Published online Feb 10, 2016. doi: 10.5306/wjco.v7.i1.114
Peer-review started: July 19, 2015
First decision: September 28, 2015
Revised: November 30, 2015
Accepted: December 8, 2015
Article in press: December 11, 2015
Published online: February 10, 2016
Breast cancer is a ubiquitous disease and one of the leading causes of death in women in western societies. With overall increasing survival rates, the number of patients who need post-mastectomy reconstruction is on the rise. Especially since its psychological benefits have been broadly recognized, breast reconstruction has become a key component of breast cancer treatment. Evolving from the early beginnings of breast reconstruction with synthetic implants in the 1960s, microsurgical tissue transfer is on the way to become the gold standard for post oncology restoration of the breast. Particularly since the advent of perforator based free flap surgery, free tissue transfer has become as safe option for breast reconstruction with low morbidity. The lower abdominal skin and subcutaneous fat tissue typically offer enough volume to create an aesthetically satisfying breast mound. Nowadays, the most commonly used flap from this donor site is the deep inferior epigastric artery perforator flap. If the lower abdomen is not available as a donor site, the gluteal area and thigh provide a number of flaps suitable for breast reconstruction. If the required breast volume is small, and there is enough tissue available on the upper medial thigh, then a transverse upper gracilis flap may be a practicable method to reconstruct the breast. In case of a higher amount of required volume, a gluteal artery perforator flap is the best choice. However, what is crucial in addition to selecting the best flap option for the individual patient is the timing of the operation. In patients with confirmed post-mastectomy radiation therapy, it is advisable to perform microvascular breast reconstruction only in a delayed fashion.
Core tip: Mastectomy is a frequent sequela of the treatment and prophylaxis of breast malignancies. Autologous microvascular breast reconstruction is becoming the gold standard in correcting these disfiguring interventions. The lower abdomen, as well as the gluteal and thigh area, is the source of multiple flaps usable for breast reconstruction. With the advent of perforator flap surgery, today’s reconstructive surgeons are able to minimize donor site morbidity whilst maximizing patient outcomes. If timed and performed correctly, microsurgical breast reconstruction is a safe procedure with low donor site morbidities and excellent aesthetic results.
- Citation: Pollhammer MS, Duscher D, Schmidt M, Huemer GM. Recent advances in microvascular autologous breast reconstruction after ablative tumor surgery. World J Clin Oncol 2016; 7(1): 114-121
- URL: https://www.wjgnet.com/2218-4333/full/v7/i1/114.htm
- DOI: https://dx.doi.org/10.5306/wjco.v7.i1.114
Breast cancer is a ubiquitous disease and one of the leading causes of death in women in western societies. While breast conservation techniques together with postoperative radiation often are successful treatment strategies for local control, mastectomy often remains unavoidable in breast cancer therapy[1-3]. Reconstructive options are divided into implant based reconstruction and reconstruction using autologous tissue or a combination of both. Spanning from the early beginnings of breast reconstruction with implants in the 1960s to the autologous microvascular breast restoration of today, plastic surgery continues to offer a great variety of options for women having suffered from mastectomy. Today, with many different methods available, it is important to find a procedure that fits the specific needs of every individual patient[4-6].
The history of breast reconstruction dates back to Vincenz Czerny, who was the first ever to successfully accomplish breast augmentation in 1895, by using a removed lumbar lipoma[7]. One year later, the latissimus dorsi myocutaneous flap was the first described autologous muscle flap for post-mastectomy breast reconstruction[8]. However, for the following decades autologous breast reconstruction was not the gold standard. Surgeons used paraffin injections and polyvinylic alcohol sponge implantation for breast augmentation, with terrible outcomes for the patients[9]. Silicone implants were first introduced in 1961 by Cronin et al[10] for aesthetic breast augmentation. Henceforth silicone implants became the leading option in breast reconstruction. Despite their considerable risk for capsular fibrosis, alloplastic implants are still used in the majority of breast reconstruction procedures worldwide at this day[12].
