10 that promote the migration of effector cells to ovarian tumors[98,108]. The use of vaccines that stimulate the expression of Th17 cells specifically in an ovarian tumor is associated with a reduction in recurrence rates, as well as in the improvement of survival rates[97,98].
Prostate cancer: Prostate cancer is the most prevalent type of cancer among the male population[109]. Chronic inflammation has been identified as a factor that is associated with the pathogenesis of various types of cancer, including prostate cancer[109,110]. In that context, IL-17 plays an important role in the inflammatory process related to the development and progression of that cancer[111]. Studies have shown that blocking IL-17 in mice inhibits the development of prostate cancer[112]. The imbalance in the proportion of CD4+ and IL-17+ cells and CD4+ Foxp3+ T cells, responsible for regulating Treg cells in the tumor microenvironment, may lead to worsening of the inflammatory process and promote carcinogenesis[113,114]. Treg cells are involved in the suppression of antitumor immune responses[115]. Blocking PD-1 can promote antitumor activity by balancing Th1/Th2 responses and stimulating Th17 cells, as well as inhibiting Treg cells and stimulating the Th17 response[116]. In that context, the use of vaccines that inhibit the action of programmed death 1 (PD-1)/PD-L1, which make up an immunological checkpoint, has demonstrated promising results[117,118]. In prostatic tumors, IL-17 has been shown to attract M1 and M2 macrophages. M1 macrophages inhibit tumor growth, whereas the M2 cells promote tumor growth[119-121].
NSCLC and small cell lung cancer: Chronic inflammation is a crucial factor in the pathogenesis of different types of cancer, including lung cancer, which has proved to be a major public health problem affecting 1.8 million patients each year[122]. Lung cancer is the leading cause of cancer-related deaths in recent decades and can be divided into two types: Small cell lung cancer and NSCLC[123]. In addition to Th17 cells and the association with tumor survival, chemokines and their receptors related to T cell migration were examined in NSCLC cases. The high expression of CCR6 was associated with shorter disease-free survival[124]. Likewise, CCL20, a chemokine known to interact with CCR6, was elevated in the tumor compared to tumor-free lung tissue. Thus, these results suggest that CCL20/CCR6 may facilitate the infiltration of Th17 cells in the NSCLC and promote tumor progression[125]. A regulatory role for Th17 cells in the tumor microenvironment of NSCLC was found to modulate the differentiation and activation of various subsets of local T cells[126]. IL-17A is associated with the concentration of VEGF in patients with NSCLC, suggesting that IL-17A may promote angiogenesis in that tumor[127]. In addition, patients with high levels of IL-17A demonstrated shorter survival compared to those with low expression of the cytokine[128]. Studies suggest that IL-17A/Th17 cells may play a pro-tumorigenic role, as an increased number of Th17 cells are found in lung cancer[129]. Th17 cells can be generated under oncogene activation or inhibition of tumor suppressors in human and murine models[130,131]. Oncogenic NSCLC models have shown a predominant pro-tumorigenic role of IL-17A[130,61], while downregulation of IL-17A in a tumor suppressor NSCLC model has been associated with antitumor activities[131].
Studies also demonstrated that IL-17A deficiency or blockade leads to the suppression of lung metastasis in experimental tumor models. This suggests that the key cytokine IL-17 produced by lung CD4+ Th17 cells plays an important role in cancer regulation[132]. In addition, anti-IL-17A treatment in pulmonary adenocarcinoma modifies cytokine responses by lung CD4+ T cells and induces production of TNF and IFN-γ by Th1 cells at the tumor site, leading to improved antitumor immune responses and suppression of tumor growth[133]. Other studies assessed the role of IL-6, a cytokine produced by Th17 cells, in a murine model of lung adenocarcinoma and human tumors, showing that IL-6 inhibits regulatory T cells and induces Th17 cells. In vivo treatment with anti-IL-17A antibodies reduced the production of IL-6 in the airways[132]. Thus, anti-IL-17A-mediated regulatory T responses can induce increased anti-tumor immune responses[134]. The main transcription factors of Foxp3 regulatory T cells, as well as the main transcription factors of Th17 in human cells, were increased in lung tumor tissues, resulting in a parallel local expansion of Th17 and Treg responses. These findings suggest a potential direct relationship between both T cell lines in lung cancer[135]. T-cell immunoglobulin-3 (TIM-3) expressed in Th1, Th17, and CD8 T cells, but not in Th2 cells, has already been described as a critical component of cell-mediated immunity against cancer[136]. Recent studies have supported an important role in the exhaustion of TIM-3 T cells in lung cancer[137]. TIM-3 as well as PD-1, another T cell exhaustion marker, are co-expressed in TCD8 in mice with lung tumors, exhibiting depleted phenotype as defined by the failure to proliferate and produce IL-2, TNF, and IFN-γ[137]. Blocking the TIM-3 and PD-1 pathways is more effective in controlling tumor growth than targeting either pathway alone, suggesting that these two pathways work synergistically in establishing T-cell exhaustion[138].
Gastric cancer: Gastric cancer is the fifth most common and the third most lethal malignancy around the world. The development of this cancer is mainly linked to factors such as the chronic inflammation induced by Helicobacter pylori infection and age[139]. It is well established that the immune response of the host infected by Helicobacter pylori leads to the activation of the IL-23 pathway, which induces the differentiation of CD4+ naive T cells into Th17 cells. This immunological pathway is called the IL-23/IL-17 axis[140]. The IL-17 acts in the endothelium, monocytes, and gastric epithelial cells producing TNF-α, IL-1, IL-6, and IL-8 that stimulate the recruitment of neutrophils to the inflammatory site[140,141]. In an interesting study, a significant increase in the levels of IL-17 was observed in the serum of patients with gastric cancer. In addition, this increase in IL-17 expression was associated with a high density of microvessels, which assist in the development of the tumor[142]. An important study also found high levels of plasma IL-17 in a patient with gastric cancer and, interestingly, the results showed an increase in the expression of IL-17 and RORγt in the cancer tissue. In addition, a 26-fold increase in IL-17 expression was observed among patients who had metastasized[143].
Moreover, a study found high levels of IL-6, TGF-β1, FoxP3, and IL-17 expression in gastric cancer patients, highlighting the importance of the Th17/Treg axis in this neoplasm. IL17 and IL-6 have been associated with tumor progression, and Foxp3 and TGF-β1 were mainly expressed in patients with advanced gastric cancer. These findings suggest that these molecules play a role in the tumor immune response evasion and cancer progression[144].
The induction of Th17 cell expression is very important in the pathophysiology of gastric cancer. Moreover, it is well described that IL-1, IL-6, IL-21, IL-23, and TGF-β induce the differentiation of T naïve cells into Th17 cells[145]. In this sense, Su et al[143] also observed increased levels of TGF-β and IL-21 in gastric cancer tissues[143], positively regulating Th17 cells and, consequently, IL-17 levels. Some studies suggest that treatment with anti-IL-17A monoclonal antibodies such as Secukinumab and Ixekizumab may be beneficial in gastric cancer therapy[146]. An experimental study with rats, which had tumor growth stimulated by injection of gastric cancer cells of the YTN16 type, showed an expressive regression of the tumor with a complete elimination of the cancer in 8 of 10 mice using a combination of anti-IL-17A and anti-PD-1 monoclonal antibodies[147]. Despite being experimental results, they point to new paths regarding the treatment of gastric cancer with immunotherapy.
Pancreatic cancer: Pancreatic cancer is the 14th most common cancer and the 7th that kills the most in the world[148]. Obesity, type 2 diabetes, and smoking are the main risk factors for the development of this cancer[149]. Th17 cells are important in tumor-associated inflammation, stimulating migration, invasion, and induction of angiogenic factors[150]. A recent study observed that IL-17 stimulates an important mediator of pancreatitis (REG3-β) in pancreatic cells and can activate the gp130-JAK2-STAT3-dependent signaling pathway, which results in a greater acinar-ductal metaplasia and in the development of lesions of early pancreatic intraepithelial neoplasia[151]. An interesting study pointed to a similarity between the IL-17A-IL-17RA pathway and the IL-17B-IL-17RB pathway in tumor malignancy. It was observed that IL17-B increased the expression of IL-8, Nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), and activating protein-1 (AP-1), which aid the tumor invasion in the pancreatic tissue and recruit neutrophils, lymphocytes, and endothelial cells[152]. Although advances have been achieved, the roles played by Th17 cells in the development of pancreatic cancer are not well understood. Previous investigations in mice suggest how this pathway works. However, the mechanism in humans needs further studies. Some studies have observed increased levels of Th17 cells in patients with pancreatic ductal adenocarcinoma, supporting theories about the role of this profile in the immune response involved in this cancer. He et al[153] reported that the frequency of these cells was higher in pancreatic tumor tissues when compared to other tissues of the organ (P = 0.031). Serum levels of IL-17 and IL-23 were significantly increased in pancreatic cancer patients when compared to healthy patients (P = 0.02)[153]. IL-17 blockade demonstrated an inhibition in neutrophil recruitment and increased activation of CD8+ T cells in the environment close to the tumor. In addition, a synergy was observed between the inhibition of IL-17 and PD-1 in the therapy against that cancer[154]. Interestingly, a study that aimed at understanding the Th17/Treg axis and its implications in pancreatic cancer found that the balance between these immune response profiles was altered in the peripheral blood of patients with this malignancy, with an important deviation to the Treg immune profile. Furthermore, it was observed that this relationship becomes even more accentuated as the disease progresses, supporting the hypothesis that Treg cells might impair the antitumor immune response and contribute to the tumorigenesis in pancreatic cancer[155]. Despite these results, studies for the clinical use of these agents are still scarce.