The rediscovery of the latissimus dorsi flap in the late seventies led to a renaissance of autologous breast reconstruction[13]. The rather limited amount of soft tissue volume that is available by this technique, however, often is not enough to replace the excised breast tissue sufficiently. Therefore, reconstruction of the breast mound with the latissimus dorsi musculocutaneous flap usually demands the addition of a silicone implant.
Striving to overcome the volume deficit of the latissimus dorsi flap and to be able to solely rely on autologous tissue, Scheflan et al[14] pioneered a pedicled abdominal flap with a transverse skin island in 1982. Not only its robust vascularization and the large arc of rotation, but also the concomitant contouring benefits of an abdominoplasty, made this technique popular among surgeons and patients alike[11]. On the contrary, herniation of the abdominal wall, bulk in unwanted regions due to its muscular pedicle as well as venous congestion are well known disadvantages of the pedicled transverse rectus abdominis muscle (TRAM) flap[15]. With the advent of perforator flaps, Koshima et al[16] revolutionized breast reconstruction by completely sparing the rectus muscle in 1989. This new technique, the deep inferior epigastric perforator (DIEP) flap, allowed the raising of large flaps without weakening of the abdominal wall. Consisting exclusively of fat tissue and skin, this autologous flap resembles the real breast tissue more closely and produces a more natural look of the reconstructed breast mound. However, experience has shown that technical challenges, variable anatomy and certain patient characteristics are limiting its applicability. Therefore, alternative flap donor sites also had to be taken into consideration.
Gluteally-based flaps such as the superior gluteal artery perforator (SGAP), the inferior gluteal artery perforator (IGAP) and the fasciocutaneous infragluteal (FCI) flap, as well as medial thigh based-flaps such as the transverse musculocutaneous gracilis (TMG) flap or the profunda artery perforator (PAP) flap have emerged as valuable alternatives if the use of abdominal flaps is precluded. Each of these flaps has particular advantages and disadvantages and indications must be assessed carefully. However, microvascular transplanted flaps should no longer be considered as a last resort, but rather begin to represent the first choice for post mastectomy breast reconstruction. Their ability to resemble a very natural breast mound has made autologous flaps an invaluable addition to the armamentarium of the reconstructive plastic surgeon.
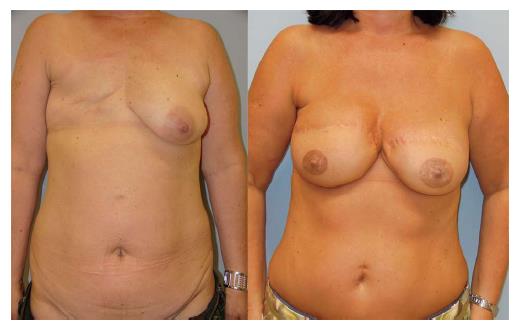
Of all donor sites available, the lower abdomen has evolved as the gold standard in microsurgical breast reconstruction. The lower abdominal skin and subcutaneous fat tissue typically offer enough volume to create an aesthetically satisfying breast mound. Nowadays, the most commonly used flap from this donor site is the deep inferior epigastric artery perforator (DIEAP) flap[17]. Prerequisites for the usage of lower abdominal flaps are enough surplus tissue in this area to match the volume of the remaining breast and the acceptance of a relatively long scar.