Colorectal cancer: Colorectal cancer is the third most common cancer and the fourth with the highest mortality in the world[156]. The main risk factors for the development of this cancer are age, genetics, obesity, type 2 diabetes, and inflammatory bowel disease[157]. Possibly, IL-17A acts to increase IL-6 and VEGF, which are important in carcinogenesis and pro-angiogenic, respectively[158]. Other studies indicate that Th17 cells stimulate immunosuppressive factors such as TGF-β, CXCR3, chemokine receptor CC 6 (CCR6), and IL-6. In addition, they decrease the anti-tumor activities of CD8+ T cells[159]. An interesting Chinese study observed the positive regulation of IL-17 in the progression of the adenoma-carcinoma sequence, with the levels of this cytokine being higher in cancer patients[160]. Although the pathogenesis of colorectal cancer has different pathways, in fact, IL-17 has an important role in the immune response and in the development of this cancer. The possible treatments that have been studied in this setting involve blocking IL-23, IL17, IL-17R, and RORγt nuclear receptor antagonists, which can inhibit the differentiation of Th17 cells[159]. However, clinical trials with these agents have not been developed and further studies are needed to understand the effectiveness of these drugs in patients at different stages of this disease.
Liver cancer: Liver cancer is the fifth and ninth most common cancer in men and women, respectively, and has a high mortality rate around the world[161]. Infection with hepatitis B and C viruses, alcoholic liver disease, and, possibly, non-alcoholic fatty liver disease are the main risk factors for the development of hepatocellular carcinoma[162]. A recent Chinese study found that IL-17 has a direct effect over hepatocellular carcinoma with the induction of IL-6/JAK2/STAT3 by activating the AKT pathway. This pathway positively regulated IL-8, matrix metalloproteinases 2 (MMP2), and VEGF, and neutrophil recruitment, neoangiogenesis, and tumor growth were observed in vivo[163]. Another study also indicated the influence of the CCL20/CCR6/Th17 cells pathway in promoting vascular invasion and metastasis[164]. A study identified that the high intramural expression of IL-17 and IL-17E were predictors of a worse survival prognosis (P = 0.016; P < 0.001)[165]. It has been well described that IL-17 is important in the development and prognosis of hepatocellular carcinoma. However, there are not many studies on immunological therapy, and the concomitant use of IL-17 inhibitors with conventional treatments, may be a promising alternative to be explored by new studies.
Non-melanoma skin cancer: According to the WHO, skin cancer has increased over the past 20 years. The global estimate is that there will be 2 to 3 million cases of non-melanoma cancer and 132000 melanoma cancers annually[166]. Previous animal model studies using the chemical inductors dimethylbenzanthracene (DMBA) and 12-O-tetradecanoylforbol-13-acetate (TPA) that promote the development of inflammation-associated skin cancer, have demonstrated that IL-17R-deficient mice are resistant to DMBA/TPA and that the depletion of this cytokine increases the immune control performed by CD8+ T cells and inhibits the promotion of inflammation in the skin tumor. It has also been found that TPA-induced inflammation increases the susceptibility of tumor growth and the development of tumor-specific IL-17-producing T cells and that IL-17 blockade can inhibit the progression of existing skin tumors stimulated by chemical reagents and cancel the induced inflammation that contributes to tumor growth[167]. In another study using the same experimental model, in addition to looking at the importance of IL-17-produced by CD4+ T cells in skin tumorigenesis, the researchers revealed an important regulatory role in the IL-17-STAT3 pathway in tumor development, verifying that IL-17 induces the oncogenic activity of STAT3 and promotes the proliferation of epidermal cells and hyperplasia. On the other hand the decrease in IL-17 reduced the activation of STAT3 and the unleashing of its protumor mechanisms[168]. In a research with samples of patients diagnosed with basal cell carcinoma (BCC) and squamous cell carcinoma (SCC) of the skin, the role of the cytokines IL-17 and IL-22 in the progression of both types of cancer was evaluated. The results showed that the tumor microenvironment in both carcinomas is enriched with IL-22+ and IL-17+ T cells. The IL-17, alone or along with TNF-α, was able to induce the production of IL-6 and IL-8, important for tumor progression in the analysis of SCC CAL27 cells. Another important finding is that IL-17 positively regulated NF-κB signaling, while IL-22 activated the STAT3 pathway and the anti-apoptotic AKT protein in both cell lines. Corroborating the in vitro findings, experiments with mice that received CAL27 also demonstrated that IL-17 and IL-22 increased the size of the tumor[169]. Another study with human BCC biopsies characterized by a moderate-to-severe inflammatory infiltrate evaluated the expression of the cytokines IFN-γ, IL-23, IL-17, and IL-22 and their expression during treatment with the drug imiquimod (IMQ) and with photodynamic therapy (PDT). The results showed high expression of all cytokines in cancer, and they were related to the severity of the inflammatory infiltrate. It was also possible to observe a correlation between IFN-γ and IL-17 expression, and both cytokines were expressed by CD4+ and CD8+ T cells. In addition, there was an increase in all cytokines in response to IMQ/PDT treatment[170].
Melanoma skin cancer: Melanoma is the 19th most frequent neoplasm in the world with an incidence rate of 3.3 per 100 thousand people[171]. Malignant melanoma is the type of skin cancer with the worst prognosis, having a high probability of spreading metastases when in advanced stage[172]. IL-17 promotes tumor growth by facilitating angiogenesis and the exit of tumor cells from their primary focus[172,173]. The expression of IL-17A represents an important target in the study of the escape of tumor cells from the immune system[171]. A research conducted in 2010 points to IL-17 as a useful biomarker for early diagnosis of melanoma in mucosae[174]. It is believed that the unregulated tissue inflammatory process may contribute to tumor expansion and metastasization[175-177]. A study analyzed the effect of IL-17 on the growth of melanoma, and wild type mice and IL-17-/-were inoculated with a B16 melanoma cell lineage. The results showed that melanoma growth was significantly inhibited in IL-17-/- mice compared with wild type mice. In this same study, the Hmgb1 (High-mobility group box 1) molecule and its receptor RAGE, which is associated with inflammation and cellular injury, were also analyzed. The growth of the B16 cell lineage was inhibited and the expression of IL-23 and IL-17 was significantly reduced in RAGE-/-mice, indicating that the Hmgb1-RAGE route contributes to the IL-17 expression dependent on the production of IL-23, and promotes tumor growth[176]. A previous study demonstrates that the production of IL-17 by B16 melanoma strains induces the production of IL-6 that activates the nuclear transcription factor STAT3, which acts activating the transcription of several genes related to cell proliferation and to an increase in the expression of VEGF, MMP9, and prostaglandins E1 and E2. Moreover, STAT3 increases the expression of anti-apoptotic genes[178]. Thus, it can be concluded that IL-17 promotes the growth of melanoma B16, while the blocking of IL-17 inhibits the growth of the tumor. On the other hand, Th17 cells might play a protumor role when they are converted into a hybrid phenotype expressing markers that characterize both this cell subtype and Treg cells. This conversion also occurs via the secretion of TGF-β and retinoic acid in the presence of suppressor cells derived from tumor-infiltrating myeloid cells. These findings reinforce that Th17 cells might play conflicting effects on melanoma, also contributing for its pathogenesis[179]. These studies support the involvement of Th17 cells and interleukins produced by this population of T lymphocytes, highlighting the cytokine IL-17, in the development and progression of skin cancer. In addition, there is also the possibility of new therapeutic approaches targeting this immune response profile. Finally, further studies are needed to better understand the relationship between Th17 responses and skin cancers.
Cervical cancer: Cervical cancer is the second leading cause of cancer death in young women worldwide. Virtually all cervical cancers begin with infection with high-risk human papillomavirus (HPV)[180]. Most HPV infections are eliminated naturally as a result of humoral and cellular immune responses[181]. However, the persistence of this infection induces an inflammatory response, which seems to contribute to tumor growth and disease progression, instead of inducing an effective immune response[182]. This response is partially induced by tumor cells that regulate the immune response negatively through the expression of human leukocyte antigen, which produces immunosuppressive cytokines, such as IL-10 and TGF-β, and attracts regulatory T cells[183].
A large number of Th17 cells was a factor that improved prognosis and survival in this type of cancer, suggesting that Th17 cells play important antitumor responses in cervical cancer settings[183]. Evidence emphasizes that the infiltrate of Th17 cells in the cervical tumor had an activated phenotype with increased expression of CCR6, the receptor for the CCL20 chemokine. It corroborates the hypothesis that the Th17/Treg axis imbalance may be involved in the promotion and progression of cervical cancer[184]. A recent study suggested that the imbalance between Th1/Th2 and Th17/Treg cells was related to the stage of cervical cancer, tumor size, metastasis, and vasoinvasion. The findings demonstrated that the peripheral immune cell levels reflect the patient's condition[185]. Although some questions can already be answered, further studies on the involvement of the Th17 response to cervical cancer are still needed.
Lymphoma: Every year, approximately 500000 people are diagnosed with non-Hodgkin's lymphoma and 80000 with Hodgkin's lymphoma, the most common cancers among the 90 lymphoma subtypes[186]. In a study conducted in China, a significantly decreased frequency of Th17 cells was observed in the peripheral blood of patients with non-Hodgkin's B-cell lymphoma compared to healthy individuals, along with an increase in Th1 cells[187]. This activity may be associated with the patient’s response to treatment and the different stages of the disease[188]. Another study revealed that the number of Th17 cells in lymphomas is influenced by the amount of mast cells and granulocytes. The IL-6 expression by these cells contributes to the establishment of a pro-inflammatory environment for Th17 cells, favoring CXCL-13 production and its interaction with CXCR3 and CXCR5 receptors expressed in mast cells[189]. It has been shown that drugs that block the IL-23/IL-17 axis, which are already available for the treatment of certain autoimmune diseases, can increase the therapeutic impact against classic Hodgkin’s lymphoma[190,191]. Moreover, a recent study demonstrated that the prognostic implication of Th17 cells depends on the type of treatment employed, since the Th17 signature did not represent a negative prognosis in the treatment with the medication Lenalidomide in non-follicular lymphoma[192,193].
Breast cancer: Breast cancer is one of the main causes of death among women[194]. This type of cancer is a heterogeneous disease with different patterns of tumor infiltrating lymphocytes, depending on the molecular subtype and other factors of the tumor microenvironment that are important for prognosis and predictive for treatment[195]. Studies point to a relevant infiltrate, characterized mainly by Foxp3+ cells and high levels of IL-6, in addition to revealing a high infiltration of IL-17-producing cells and a low amount of CD8+ cells, suggesting that Th17 cells participate in an effective immune response to eliminate the tumor in patients with breast cancer[196,197]. IL-6 is expressed in breast cancer patients, and its levels are positively associated with the number of Th17 cells. In the breast cancer microenvironment, IL-6 enhances the differentiation and expansion of Th17 cells[198]. Another study showed that Th17 cells positively regulate the production of CXCL1 during the progression of breast cancer. CXCL1, which is produced by breast cancer cells, might promote the growth and development of cancer[199]. In a recent study carried out in China, the involvement of high salt intake was evidenced as a factor that accelerated the growth of breast cancer in addition to increasing Th17 cells circulation in mice. It was also demonstrated in an in vitro study that the elevation of Th17 cells was reversed with the application of 1.25 Vitamin D3, inhibiting the differentiation of these cells (P < 0.001)[200]. Although some studies point to the important protective role of Th17 cells, further investigation is needed in that context[201].
Bone-related cancers: Studies evaluating the relationship between Th17/Treg axis and bone marrow cancer concluded that deregulations in this axis leading to immune tolerance or impaired immune response might contribute to bone tumorigenesis. In that context, a study evaluating peripheral blood mononuclear cells and bone-marrow mononuclear cells from patients with multiple myeloma and healthy controls observed an enhanced expression of Th17 cell-related cytokines in the affected individuals. Moreover, that study demonstrated that the IL-17 has the potential to promote the growth of myeloma cells and colony formation through the activation of IL-17 receptors as well as to inhibit the Th1 immune system profile along with the IL-22[202]. Otherwise, studies have observed an increased number of Treg cells in patients with acute myelogenous leukemia (AML) compared to controls, which suggests that the Treg immune profile might contribute for an improper immune response against the malignancy and to a consequent progression of the AML[203,204]. Furthermore, a study investigating the role of IL-22 produced by Th17 and Th22 cells in osteosarcoma demonstrated that its levels were enhanced in osteosarcoma cells and that it stimulates the proliferation and invasion of tumor cells via STAT3 signaling[205]. Finally, the aforementioned studies suggest that the understanding of the role of the Th17/Treg axis is essential for the study of bone-related cancers and should be explored as a potential therapeutic target in the treatment of those malignancies. Interestingly, vitamin D3 can act therapeutically over Th17 cells, reducing IL-17A and IFN-γ levels in rheumatoid arthritis. Therefore, vitamin D3 might could be used for the development of a therapeutic approach against bone-related cancers through the modulation of the Th17 immune profile (Figure 2)[206].
The Th17 response is intimately linked to the development of cancers and, in the last few years, the knowledge on the role of Th17 cells in the tumor microenvironment have significantly increased. However, much still has to be done in order to achieve a broader understanding on this issue. The IL-17 stands out among the Th17 cells-related inflammatory cytokines, being involved mainly in processes that promote tumorigenesis. In addition, the plasticity of Th17 cells, which allows a broader dynamics of the Th17/Treg axis in different tumor activities, and the Th17/Th1 axis, which is associated with antitumor mechanisms, are important issues to be taken into account in the immune-oncology field. The modulation of these immune system interplays might be a potential alternative for the development of new therapeutic interventions for various malignancies. Moreover, the role of the IL-27 should be further studied in various types of cancer since an important antitumor effect has been associated with this interleukin in some malignancies. Because IL-17 has shown to be so important in the pathogenesis of several malignant tumors, anti-IL-17 monoclonal antibodies are promising drugs that should be evaluated in numerous neoplasms. Finally, it has to be emphasized that there are several similarities and differences between Th17 responses in various cancers, being it highly dependent on the tumor context. The comprehension of the Th17 immune response in cancer is important not only to predict prognosis, but also to identify new therapeutic possibilities.
Manuscript source: Invited manuscript
Specialty type: Oncology
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li X, Mi Y S-Editor: Fan JR L-Editor: A P-Editor: Li JH
| 1. | Mattiuzzi C, Lippi G. Current Cancer Epidemiology. J Epidemiol Glob Health. 2019;9:217-222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 570] [Article Influence: 142.5] [Reference Citation Analysis (0)] |
| 2. | Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321-330. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2373] [Cited by in F6Publishing: 3039] [Article Influence: 434.1] [Reference Citation Analysis (0)] |
| 3. | Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1312] [Cited by in F6Publishing: 1403] [Article Influence: 107.9] [Reference Citation Analysis (0)] |
| 4. | Stadhouders R, Lubberts E, Hendriks RW. A cellular and molecular view of T helper 17 cell plasticity in autoimmunity. J Autoimmun. 2018;87:1-15. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 185] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 5. | Couturier M, Lamarthée B, Arbez J, Renauld JC, Bossard C, Malard F, Bonnefoy F, Mohty M, Perruche S, Tiberghien P, Saas P, Gaugler B. IL-22 deficiency in donor T cells attenuates murine acute graft-versus-host disease mortality while sparing the graft-versus-leukemia effect. Leukemia. 2013;27:1527-1537. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 6. | Zheng HL, Shi BY, Du GS, Wang Z. Changes in Th17 and IL-17 levels during acute rejection after mouse skin transplantation. Eur Rev Med Pharmacol Sci. 2014;18:2720-2786. [PubMed] [Cited in This Article: ] |
| 7. | Annunziato F, Cosmi L, Romagnani S. Human and murine Th17. Curr Opin HIV AIDS. 2010;5:114-119. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Chang SH. T helper 17 (Th17) cells and interleukin-17 (IL-17) in cancer. Arch Pharm Res. 2019;42:549-559. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 51] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 9. | Ye J, Su X, Hsueh EC, Zhang Y, Koenig JM, Hoft DF, Peng G. Human tumor-infiltrating Th17 cells have the capacity to differentiate into IFN-γ+ and FOXP3+ T cells with potent suppressive function. Eur J Immunol. 2011;41:936-951. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Bending D, De la Peña H, Veldhoen M, Phillips JM, Uyttenhove C, Stockinger B, Cooke A. Highly purified Th17 cells from BDC2.5NOD mice convert into Th1-like cells in NOD/SCID recipient mice. J Clin Invest. 2009;119:565-572. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 426] [Cited by in F6Publishing: 427] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 11. | Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5130] [Cited by in F6Publishing: 5309] [Article Influence: 294.9] [Reference Citation Analysis (0)] |