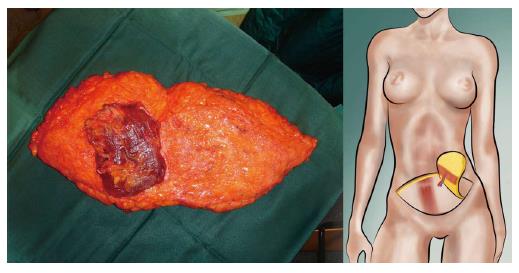
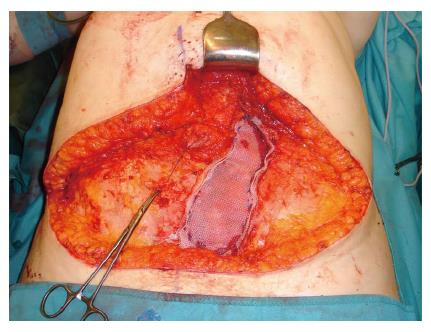
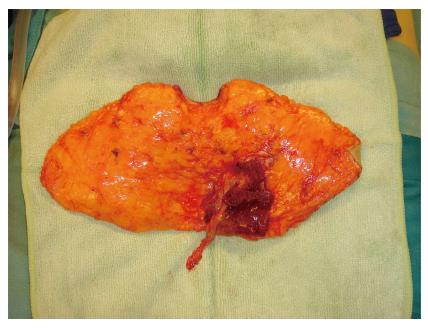
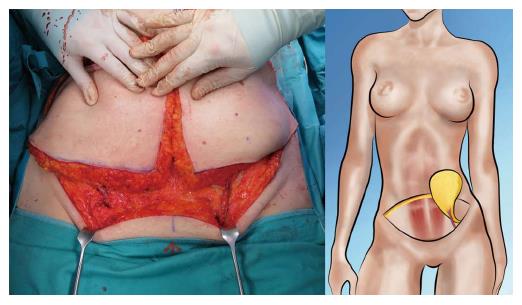
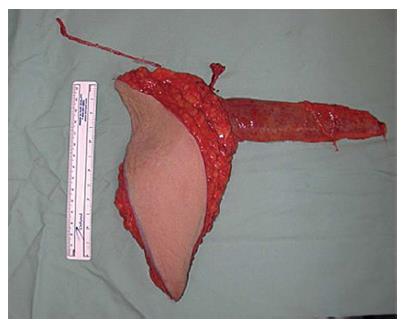
Both, the pedicled TRAM-flap by Scheflan et al[14] and the free TRAM flap by Holmström[18] have been widely used for autologous breast reconstruction in the past decades. The free TRAM flap is composed of a lower abdominal skin island overlying usually one rectus abdominis muscle (Figure 1). The overlying skin island and its subcutaneous fat tissue is supplied by the perforating vessels from the deep inferior epigastric artery (DIEA) travelling through the muscle. It covers approximately the area typically excised in an aesthetic abdominoplasty procedure. The DIEA is dissected together with a 5-6 cm strip of rectus muscle and anastomosed microscopically to the recipient vessel, which is typically the internal mammary artery (IMA) or, less frequently, the thoracodorsal artery. The fascial defect in the lower abdominal wall is typically repaired with an alloplastic mesh (Figure 2) and the wound is closed similarly to an abdominoplasty incision. Significant donor-site morbidity remained a major concern with this technique and resulted in an effort towards conserving as much muscle as possible[19]. Over time, the free TRAM flap sacrificing the whole muscle evolved into the muscle-sparing (ms-) TRAM flap and in further consequence into the DIEP flap with the main goal to minimize donor-site morbidity by harvesting less muscle and fascia. The ms-TRAM flap, in which only a small cuff of muscle around the vascular pedicle is harvested (Figure 3), is still a widely used method in breast reconstruction as it is technically less challenging than the DIEP flap with a comparable donor site morbidity[20].
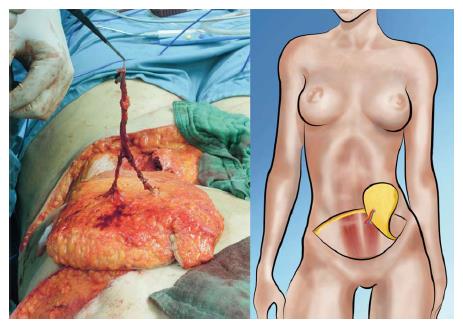
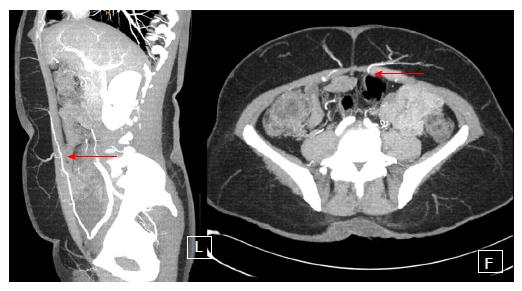
The popularity of the DIEP flap has increased widely over the last decade. Currently it is the gold standard in autologous breast reconstruction not only because of its low donor site morbidity but also because of the satisfying aesthetic outcomes it can achieve. The DIEP flap utilizes the same skin island as the TRAM flap without harvesting the abdominal rectus muscle and anterior rectus sheath. Harvesting DIEP flaps requires considerable expertise in perforator pedicle dissection. Especially during the intramuscular part of the preparation one must dissect carefully to avoid damaging of the perforator vessels, which would subsequently lead to compromised flap circulation[21]. The DIEP flap is generally based on 1-3 perforating vessels and their connection to the DIEA (Figure 4). Unlike the TRAM flap dissection, these perforating vessels are carefully traced through the abdominal rectus muscle to their connection with the DIEA. Prior to surgery, the major perforating vessels can be identified via computed tomographic (CT)-angiography (Figure 5), Doppler ultrasonography or, if available, magnetic resonance imaging-angiography. Preoperative CT-angiography has been shown to reduce operative time, and can help to avoid injuries of the vulnerable pedicle[20]. DIEP flaps are typically outlined similar to aesthetic abdominoplasty procedures and surgical marking takes place in a standing position.
Simultaneous raising of the flap and preparation of the recipient vessels typically requires a two team approach. The IMA and vein are also here the preferred recipient vessels, while the thoracodorsal artery and vein remaining second choice. The IMA is typically approached in the second or third intercostal space. In case of a tight rib interspace, a small part of the rib cartilage can be removed for a better insetting of the pedicle.
After the skin incisions are made the superficial inferior epigastric artery (SIEA) and vein are first approached. In case of sufficient size and quality, they can be dissected down to their origin from the common femoral artery and a SIEA flap can be performed (see below). In case of an unsuitable SIEA, the abdominal skin island is raised carefully from lateral to medial until the lateral row of perforators is reached. Vessel diameter is the key factor in selecting a perforator. Once a perforator has been chosen, the anterior rectus fascia is incised and the perforator is dissected through the muscle until sufficient caliber of the pedicle is reached. Additional perforators in the same row may also be dissected out and included for further blood supply. However, intercostal motor nerves supplying the rectus muscle have been shown to enter the muscle just medial to the lateral row perforators and therefore harvesting of the lateral row perforators may decrease abdominal wall stability.
After completion of dissection, the flap is divided from its pedicle and passed to the chest for anastomosis and inset. The recipient vein and the vein of the flap are typically connected under magnification using an anastomotic coupling device. The arterial anastomosis is generally performed with a 9.0 suture. Upon completion of the anastomosis, the flap is checked for capillary refill. Finally, the incision in the anterior rectus fascia is closed directly without the need of a mesh.
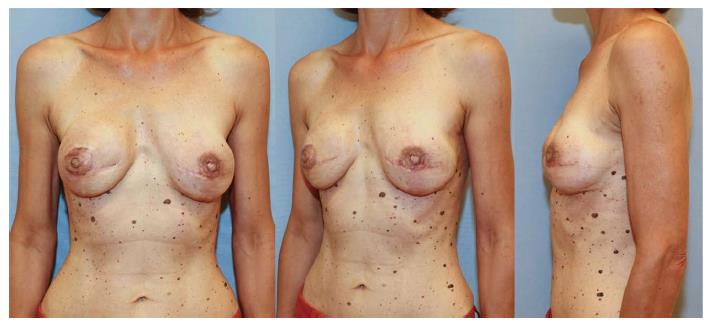
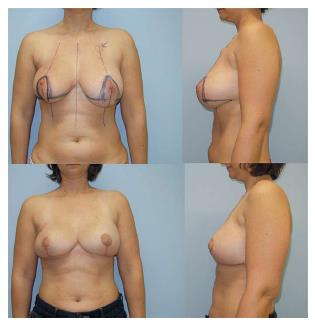
DIEP flaps can be used in uni- and bilateral post-mastectomy breast reconstructions (Figure 6) and may also be used for reconstruction of congenital thoracic defects. There are only a few contraindications for the use of a DIEP flap. In the past, absolute contraindications included history of previous abdominoplasty, liposuction, or active smoking. Large transverse or oblique abdominal incisions were regarded as relative contraindications. Today, as a result of technical refinements, the only real absolute contraindications to DIEP flap reconstructions are non-compliant patients and/or a poor general condition. Obesity itself is not a contraindication, although it may lead to less aesthetically pleasing outcomes[22-24].