| 12. | Su X, Ye J, Hsueh EC, Zhang Y, Hoft DF, Peng G. Tumor microenvironments direct the recruitment and expansion of human Th17 cells. J Immunol. 2010;184:1630-1641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 244] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 13. | Chen D, Jiang R, Mao C, Shi L, Wang S, Yu L, Hu Q, Dai D, Xu H. Chemokine/chemokine receptor interactions contribute to the accumulation of Th17 cells in patients with esophageal squamous cell carcinoma. Hum Immunol. 2012;73:1068-1072. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Li J, Mo HY, Xiong G, Zhang L, He J, Huang ZF, Liu ZW, Chen QY, Du ZM, Zheng LM, Qian CN, Zeng YX. Tumor microenvironment macrophage inhibitory factor directs the accumulation of interleukin-17-producing tumor-infiltrating lymphocytes and predicts favorable survival in nasopharyngeal carcinoma patients. J Biol Chem. 2012;287:35484-35495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 15. | Yu Q, Lou XM, He Y. Preferential recruitment of Th17 cells to cervical cancer via CCR6-CCL20 pathway. PLoS One. 2015;10:e0120855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Ankathatti Munegowda M, Deng Y, Mulligan SJ, Xiang J. Th17 and Th17-stimulated CD8⁺ T cells play a distinct role in Th17-induced preventive and therapeutic antitumor immunity. Cancer Immunol Immunother. 2011;60:1473-1484. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, Hunter CA, Cua DJ, Kastelein RA. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748-5756. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 256] [Cited by in F6Publishing: 256] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 18. | Patel SA, Dave MA, Bliss SA, Giec-Ujda AB, Bryan M, Pliner LF, Rameshwar P. Treg/Th17 polarization by distinct subsets of breast cancer cells is dictated by the interaction with mesenchymal stem cells. J Cancer Stem Cell Res. 2014;2014. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007-1010. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1239] [Cited by in F6Publishing: 1224] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 20. | Lotti F, Jarrar AM, Pai RK, Hitomi M, Lathia J, Mace A, Gantt GA Jr, Sukhdeo K, DeVecchio J, Vasanji A, Leahy P, Hjelmeland AB, Kalady MF, Rich JN. Chemotherapy activates cancer-associated fibroblasts to maintain colorectal cancer-initiating cells by IL-17A. J Exp Med. 2013;210:2851-2872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 222] [Cited by in F6Publishing: 298] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 21. | Shahid A, Bharadwaj M. The connection between the Th17 cell related cytokines and cancer stem cells in cancer: Novel therapeutic targets. Immunol Lett. 2019;213:9-20. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 22. | Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1247] [Cited by in F6Publishing: 1291] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 23. | Ruan Q, Kameswaran V, Zhang Y, Zheng S, Sun J, Wang J, DeVirgiliis J, Liou HC, Beg AA, Chen YH. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J Exp Med. 2011;208:2321-2333. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 194] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 24. | Downs-Canner S, Berkey S, Delgoffe GM, Edwards RP, Curiel T, Odunsi K, Bartlett DL, Obermajer N. Suppressive IL-17A+Foxp3+ and ex-Th17 IL-17AnegFoxp3+ Treg cells are a source of tumour-associated Treg cells. Nat Commun. 2017;8:14649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 25. | Romagnani S. Human Th17 cells. Arthritis Res Ther. 2008;10:206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 118] [Cited by in F6Publishing: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 26. | Santarlasci V, Maggi L, Capone M, Frosali F, Querci V, De Palma R, Liotta F, Cosmi L, Maggi E, Romagnani S, Annunziato F. TGF-beta indirectly favors the development of human Th17 cells by inhibiting Th1 cells. Eur J Immunol. 2009;39:207-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 119] [Cited by in F6Publishing: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 27. | Monin L, Gaffen SL. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb Perspect Biol. 2018;10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 151] [Cited by in F6Publishing: 199] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 28. | Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345-350. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1119] [Cited by in F6Publishing: 1144] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 29. | Shabgah AG, Fattahi E, Shahneh FZ. Interleukin-17 in human inflammatory diseases. Postepy Dermatol Alergol. 2014;31:256-261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 110] [Cited by in F6Publishing: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 30. | Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454-467. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1316] [Cited by in F6Publishing: 1275] [Article Influence: 79.7] [Reference Citation Analysis (0)] |
| 31. | Dudakov JA, Hanash AM, van den Brink MR. Interleukin-22: immunobiology and pathology. Annu Rev Immunol. 2015;33:747-785. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 612] [Cited by in F6Publishing: 592] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 32. | Yao Z, Painter SL, Fanslow WC, Ulrich D, Macduff BM, Spriggs MK, Armitage RJ. Human IL-17: a novel cytokine derived from T cells. J Immunol. 1995;155:5483-5486. [PubMed] [Cited in This Article: ] |
| 33. | Morita R, Schmitt N, Bentebibel SE, Ranganathan R, Bourdery L, Zurawski G, Foucat E, Dullaers M, Oh S, Sabzghabaei N, Lavecchio EM, Punaro M, Pascual V, Banchereau J, Ueno H. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity. 2011;34:108-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1104] [Cited by in F6Publishing: 1195] [Article Influence: 91.9] [Reference Citation Analysis (0)] |
| 34. | Asadzadeh Z, Mohammadi H, Safarzadeh E, Hemmatzadeh M, Mahdian-Shakib A, Jadidi-Niaragh F, Azizi G, Baradaran B. The paradox of Th17 cell functions in tumor immunity. Cell Immunol. 2017;322:15-25. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 138] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 35. | Phillips JD, Knab LM, Blatner NR, Haghi L, DeCamp MM, Meyerson SL, Heiferman MJ, Heiferman JR, Gounari F, Bentrem DJ, Khazaie K. Preferential expansion of pro-inflammatory Tregs in human non-small cell lung cancer. Cancer Immunol Immunother. 2015;64:1185-1191. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Xin L, Gao J, Ge X, Tian C, Ma W, Tian Z, Zheng X, Hou J. Increased pro-inflammatory cytokine-secreting regulatory T cells are correlated with the plasticity of T helper cell differentiation and reflect disease status in asthma. Respir Med. 2018;143:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 37. | Bhaumik S, Basu R. Cellular and Molecular Dynamics of Th17 Differentiation and its Developmental Plasticity in the Intestinal Immune Response. Front Immunol. 2017;8:254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 81] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 38. | Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 829] [Cited by in F6Publishing: 873] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 39. | Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci U S A. 2015;112:7061-7066. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 40. | Agalioti T, Villablanca EJ, Huber S, Gagliani N. TH17 cell plasticity: The role of dendritic cells and molecular mechanisms. J Autoimmun. 2018;87:50-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 41. | Seidl A, Panzer M, Voehringer D. Protective immunity against the gastrointestinal nematode Nippostrongylus brasiliensis requires a broad T-cell receptor repertoire. Immunology. 2011;134:214-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Cosmi L, Maggi L, Santarlasci V, Capone M, Cardilicchia E, Frosali F, Querci V, Angeli R, Matucci A, Fambrini M, Liotta F, Parronchi P, Maggi E, Romagnani S, Annunziato F. Identification of a novel subset of human circulating memory CD4(+) T cells that produce both IL-17A and IL-4. J Allergy Clin Immunol. 2010;125:222-30.e1. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 240] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 43. | Guéry L, Hugues S. Th17 Cell Plasticity and Functions in Cancer Immunity. Biomed Res Int. 2015;2015:314620. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 157] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 44. | Gagliani N, Amezcua Vesely MC, Iseppon A, Brockmann L, Xu H, Palm NW, de Zoete MR, Licona-Limón P, Paiva RS, Ching T, Weaver C, Zi X, Pan X, Fan R, Garmire LX, Cotton MJ, Drier Y, Bernstein B, Geginat J, Stockinger B, Esplugues E, Huber S, Flavell RA. Th17 cells transdifferentiate into regulatory T cells during resolution of inflammation. Nature. 2015;523:221-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 534] [Cited by in F6Publishing: 575] [Article Influence: 63.9] [Reference Citation Analysis (0)] |
| 45. | Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014-1022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2228] [Cited by in F6Publishing: 2680] [Article Influence: 243.6] [Reference Citation Analysis (0)] |
| 46. | Ma S, Cheng Q, Cai Y, Gong H, Wu Y, Yu X, Shi L, Wu D, Dong C, Liu H. IL-17A produced by γδ T cells promotes tumor growth in hepatocellular carcinoma. Cancer Res. 2014;74:1969-1982. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 181] [Cited by in F6Publishing: 195] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 47. | Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, Tonc E, Schreiber RD, Pearce EJ, Pearce EL. Metabolic Competition in the Tumor Microenvironment Is a Driver of Cancer Progression. Cell. 2015;162:1229-1241. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1558] [Cited by in F6Publishing: 1943] [Article Influence: 215.9] [Reference Citation Analysis (0)] |
| 48. | Muranski P, Borman ZA, Kerkar SP, Klebanoff CA, Ji Y, Sanchez-Perez L, Sukumar M, Reger RN, Yu Z, Kern SJ, Roychoudhuri R, Ferreyra GA, Shen W, Durum SK, Feigenbaum L, Palmer DC, Antony PA, Chan CC, Laurence A, Danner RL, Gattinoni L, Restifo NP. Th17 cells are long lived and retain a stem cell-like molecular signature. Immunity. 2011;35:972-985. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 321] [Cited by in F6Publishing: 345] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 49. | Bailey SR, Nelson MH, Himes RA, Li Z, Mehrotra S, Paulos CM. Th17 cells in cancer: the ultimate identity crisis. Front Immunol. 2014;5:276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 208] [Cited by in F6Publishing: 224] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 50. | Yang S, Wang B, Guan C, Wu B, Cai C, Wang M, Zhang B, Liu T, Yang P. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J Leukoc Biol. 2011;89:85-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Ma C, Dong X. Colorectal cancer-derived Foxp3(+) IL-17(+) T cells suppress tumour-specific CD8+ T cells. Scand J Immunol. 2011;74:47-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 52. | Sharma MD, Hou DY, Liu Y, Koni PA, Metz R, Chandler P, Mellor AL, He Y, Munn DH. Indoleamine 2,3-dioxygenase controls conversion of Foxp3+ Tregs to TH17-like cells in tumor-draining lymph nodes. Blood. 2009;113:6102-6111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 300] [Cited by in F6Publishing: 316] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 53. | Liu H, Ji Y, Ma X, He A, Zhao W, Zhang P, Gu L, Lei B, Zhang Y, Wang Y, Zhang W, Wang J. Effects of CpG oligodeoxynucleotides on the differentiation of Treg/Th17 cells. Mol Immunol. 