Complications occur infrequently. Partial flap loss has been reported as low as only 2.5%, total flap loss with less than 1%[25]. Problems regarding the venous anastomosis appear much more likely than problems with the arterial anastomosis. The most common complications include necrosis of the fat tissue of the flaps and seroma formation. The risk of bulging in DIEP flaps is reported as two thirds lower in comparison to TRAM flap reconstructions. Abdominal herniation occurred in fewer than 1% of all DIEP flaps compared with 3.9% in TRAM flaps[20,24,25]. However, current literature fails to prove a significant difference in the risk of developing abdominal bulge/hernia between ms-TRAM flap and DIEP flap based reconstructions[26]. Additionally, there is no significant difference in the risk of donor site morbidity between bilateral and unilateral DIEP flap breast reconstruction[26].
The SIEA flap is the least invasive option of free abdominal tissue transfer for breast reconstruction. It provides the same tissue as the DIEP flap, without injuring the anterior rectus sheath and no vessel dissection needs to be performed through the muscle (Figure 7). Consequently, the SIEA flap causes the least donor site morbidity of all abdominal flaps, eliminating the risk of developing unwanted abdominal bulk or herniation[27]. However, the dissection of the vascular pedicle requires a high level of expertise, mainly because of its variability in existence, location, and caliber. The superficial inferior epigastric vessels have noted to be absent in 13%-42% of dissections. Furthermore, vessels are often not in appropriate caliber to sufficiently support the perfusion of the tissue volume needed for breast reconstruction. As a consequence, the amount of skin and fat tissue, which may be transferred utilizing a SIEA flap, is limited[22,24,28].
If the lower abdomen is not available as a donor site, the gluteal area and thigh provide a number of flaps suitable for breast reconstruction. If the required breast volume is small, and there is enough tissue available on the upper medial thigh, a transverse upper gracilis flap may be a practicable method to reconstruct the breast. In case of a higher amount of required volume, a gluteal artery perforator flap is the best choice[22,29].
Other more exotic examples of free flaps in use for breast reconstruction, which will not be reviewed here in detail, include the anterolateral thigh flap[30,31] and the recently described PAP flap[32].
The first free gluteal myocutaneous flap for breast reconstruction was performed by Fujino et al[33] in 1975. As the development of the perforator flap technique revolutionized the applicability of soft tissue transfer, gluteal flaps became popular in the 1990s[11]. Gluteal perforator flaps offer a great alternative for breast reconstruction in women who have more tissue available in the buttock area than in the lower abdomen. Similar to the DIEP and SIEA flaps, no sacrifice of muscle is required using this technique.
The superior and inferior GAP (S/I-GAP) flaps have been specified and modified by Allen et al[34-36]. The surgical procedure is similar to the DIEP flap. The gluteal vessel pedicle is dissected throughout the gluteus maximus muscle to their origin in the internal iliac artery. The pedicle is generally about 5-8 cm long, which provides sufficient length for a comfortable anastomosis and inset at the chest wall. A clear disadvantage of this method is in the necessary rearrangement of the patient from abdominal to supine position during surgery. GAP flaps are generally a safe reconstructive option. Vascular complications are reported in approximately 6%, total lap loss in 2% of all cases. However, approximately 4% of all patients require revisions because of donor site problems, such as contour deformity after SGAP, or scar issues after IGAP flaps[12,22,24].
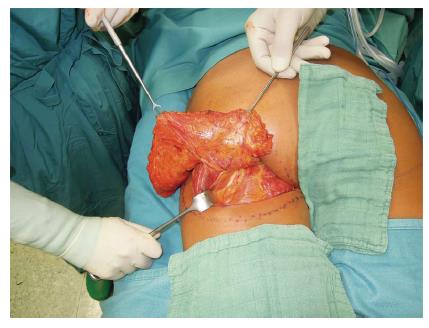
Another, in our opinion more valuable flap of the gluteal region is the FCI flap. The FCI flap is based on the descending branch of the inferior gluteal artery with a very reliable location, good caliber and a pedicle length ranging up to 18 cm (Figure 8). Unlike other gluteal flaps, the vessels curl around the lower border of the gluteus maximus muscle. Therefore, no perforator dissection through the muscle is necessary. Studies demonstrated that even thin patients offer enough tissue to allow breast reconstruction with adequate volume[37], making the FCI flap a convincing alternative to standard free tissue breast reconstruction in certain cases. The tissue from this region is more compact and not as soft compared to the lower abdomen making it more suitable for reconstruction of firm breasts in younger patients (Figure 9).
Since its first description for free tissue transfer in 1976[38] the gracilis flap has become a workhorse in autologous breast reconstruction. The TMG flap is an alternative method for patients who do not have adequate abdominal tissue, or seek to avoid abdominal scarring. The TMG flap is ideally suited for reconstruction of small to moderate sized breasts, providing excellent contour and projection[39]. The donor site is well hidden and harvesting the flap has no functional consequences[40].
The flap is designed with a semilunar skin island in the inner thigh with a mean volume of around 300 cc. The raising of the skin island is started close to the groin and harvested with the underlying fascia. The average pedicle length is reported as 6-8 cm, with an arterial diameter of 1.2 mm and a venous diameter of 2.8 mm. The artery is usually slightly smaller than the IMA, which usually does not preclude a safe anastomosis[41] (Figure 10).
Performing a unilateral TMG flap may cause asymmetry. The use in bilateral reconstruction is reported as one of the strongest advantages of the flap and even recommended as first choice, except for patients that would clearly benefit from an abdominoplasty (Figure 11). As with all flaps including a muscular component, the TMG flap is prone to volume loss resulting from neurogenic atrophy, but a true impact on cosmetic appearance has yet to be demonstrated[22,42]. A true disadvantage of the gracilis flap is the small size of the skin island. A single TMG flap may provide not enough tissue for sufficient reconstruction of a large breast. In this case, two TMG flaps or the usage of a combined infragluteal-transverse myocutaneus gracilis flap can be a viable option[43].
Breast reconstruction can be divided into 3 approaches: Immediate, delayed-immediate and delayed[22]. The advantage of immediate breast reconstruction is the preservation of the breast skin and inframammary fold, which results in optimized aesthetic outcomes and minimized psychological burden of the patient by eliminating a period without a breast. The disadvantage is the uncertainty regarding radiotherapy and chemotherapy and their effects on autologous breast reconstruction. An increased incidence of fat necrosis and a higher rate of surgical revisions are reported in patients with free tissue transfer and further anti cancer therapy[44].
Therefore, a delayed-immediate reconstruction may be a good alternative. Expander implants are inserted as a temporary placeholder to maintain the breast skin envelope. Breast reconstruction is performed once radiotherapy is complete. In contrast, a fully delayed reconstruction offers the advantage of a typically very motivated patient with completed oncological treatment. The obvious disadvantage is the reduction of skin tissue and the presence of significant scarring[45].
The optimal timing of breast reconstruction in relation to radiation therapy is controversially discussed. Therefore, it is advisable to perform autologous microvascular breast reconstruction in patients, who are confirmed for post-mastectomy radiation therapy, only in a delayed fashion. If post-mastectomy radiation therapy is likely but not certain, delayed-immediate breast reconstruction may be the better choice. Nonetheless, because of the mostly satisfying aesthetic outcome and the contradictory literature regarding this topic, immediate autologous breast reconstruction should at least be considered and discussed with the patient, even when post-mastectomy radiotherapy is anticipated.
Autologous microvascular breast reconstruction is becoming more and more accepted as a key component of breast cancer treatment after mastectomy. The abdominal wall or, alternatively, the gluteal and thigh areas provide numerous flaps suitable for breast reconstruction with appealing results. Recent advances in surgical technique enable today’s reconstructive surgeons to minimize donor site morbidity whilst maximizing patient outcomes. If timed and performed correctly, microsurgical breast reconstruction is able to provide the best aesthetic outcomes with low complication rates and donor site morbidity.