2021;132:199-208. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5] [Cited by in F6Publishing: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 54. | Wang L, Zhang Y, Xie F. T-regulatory cell/T helper 17 cell imbalance functions as prognostic biomarker of oral squamous cell carcinoma - CONSORT. Medicine (Baltimore). 2020;99:e23145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 55. | Cluxton D, Petrasca A, Moran B, Fletcher JM. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front Immunol. 2019;10:115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 56. | Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92-107. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 789] [Cited by in F6Publishing: 829] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 57. | Ma Y, Shurin GV, Peiyuan Z, Shurin MR. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4:36-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 217] [Cited by in F6Publishing: 227] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 58. | Jiao S, Subudhi SK, Aparicio A, Ge Z, Guan B, Miura Y, Sharma P. Differences in Tumor Microenvironment Dictate T Helper Lineage Polarization and Response to Immune Checkpoint Therapy. Cell. 2019;179:1177-1190.e13. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 167] [Cited by in F6Publishing: 226] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 59. | Benchetrit F, Ciree A, Vives V, Warnier G, Gey A, Sautès-Fridman C, Fossiez F, Haicheur N, Fridman WH, Tartour E. Interleukin-17 inhibits tumor cell growth by means of a T-cell-dependent mechanism. Blood. 2002;99:2114-2121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 263] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 60. | Wu L, Awaji M, Saxena S, Varney ML, Sharma B, Singh RK. IL-17-CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am J Pathol. 2020;190:222-233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 61. | Akbay EA, Koyama S, Liu Y, Dries R, Bufe LE, Silkes M, Alam MM, Magee DM, Jones R, Jinushi M, Kulkarni M, Carretero J, Wang X, Warner-Hatten T, Cavanaugh JD, Osa A, Kumanogoh A, Freeman GJ, Awad MM, Christiani DC, Bueno R, Hammerman PS, Dranoff G, Wong KK. Interleukin-17A Promotes Lung Tumor Progression through Neutrophil Attraction to Tumor Sites and Mediating Resistance to PD-1 Blockade. J Thorac Oncol. 2017;12:1268-1279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 62. | Chen CL, Wang Y, Huang CY, Zhou ZQ, Zhao JJ, Zhang XF, Pan QZ, Wu JX, Weng DS, Tang Y, Zhu Q, Yuan LP, Xia JC. IL-17 induces antitumor immunity by promoting beneficial neutrophil recruitment and activation in esophageal squamous cell carcinoma. Oncoimmunology. 2017;7:e1373234. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 63. | Oberg HH, Wesch D, Kalyan S, Kabelitz D. Regulatory Interactions Between Neutrophils, Tumor Cells and T Cells. Front Immunol. 2019;10:1690. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 64. | Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 527] [Cited by in F6Publishing: 550] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 65. | Witowski J, Pawlaczyk K, Breborowicz A, Scheuren A, Kuzlan-Pawlaczyk M, Wisniewska J, Polubinska A, Friess H, Gahl GM, Frei U, Jörres A. Interleukin-17 stimulates intraperitoneal neutrophil infiltration through the release of the chemokine GROα from peritoneal mesothelial cells. Crit Care. 2000;4(Suppl 1):P60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 255] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 66. | Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1322] [Cited by in F6Publishing: 1366] [Article Influence: 80.4] [Reference Citation Analysis (0)] |
| 67. | Eyerich K, Dimartino V, Cavani A. IL-17 and IL-22 in immunity: Driving protection and pathology. Eur J Immunol. 2017;47:607-614. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 226] [Cited by in F6Publishing: 226] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 68. | Huber S, Gagliani N, Zenewicz LA, Huber FJ, Bosurgi L, Hu B, Hedl M, Zhang W, O'Connor W Jr, Murphy AJ, Valenzuela DM, Yancopoulos GD, Booth CJ, Cho JH, Ouyang W, Abraham C, Flavell RA. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature. 2012;491:259-263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 580] [Cited by in F6Publishing: 558] [Article Influence: 46.5] [Reference Citation Analysis (0)] |
| 69. | Khosravi N, Caetano MS, Cumpian AM, Unver N, De la Garza Ramos C, Noble O, Daliri S, Hernandez BJ, Gutierrez BA, Evans SE, Hanash S, Alekseev AM, Yang Y, Chang SH, Nurieva R, Kadara H, Chen J, Ostrin EJ, Moghaddam SJ. IL22 Promotes Kras-Mutant Lung Cancer by Induction of a Protumor Immune Response and Protection of Stemness Properties. Cancer Immunol Res. 2018;6:788-797. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 53] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 70. | Qian X, Chen H, Wu X, Hu L, Huang Q, Jin Y. Interleukin-17 acts as double-edged sword in anti-tumor immunity and tumorigenesis. Cytokine. 2017;89:34-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Chen G, Zhang PG, Li JS, Duan JJ, Su W, Guo SP, Wang YF, Sun JN, Yang XT. Th17 cell frequency and IL-17A production in peripheral blood of patients with non-small-cell lung cancer. J Int Med Res. 2020;48:300060520925948. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 72. | Numasaki M, Watanabe M, Suzuki T, Takahashi H, Nakamura A, McAllister F, Hishinuma T, Goto J, Lotze MT, Kolls JK, Sasaki H. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2-dependent angiogenesis. J Immunol. 2005;175:6177-6189. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 295] [Cited by in F6Publishing: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 73. | Wilke CM, Kryczek I, Wei S, Zhao E, Wu K, Wang G, Zou W. Th17 cells in cancer: help or hindrance? Carcinogenesis. 2011;32:643-649. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 132] [Cited by in F6Publishing: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 74. | Protopsaltis NJ, Liang W, Nudleman E, Ferrara N. Interleukin-22 promotes tumor angiogenesis. Angiogenesis. 2019;22:311-323. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 50] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 75. | Punt S, Langenhoff JM, Putter H, Fleuren GJ, Gorter A, Jordanova ES. The correlations between IL-17 vs. Th17 cells and cancer patient survival: a systematic review. Oncoimmunology. 2015;4:e984547. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 72] [Cited by in F6Publishing: 87] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 76. | Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells vs suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377-5385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 77. | Rocha GA, de Melo FF, Cabral MMDA, de Brito BB, da Silva FAF, Queiroz DMM. Interleukin-27 is abrogated in gastric cancer, but highly expressed in other Helicobacter pylori-associated gastroduodenal diseases. Helicobacter. 2020;25:e12667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 78. | Duan M, Ning Z, Fu Z, Zhang J, Liu G, Wei Q, Zheng X. Decreased IL-27 Negatively Correlated with Th17 Cells in Non-Small-Cell Lung Cancer Patients. Mediators Inflamm. 2015;2015:802939. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 79. | Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16:225-238. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 883] [Cited by in F6Publishing: 1021] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 80. | Terraza-Aguirre C, Campos-Mora M, Elizondo-Vega R, Contreras-López RA, Luz-Crawford P, Jorgensen C, Djouad F. Mechanisms behind the Immunoregulatory Dialogue between Mesenchymal Stem Cells and Th17 Cells. Cells. 2020;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 81. | Chaudhry A, Rudra D, Treuting P, Samstein RM, Liang Y, Kas A, Rudensky AY. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986-991. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 767] [Cited by in F6Publishing: 789] [Article Influence: 52.6] [Reference Citation Analysis (0)] |
| 82. | Dingli D, Michor F. Successful therapy must eradicate cancer stem cells. Stem Cells. 2006;24:2603-2610. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 194] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 83. | La Vecchia C. Ovarian cancer: epidemiology and risk factors. Eur J Cancer Prev. 2017;26:55-62. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 141] [Cited by in F6Publishing: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 84. | Bilska M, Pawłowska A, Zakrzewska E, Chudzik A, Suszczyk D, Gogacz M, Wertel I. Th17 Cells and IL-17 As Novel Immune Targets in Ovarian Cancer Therapy. J Oncol. 2020;2020:8797683. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 85. | Erratum for the Research Article: "The brain microenvironment mediates resistance in luminal breast cancer to PI3K inhibition through HER3 activation" by D. P. Kodack, V. Askoxylakis, G. B. Ferraro, Q. Sheng, M. Badeaux, S. Goel, X. Qi, R. Shankaraiah, Z. A. Cao, R. R. Ramjiawan, D. Bezwada, B. Patel, Y. Song, C. Costa, K. Naxerova, C. S. F. Wong, J. Kloepper, R. Das, A. Tam, J. Tanboon, D. G. Duda, C. R. Miller, M. B. Siegel, C. K. Anders, M. Sanders, M. V. Estrada, R. Schlegel, C. L. Arteaga, E. Brachtel, A. Huang, D. Fukumura, J. A. Engelman, R. K. Jain. Sci Transl Med. 2019;11. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 86. | Klinke DJ 2nd. An evolutionary perspective on anti-tumor immunity. Front Oncol. 2012;2:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 87. | Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, Chen W, Selbach M. Global quantification of mammalian gene expression control. Nature. 2011;473:337-342. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4346] [Cited by in F6Publishing: 4367] [Article Influence: 335.9] [Reference Citation Analysis (0)] |