P- Reviewer: Li Y S- Editor: Gong XM L- Editor: A E- Editor: Jiao XK
| 1. | Iwuchukwu OC, Harvey JR, Dordea M, Critchley AC, Drew PJ. The role of oncoplastic therapeutic mammoplasty in breast cancer surgery--a review. Surg Oncol. 2012;21:133-141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 2. | Berry MG, Fitoussi AD, Curnier A, Couturaud B, Salmon RJ. Oncoplastic breast surgery: a review and systematic approach. J Plast Reconstr Aesthet Surg. 2010;63:1233-1243. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 79] [Cited by in F6Publishing: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 3. | McLaughlin SA. Surgical management of the breast: breast conservation therapy and mastectomy. Surg Clin North Am. 2013;93:411-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Noone RB. Thirty-five years of breast reconstruction: eleven lessons to share. Plast Reconstr Surg. 2009;124:1820-1827. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Preminger BA, Lemaine V, Sulimanoff I, Pusic AL, McCarthy CM. Preoperative patient education for breast reconstruction: a systematic review of the literature. J Cancer Educ. 2011;26:270-276. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Sbitany H, Amalfi AN, Langstein HN. Preferences in choosing between breast reconstruction options: a survey of female plastic surgeons. Plast Reconstr Surg. 2009;124:1781-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Goldwyn RM. Vincenz Czerny and the beginnings of breast reconstruction. Plast Reconstr Surg. 1978;61:673-681. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Olivari N. The latissimus flap. Br J Plast Surg. 1976;29:126-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 288] [Cited by in F6Publishing: 296] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Glatt BS, Afifi G, Noone RB. Long-term follow-up of a sponge breast implant and review of the literature. Ann Plast Surg. 1999;42:196-201. [PubMed] [Cited in This Article: ] |
| 10. | Cronin TD, Greenberg RL. Our experiences with the silastic gel breast prosthesis. Plast Reconstr Surg. 1970;46:1-7. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 56] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Champaneria MC, Wong WW, Hill ME, Gupta SC. The evolution of breast reconstruction: a historical perspective. World J Surg. 2012;36:730-742. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Pelzer M, Reichenberger MA, Germann G. [Microsurgical techniques for breast reconstruction]. Chirurg. 2011;82:807-812. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Olivari N. Use of thirty latissimus dorsi flaps. Plast Reconstr Surg. 1979;64:654-661. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 48] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Scheflan M, Hartrampf CR, Black PW. Breast reconstruction with a transverse abdominal island flap. Plast Reconstr Surg. 1982;69:908-909. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 15. | Ozkan A, Cizmeci O, Aydin H, Ozden BC, Tümerdem B, Emekli U, Asoğlu O, Bozfakioğlu Y. The use of the ipsilateral versus contralateral pedicle and vertical versus horizontal flap inset models in TRAM flap breast reconstruction: the aesthetic outcome. Aesthetic Plast Surg. 2002;26:451-456. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Koshima I, Soeda S. Inferior epigastric artery skin flaps without rectus abdominis muscle. Br J Plast Surg. 1989;42:645-648. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 800] [Cited by in F6Publishing: 753] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 17. | Gurunluoglu R, Gurunluoglu A, Williams SA, Tebockhorst S. Current trends in breast reconstruction: survey of American Society of Plastic Surgeons 2010. Ann Plast Surg. 2013;70:103-110. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 18. | Holmström H. The free abdominoplasty flap and its use in breast reconstruction. An experimental study and clinical case report. Scand J Plast Reconstr Surg. 1979;13:423-427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 311] [Cited by in F6Publishing: 327] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 19. | Kroll SS, Schusterman MA, Reece GP, Miller MJ, Robb G, Evans G. Abdominal wall strength, bulging, and hernia after TRAM flap breast reconstruction. Plast Reconstr Surg. 1995;96:616-619. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 177] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 20. | Egeberg A, Rasmussen MK, Sørensen JA. Comparing the donor-site morbidity using DIEP, SIEA or MS-TRAM flaps for breast reconstructive surgery: a meta-analysis. J Plast Reconstr Aesthet Surg. 2012;65:1474-1480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 21. | Man LX, Selber JC, Serletti JM. Abdominal wall following free TRAM or DIEP flap reconstruction: a meta-analysis and critical review. Plast Reconstr Surg. 2009;124:752-764. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 159] [Cited by in F6Publishing: 170] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 22. | Healy C, Ramakrishnan V. Autologous microvascular breast reconstruction. Arch Plast Surg. 2013;40:3-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 23. | Serletti JM, Moran SL. Microvascular reconstruction of the breast. Semin Surg Oncol. 2000;19:264-271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 24. | Granzow JW, Levine JL, Chiu ES, Allen RJ. Breast reconstruction with the deep inferior epigastric perforator flap: history and an update on current technique. J Plast Reconstr Aesthet Surg. 2006;59:571-579. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Gill PS, Hunt JP, Guerra AB, Dellacroce FJ, Sullivan SK, Boraski J, Metzinger SE, Dupin CL, Allen RJ. A 10-year retrospective review of 758 DIEP flaps for breast reconstruction. Plast Reconstr Surg. 2004;113:1153-1160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 400] [Cited by in F6Publishing: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 26. | Chang EI, Chang EI, Soto-Miranda MA, Zhang H, Nosrati N, Robb GL, Chang DW. Comprehensive analysis of donor-site morbidity in abdominally based free flap breast reconstruction. Plast Reconstr Surg. 2013;132:1383-1391. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 27. | Hamdi M, Larsen M, Craggs B, Vanmierlo B, Zeltzer A. Harvesting free abdominal perforator flaps in the presence of previous upper abdominal scars. J Plast Reconstr Aesthet Surg. 2014;67:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Hadad I, Ibrahim AM, Lin SJ, Lee BT. Augmented SIEA flap for microvascular breast reconstruction after prior ligation of bilateral deep inferior epigastric arteries. J Plast Reconstr Aesthet Surg. 2013;66:845-847. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Yu SC, Kleiber GM, Song DH. An algorithmic approach to total breast reconstruction with free tissue transfer. Arch Plast Surg. 2013;40:173-180. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Wei FC, Suominen S, Cheng MH, Celik N, Lai YL. Anterolateral thigh flap for postmastectomy breast reconstruction. Plast Reconstr Surg. 2002;110:82-88. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Rosenberg JJ, Chandawarkar R, Ross MI, Chevray PM. Bilateral anterolateral thigh flaps for large-volume breast reconstruction. Microsurgery. 2004;24:281-284. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Allen RJ, Haddock NT, Ahn CY, Sadeghi A. Breast reconstruction with the profunda artery perforator flap. Plast Reconstr Surg. 2012;129:16e-23e. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 157] [Cited by in F6Publishing: 174] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 33. | Fujino T, Harashina T, Enomoto K. Primary breast reconstruction after a standard radical mastectomy by a free flap transfer. Case report. Plast Reconstr Surg. 1976;58:371-374. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 55] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Allen RJ. The superior gluteal artery perforator flap. Clin Plast Surg. 1998;25:293-302. [PubMed] [Cited in This Article: ] |
| 35. | Allen RJ, Levine JL, Granzow JW. The in-the-crease inferior gluteal artery perforator flap for breast reconstruction. Plast Reconstr Surg. 2006;118:333-339. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 36. | Allen RJ, Tucker C. Superior gluteal artery perforator free flap for breast reconstruction. Plast Reconstr Surg. 1995;95:1207-1212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 255] [Cited by in F6Publishing: 225] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Papp C, Windhofer C, Michlits W. Autologous breast augmentation with the deepithelialized fasciocutaneous infragluteal free flap: a 10-year experience. Ann Plast Surg. 2011;66:587-592. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 13] [Cited by in F6Publishing: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 38. | Harii K, Ohmori K, Sekiguchi J. The free musculocutaneous flap. Plast Reconstr Surg. 1976;57:294-303. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 84] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Buntic RF, Horton KM, Brooks D, Althubaiti GA. Transverse upper gracilis flap as an alternative to abdominal tissue breast reconstruction: technique and modifications. Plast Reconstr Surg. 2011;128:607e-613e. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 40. | Schoeller T, Huemer GM, Wechselberger G. The transverse musculocutaneous gracilis flap for breast reconstruction: guidelines for flap and