| 88. | Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457-1464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 611] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 89. | Ogura H, Murakami M, Okuyama Y, Tsuruoka M, Kitabayashi C, Kanamoto M, Nishihara M, Iwakura Y, Hirano T. Interleukin-17 promotes autoimmunity by triggering a positive-feedback loop via interleukin-6 induction. Immunity. 2008;29:628-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 393] [Cited by in F6Publishing: 425] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 90. | Hamanishi J, Mandai M, Matsumura N, Abiko K, Baba T, Konishi I. PD-1/PD-L1 blockade in cancer treatment: perspectives and issues. Int J Clin Oncol. 2016;21:462-473. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 219] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 91. | Miyahara Y, Odunsi K, Chen W, Peng G, Matsuzaki J, Wang RF. Generation and regulation of human CD4+ IL-17-producing T cells in ovarian cancer. Proc Natl Acad Sci U S A. 2008;105:15505-15510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 211] [Cited by in F6Publishing: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 92. | Kato T, Furumoto H, Ogura T, Onishi Y, Irahara M, Yamano S, Kamada M, Aono T. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282:735-738. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 115] [Cited by in F6Publishing: 127] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 93. | Kryczek I, Lange A, Mottram P, Alvarez X, Cheng P, Hogan M, Moons L, Wei S, Zou L, Machelon V, Emilie D, Terrassa M, Lackner A, Curiel TJ, Carmeliet P, Zou W. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465-472. [PubMed] [Cited in This Article: ] |
| 94. | Curiel TJ, Cheng P, Mottram P, Alvarez X, Moons L, Evdemon-Hogan M, Wei S, Zou L, Kryczek I, Hoyle G, Lackner A, Carmeliet P, Zou W. Dendritic cell subsets differentially regulate angiogenesis in human ovarian cancer. Cancer Res. 2004;64:5535-5538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 196] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 95. | Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869-881. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 105] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 96. | Munn DH. Th17 cells in ovarian cancer. Blood. 2009;114:1134-1135. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 97. | Block MS, Dietz AB, Gustafson MP, Kalli KR, Erskine CL, Youssef B, Vijay GV, Allred JB, Pavelko KD, Strausbauch MA, Lin Y, Grudem ME, Jatoi A, Klampe CM, Wahner-Hendrickson AE, Weroha SJ, Glaser GE, Kumar A, Langstraat CL, Solseth ML, Deeds MC, Knutson KL, Cannon MJ. Th17-inducing autologous dendritic cell vaccination promotes antigen-specific cellular and humoral immunity in ovarian cancer patients. Nat Commun. 2020;11:5173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 98. | Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141-1149. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 541] [Cited by in F6Publishing: 585] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 99. | Winkler I, Gogacz M, Rechberger T. [Do Th17 cells play an important role in the pathogenesis and prognosis of ovarian cancer? Ginekol Pol. 2012;83:295-300. [PubMed] [Cited in This Article: ] |
| 100. | Cardenas H, Vieth E, Lee J, Segar M, Liu Y, Nephew KP, Matei D. TGF-β induces global changes in DNA methylation during the epithelial-to-mesenchymal transition in ovarian cancer cells. Epigenetics. 2014;9:1461-1472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 101. | Zhou J, Li X, Wu X, Zhang T, Zhu Q, Wang X, Wang H, Wang K, Lin Y. Exosomes Released from Tumor-Associated Macrophages Transfer miRNAs That Induce a Treg/Th17 Cell Imbalance in Epithelial Ovarian Cancer. Cancer Immunol Res. 2018;6:1578-1592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 176] [Cited by in F6Publishing: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 102. | Liu J, Zhang N, Li Q, Zhang W, Ke F, Leng Q, Wang H, Chen J. Tumor-associated macrophages recruit CCR6+ regulatory T cells and promote the development of colorectal cancer via enhancing CCL20 production in mice. PLoS One. 2011;6:e19495. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 103. | Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838-848. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 808] [Cited by in F6Publishing: 848] [Article Influence: 60.6] [Reference Citation Analysis (0)] |
| 104. | Takikawa T, Masamune A, Yoshida N, Hamada S, Kogure T, Shimosegawa T. Exosomes Derived From Pancreatic Stellate Cells: MicroRNA Signature and Effects on Pancreatic Cancer Cells. Pancreas. 2017;46:19-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 83] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 105. | Wacleche VS, Landay A, Routy JP, Ancuta P. The Th17 Lineage: From Barrier Surfaces Homeostasis to Autoimmunity, Cancer, and HIV-1 Pathogenesis. Viruses. 2017;9. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 106. | Planas D, Routy JP, Ancuta P. New Th17-specific therapeutic strategies for HIV remission. Curr Opin HIV AIDS. 2019;14:85-92. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 107. | Kalli KR, Oberg AL, Keeney GL, Christianson TJ, Low PS, Knutson KL, Hartmann LC. Folate receptor alpha as a tumor target in epithelial ovarian cancer. Gynecol Oncol. 2008;108:619-626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 307] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 108. | Cochaud S, Giustiniani J, Thomas C, Laprevotte E, Garbar C, Savoye AM, Curé H, Mascaux C, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A, Bastid J. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci Rep. 2013;3:3456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 90] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 109. | Zhang Q, Liu S, Ge D, Cunningham DM, Huang F, Ma L, Burris TP, You Z. Targeting Th17-IL-17 Pathway in Prevention of Micro-Invasive Prostate Cancer in a Mouse Model. Prostate. 2017;77:888-899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 110. | Shacter E, Weitzman SA. Chronic inflammation and cancer. Oncology (Williston Park). 2002;16:217-226, 229; discussion 230. [PubMed] [Cited in This Article: ] |
| 111. | Sfanos KS, De Marzo AM. Prostate cancer and inflammation: the evidence. Histopathology. 2012;60:199-215. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 391] [Cited by in F6Publishing: 431] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 112. | Zhang Q, Liu S, Ge D, Zhang Q, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72:2589-2599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 113. | Radej S, Płaza P, Olender A, Szewc M, Bar K, Maciejewski R. Infiltrating Treg and Th17 Cells of the Prostate Hypertrophy Gland Associated with Propionibacterium Acnes Infection. Res Rep Urol. 2020;12:593-597. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 114. | Saleh R, Elkord E. FoxP3+ T regulatory cells in cancer: Prognostic biomarkers and therapeutic targets. Cancer Lett. 2020;490:174-185. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 145] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 115. | Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804-811. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 516] [Cited by in F6Publishing: 536] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 116. | Koenen HJ, Smeets RL, Vink PM, van Rijssen E, Boots AM, Joosten I. Human CD25highFoxp3pos regulatory T cells differentiate into IL-17-producing cells. Blood. 2008;112:2340-2352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 570] [Cited by in F6Publishing: 585] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 117. | Baxevanis CN, Fortis SP, Perez SA. Prostate cancer: any room left for immunotherapies? Immunotherapy. 2019;11:69-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 118. | Handy CE, Antonarakis ES. Sipuleucel-T for the treatment of prostate cancer: novel insights and future directions. Future Oncol. 2018;14:907-917. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 97] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 119. | Duan Z, Miller HD, Fu X, Ge D, Jin B, Moustafa AA, Lan R, Zhang K, Chen Z, You Z. Th17 cells promote tumor growth in an immunocompetent orthotopic mouse model of prostate cancer. Am J Clin Exp Urol. 2019;7:249-261. [PubMed] [Cited in This Article: ] |
| 120. | Liu L, Ge D, Ma L, Mei J, Liu S, Zhang Q, Ren F, Liao H, Pu Q, Wang T, You Z. Interleukin-17 and prostaglandin E2 are involved in formation of an M2 macrophage-dominant microenvironment in lung cancer. J Thorac Oncol. 2012;7:1091-1100. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 79] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 121. | Zhang ZY, Schluesener HJ. HDAC inhibitor MS-275 attenuates the inflammatory reaction in rat experimental autoimmune prostatitis. Prostate. 2012;72:90-99. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 122. | Wang XF, Zhu YT, Wang JJ, Zeng DX, Mu CY, Chen YB, Lei W, Zhu YH, Huang JA. The prognostic value of interleukin-17 in lung cancer: A systematic review with meta-analysis based on Chinese patients. PLoS One. 2017;12:e0185168. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 123. | Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, Jemal A. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177-193. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2526] [Cited by in F6Publishing: 2757] [Article Influence: 393.9] [Reference Citation Analysis (2)] |
| 124. | Singh SP, Zhang HH, Foley JF, Hedrick MN, Farber JM. Human T cells that are able to produce IL-17 express the chemokine receptor CCR6. J Immunol. 2008;180:214-221. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 280] [Cited by in F6Publishing: 290] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 125. | Kirshberg S, Izhar U, Amir G, Demma J, Vernea F, Beider K, Shlomai Z, Wald H, Zamir G, Shapira OM, Peled A, Wald O. Involvement of CCR6/CCL20/IL-17 axis in NSCLC disease progression. PLoS One. 2011;6:e24856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 126. | Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845-858. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 723] [Cited by in F6Publishing: 774] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 127. | Pan B, Che D, Cao J, Shen J, Jin S, Zhou Y, Liu F, Gu K, Man Y, Shang L, Yu Y. Interleukin-17 levels correlate with poor prognosis and vascular endothelial growth factor concentration in the serum of patients with non-small cell lung cancer. Biomarkers. 2015;20:232-239. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 27] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | Xu C, Hao K, Yu L, Zhang X. Serum interleukin-17 as a diagnostic and prognostic marker for non-small cell lung cancer. Biomarkers. 2014;19:287-290. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 129. | Jin W, Dong C. IL-17 cytokines in immunity and inflammation. Emerg Microbes Infect. 2013;2:e60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 319] [Cited by in F6Publishing: 371] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 130. | Chang SH, Mirabolfathinejad SG, Katta H, Cumpian AM, Gong L, Caetano MS, Moghaddam SJ, Dong C. T helper 17 cells play a critical pathogenic role in lung cancer. Proc Natl Acad Sci U S A. 2014;111:5664-5669. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 203] [Cited by in F6Publishing: 234] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 131. | You R, DeMayo FJ, Liu J, Cho SN, Burt BM, Creighton CJ, Casal RF, Lazarus DR, Lu W, Tung HY, Yuan X, Hill-McAlester A, Kim M, Perusich S, Cornwell L, Rosen D, Song LZ, Paust S, Diehl G, Corry D, Kheradmand F. IL17A Regulates Tumor Latency and Metastasis in Lung Adeno and Squamous SQ.2b and AD.1 Cancer. Cancer Immunol Res. 2018;6:645-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 27] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Carmi Y, Rinott G, Dotan S, Elkabets M, Rider P, Voronov E, Apte RN. Microenvironment-derived IL-1 and IL-17 interact in the control of lung metastasis. J Immunol. 2011;186:3462-3471. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 84] [Cited by in F6Publishing: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 133. | Reppert S, Boross I, Koslowski M, Türeci Ö, Koch S, Lehr HA, Finotto S. A role for T-bet-mediated tumour immune surveillance in anti-IL-17A treatment of lung cancer. Nat Commun. 2011;2:600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 134. | Neurath MF, Finotto S. The emerging role of T cell cytokines in non-small cell lung cancer. Cytokine Growth Factor Rev. 2012;23:315-322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 135. | Maxeiner JH, Karwot R, Sauer K, Scholtes P, Boross I, Koslowski M, Türeci O, Wiewrodt R, Neurath MF, Lehr HA, Finotto S. A key regulatory role of the transcription factor NFATc2 in bronchial adenocarcinoma via CD8+ T lymphocytes. Cancer Res. 2009;69:3069-3076. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 136. | Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoué F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pagès F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960-1964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4318] [Cited by in F6Publishing: 4595] [Article Influence: 255.3] [Reference Citation Analysis (0)] |
| 137. | Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med. 2010;207:2175-2186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 890] [Cited by in F6Publishing: 974] [Article Influence: 69.6] [Reference Citation Analysis (0)] |
| 138. | Gao X, Zhu Y, Li G, Huang H, Zhang G, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PLoS One. 2012;7:e30676. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 278] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 139. | Smyth EC, Nilsson M, Grabsch HI, van Grieken NC, Lordick F. Gastric cancer. Lancet. 2020;396:635-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1150] [Cited by in F6Publishing: 1793] [Article Influence: 448.3] [Reference Citation Analysis (0)] |
| 140. | Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218-1222. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 708] [Cited by in F6Publishing: 739] [Article Influence: 41.1] [Reference Citation Analysis (1)] |
| 141. | Bagheri N, Azadegan-Dehkordi F, Shirzad H, Rafieian-Kopaei M, Rahimian G, Razavi A. The biological functions of IL-17 in different clinical expressions of Helicobacter pylori-infection. Microb Pathog. 2015;81:33-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 142. | Meng XY, Zhou CH, Ma J, Jiang C, Ji P. Expression of interleukin-17 and its clinical significance in gastric cancer patients. Med Oncol. 2012;29:3024-3028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 143. | Su Z, Sun Y, Zhu H, Liu Y, Lin X, Shen H, Chen J, Xu W, Xu H. Th17 cell expansion in gastric cancer may contribute to cancer development and metastasis. Immunol Res. 2014;58:118-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 144. | Meng X, Zhu S, Dong Q, Zhang S, Ma J, Zhou C. Expression of Th17/Treg related molecules in gastric cancer tissues. Turk J Gastroenterol. 2018;29:45-51. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 145. | Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888-898. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1059] [Cited by in F6Publishing: 1029] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 146. | Nguyen PM, Putoczki TL. Could the inhibition of IL-17 or IL-18 be a potential therapeutic opportunity for gastric cancer? Cytokine. 2019;118:8-18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 147. | Nagaoka K, Shirai M, Taniguchi K, Hosoi A, Sun C, Kobayashi Y, Maejima K, Fujita M, Nakagawa H, Nomura S, Kakimi K. Deep immunophenotyping at the single-cell level identifies a combination of anti-IL-17 and checkpoint blockade as an effective treatment in a preclinical model of data-guided personalized immunotherapy. J Immunother Cancer. 2020;8. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 42] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 148. | McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846-4861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in CrossRef: 906] [Cited by in F6Publishing: 968] [Article Influence: 161.3] [Reference Citation Analysis (30)] |
| 149. | Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008-2020. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 869] [Cited by in F6Publishing: 1207] [Article Influence: 301.8] [Reference Citation Analysis (0)] |
| 150. | McAllister F, Leach SD. Targeting IL-17 for pancreatic cancer prevention. Oncotarget. 2014;5:9530-9531. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 151. | Loncle C, Bonjoch L, Folch-Puy E, Lopez-Millan MB, Lac S, Molejon MI, Chuluyan E, Cordelier P, Dubus P, Lomberk G, Urrutia R, Closa D, Iovanna JL. IL17 Functions through the Novel REG3β-JAK2-STAT3 Inflammatory Pathway to Promote the Transition from Chronic Pancreatitis to Pancreatic Cancer. Cancer Res. 2015;75:4852-4862. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 70] [Cited by in F6Publishing: 79] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 152. | Wu HH, Hwang-Verslues WW, Lee WH, Huang CK, Wei PC, Chen CL, Shew JY, Lee EY, Jeng YM, Tien YW, Ma C. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J Exp Med. 2015;212:333-349. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 153. | He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of Th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424-7437. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 154. | Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, Zoltan M, Arora N, Baydogan S, Horne W, Burks J, Xu H, Hussain P, Wang H, Gupta S, Maitra A, Bailey JM, Moghaddam SJ, Banerjee S, Sahin I, Bhattacharya P, McAllister F. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 199] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 155. | Wang X, Wang L, Mo Q, Dong Y, Wang G, Ji A. Changes of Th17/Treg cell and related cytokines in pancreatic cancer patients. Int J Clin Exp Pathol. 2015;8:5702-5708. [PubMed] [Cited in This Article: ] |
| 156. | Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20108] [Cited by in F6Publishing: 19781] [Article Influence: 2197.9] [Reference Citation Analysis (17)] |
| 157. | Lucas C, Barnich N, Nguyen HTT. Microbiota, Inflammation and Colorectal Cancer. Int J Mol Sci. 2017;18. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 191] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 158. | Shi Y, Lin H, Cui J, Qi H, Florholmen J, Liu Z, Cui G. The role of interleukin-17A in colorectal tumorigenesis. Cancer Biother Radiopharm. 2013;28:429-432. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 159. | Razi S, Baradaran Noveiry B, Keshavarz-Fathi M, Rezaei N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine. 2019;116:7-12. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 160. | Cui G, Yang H, Zhao J, Yuan A, Florholmen J. Elevated proinflammatory cytokine IL-17A in the adjacent tissues along the adenoma-carcinoma sequence. Pathol Oncol Res. 2015;21:139-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 161. | Zeller FP, Spinler SA. Bepridil: a new long-acting calcium channel blocking agent. Drug Intell Clin Pharm. 1987;21:487-492. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 162. | El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118-1127. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 2881] [Cited by in F6Publishing: 2983] [Article Influence: 229.5] [Reference Citation Analysis (0)] |
| 163. | Gu FM, Li QL, Gao Q, Jiang JH, Zhu K, Huang XY, Pan JF, Yan J, Hu JH, Wang Z, Dai Z, Fan J, Zhou J. IL-17 induces AKT-dependent IL-6/JAK2/STAT3 activation and tumor progression in hepatocellular carcinoma. Mol Cancer. 2011;10:150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 129] [Cited by in F6Publishing: 142] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 164. | Chang X, Wang L, Zang M, Rong W, Wu Z, Liu L, Du J, Liu J, Wu J, Qu C. [Relationship between CCL20/CCR6/Th17 axis and vascular invasion and metastasis in patients with primary hepatocellular carcinoma]. Zhonghua Zhong Liu Za Zhi. 2015;37:5-10. [PubMed] [Cited in This Article: ] |
| 165. | Liao R, Sun J, Wu H, Yi Y, Wang JX, He HW, Cai XY, Zhou J, Cheng YF, Fan J, Qiu SJ. High expression of IL-17 and IL-17RE associate with poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2013;32:3. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 99] [Cited by in F6Publishing: 119] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 166. | World Health Organization. Radiation: Ultraviolet (UV) radiation and skin cancer. [cited 3 February 2021]. Available from: https://www.who.int/. [Cited in This Article: ] |
| 167. | He D, Li H, Yusuf N, Elmets CA, Athar M, Katiyar SK, Xu H. IL-17 mediated inflammation promotes tumor growth and progression in the skin. PLoS One. 2012;7:e32126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 168. | Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010;70:10112-10120. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 169. | Nardinocchi L, Sonego G, Passarelli F, Avitabile S, Scarponi C, Failla CM, Simoni S, Albanesi C, Cavani A. Interleukin-17 and interleukin-22 promote tumor progression in human nonmelanoma skin cancer. Eur J Immunol. 2015;45:922-931. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 170. | Pellegrini C, Orlandi A, Costanza G, Di Stefani A, Piccioni A, Di Cesare A, Chiricozzi A, Ferlosio A, Peris K, Fargnoli MC. Expression of IL-23/Th17-related cytokines in basal cell carcinoma and in the response to medical treatments. PLoS One. 2017;12:e0183415. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 171. | Rastrelli M, Tropea S, Rossi CR, Alaibac M. Melanoma: epidemiology, risk factors, pathogenesis, diagnosis and classification. In Vivo. 2014;28:1005-1011. [PubMed] [Cited in This Article: ] |
| 172. | Nunes LM, Sobrinho HMR, Silva CMN, Modesto DC, Marinho LC, Wastowski IJ. The IL-17A expression in the molecular mechanisms on tumor escape from immune response by neoplastic melanocytes. EVS. 2016;43:54-61. [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 173. | Nasso M, Fedele G, Spensieri F, Palazzo R, Costantino P, Rappuoli R, Ausiello CM. Genetically detoxified pertussis toxin induces Th1/Th17 immune response through MAPKs and IL-10-dependent mechanisms. J Immunol. 2009;183:1892-1899. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 174. | Sirikanjanapong S, Lanson B, Amin M, Martiniuk F, Kamino H, Wang BY. Collision tumor of primary laryngeal mucosal melanoma and invasive squamous cell carcinoma with IL-17A and CD70 gene over-expression. Head Neck Pathol. 2010;4:295-299. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 175. | Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Roliński J, Radwan P, Fang J, Wang G, Zou W. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388-4395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 184] [Cited by in F6Publishing: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 176. | Tang Q, Li J, Zhu H, Li P, Zou Z, Xiao Y. Hmgb1-IL-23-IL-17-IL-6-Stat3 axis promotes tumor growth in murine models of melanoma. Mediators Inflamm. 2013;2013:713859. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 177. | Hayata K, Iwahashi M, Ojima T, Katsuda M, Iida T, Nakamori M, Ueda K, Nakamura M, Miyazawa M, Tsuji T, Yamaue H. Inhibition of IL-17A in tumor microenvironment augments cytotoxicity of tumor-infiltrating lymphocytes in tumor-bearing mice. PLoS One. 2013;8:e53131. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 178. | Hirano T, Ishihara K, Hibi M. Roles of STAT3 in mediating the cell growth, differentiation and survival signals relayed through the IL-6 family of cytokine receptors. Oncogene. 2000;19:2548-2556. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 889] [Cited by in F6Publishing: 911] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 179. | Chen C, Gao FH. Th17 Cells Paradoxical Roles in Melanoma and Potential Application in Immunotherapy. Front Immunol. 2019;10:187. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 180. | Small W Jr, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN, Gaffney DK. Cervical cancer: A global health crisis. Cancer. 2017;123:2404-2412. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 480] [Cited by in F6Publishing: 660] [Article Influence: 94.3] [Reference Citation Analysis (0)] |
| 181. | Lin W, Niu Z, Zhang H, Kong Y, Wang Z, Yang X, Yuan F. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int J Clin Exp Pathol. 2019;12:3604-3612. [PubMed] [Cited in This Article: ] |
| 182. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 5245] [Cited by in F6Publishing: 5425] [Article Influence: 235.9] [Reference Citation Analysis (0)] |
| 183. | Punt S, Fleuren GJ, Kritikou E, Lubberts E, Trimbos JB, Jordanova ES, Gorter A. Angels and demons: Th17 cells represent a beneficial response, while neutrophil IL-17 is associated with poor prognosis in squamous cervical cancer. Oncoimmunology. 2015;4:e984539. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 184. | Alves JJP, De Medeiros Fernandes TAA, De Araújo JMG, Cobucci RNO, Lanza DCF, Bezerra FL, Andrade VS, Fernandes JV. Th17 response in patients with cervical cancer. Oncol Lett. 2018;16:6215-6227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 185. | Lin W, Zhang HL, Niu ZY, Wang Z, Kong Y, Yang XS, Yuan F. The disease stage-associated imbalance of Th1/Th2 and Th17/Treg in uterine cervical cancer patients and their recovery with the reduction of tumor burden. BMC Womens Health. 2020;20:126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 186. | Khanmohammadi S, Shabani M, Tabary M, Rayzan E, Rezaei N. Lymphoma in the setting of autoimmune diseases: A review of association and mechanisms. Crit Rev Oncol Hematol. 2020;150:102945. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 10] [Cited by in F6Publishing: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 187. | Lu T, Yu S, Liu Y, Yin C, Ye J, Liu Z, Ma D, Ji C. Aberrant Circulating Th17 Cells in Patients with B-Cell Non-Hodgkin's Lymphoma. PLoS One. 2016;11:e0148044. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 188. | Yang ZZ, Novak AJ, Ziesmer SC, Witzig TE, Ansell SM. Malignant B cells skew the balance of regulatory T cells and TH17 cells in B-cell non-Hodgkin's lymphoma. Cancer Res. 2009;69:5522-5530. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 123] [Cited by in F6Publishing: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 189. | Tripodo C, Gri G, Piccaluga PP, Frossi B, Guarnotta C, Piconese S, Franco G, Vetri V, Pucillo CE, Florena AM, Colombo MP, Pileri SA. Mast cells and Th17 cells contribute to the lymphoma-associated pro-inflammatory microenvironment of angioimmunoblastic T-cell lymphoma. Am J Pathol. 2010;177:792-802. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 190. | Menter T, Hayoz S, Zucca E, Kimby E, Dirnhofer S, Tzankov A. Immunomodulatory drugs may overcome the negative prognostic role of active Th17 axis in follicular lymphoma: evidence from the SAKK35/10 trial. Br J Haematol. 2020;190:e258-e261. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 191. | Duffield AS, Ascierto ML, Anders RA, Taube JM, Meeker AK, Chen S, McMiller TL, Phillips NA, Xu H, Ogurtsova A, Berger AE, Pardoll DM, Topalian SL, Ambinder RF. Th17 immune microenvironment in Epstein-Barr virus-negative Hodgkin lymphoma: implications for immunotherapy. Blood Adv. 2017;1:1324-1334. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 192. | Ferrarini I, Rigo A, Zamò A, Vinante F. Classical Hodgkin lymphoma cells may promote an IL-17-enriched microenvironment. Leuk Lymphoma. 2019;60:3395-3405. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 193. | Zhong W, Xu X, Zhu Z, Du Q, Du H, Yang L, Ling Y, Xiong H, Li Q. Increased expression of IRF8 in tumor cells inhibits the generation of Th17 cells and predicts unfavorable survival of diffuse large B cell lymphoma patients. Oncotarget. 2017;8:49757-49772. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 194. | Avalos-Navarro G, Muñoz-Valle JF, Daneri-Navarro A, Quintero-Ramos A, Franco-Topete RA, Morán-Mendoza AJ, Oceguera-Villanueva A, Bautista-Herrera LA, Topete-Camacho A, Del Toro-Arreola A. Circulating soluble levels of MIF in women with breast cancer in the molecular subtypes: relationship with Th17 cytokine profile. Clin Exp Med. 2019;19:385-391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 14] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 195. | Dushyanthen S, Beavis PA, Savas P, Teo ZL, Zhou C, Mansour M, Darcy PK, Loi S. Relevance of tumor-infiltrating lymphocytes in breast cancer. BMC Med. 2015;13:202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 136] [Cited by in F6Publishing: 155] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 196. | Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol. 2015;68:506-510. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 197. | Agahozo MC, Hammerl D, Debets R, Kok M, van Deurzen CHM. Tumor-infiltrating lymphocytes and ductal carcinoma in situ of the breast: friends or foes? Mod Pathol. 2018;31:1012-1025. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 198. | Neurath MF, Finotto S. IL-6 signaling in autoimmunity, chronic inflammation and inflammation-associated cancer. Cytokine Growth Factor Rev. 2011;22:83-89. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 368] [Cited by in F6Publishing: 400] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 199. | Ma K, Yang L, Shen R, Kong B, Chen W, Liang J, Tang G, Zhang B. Th17 cells regulate the production of CXCL1 in breast cancer. Int Immunopharmacol. 2018;56:320-329. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 30] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 200. | Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature. 2013;496:518-522. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 884] [Cited by in F6Publishing: 942] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 201. | Alinejad V, Dolati S, Motallebnezhad M, Yousefi M. The role of IL17B-IL17RB signaling pathway in breast cancer. Biomed Pharmacother. 2017;88:795-803. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 202. | Prabhala RH, Pelluru D, Fulciniti M, Prabhala HK, Nanjappa P, Song W, Pai C, Amin S, Tai YT, Richardson PG, Ghobrial IM, Treon SP, Daley JF, Anderson KC, Kutok JL, Munshi NC. Elevated IL-17 produced by TH17 cells promotes myeloma cell growth and inhibits immune function in multiple myeloma. Blood. 2010;115:5385-5392. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 265] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 203. | Ustun C, Miller JS, Munn DH, Weisdorf DJ, Blazar BR. Regulatory T cells in acute myelogenous leukemia: is it time for immunomodulation? Blood. 2011;118:5084-5095. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 150] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 204. | Szczepanski MJ, Szajnik M, Czystowska M, Mandapathil M, Strauss L, Welsh A, Foon KA, Whiteside TL, Boyiadzis M. Increased frequency and suppression by regulatory T cells in patients with acute myelogenous leukemia. Clin Cancer Res. 2009;15:3325-3332. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 205. | Li P, Shi X, Xu Y, Zhong B, Lu Y, Sun Y. Interleukin-22 Promotes Osteosarcoma Cell Proliferation and Invasion via STAT3 Activation. Med Sci Monit. 2018;24:7802-7808. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 206. | Iwamoto J, Takeda T, Ichimura S. Treatment with vitamin D3 and/or vitamin K2 for postmenopausal osteoporosis. Keio J Med. 2003;52:147-150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |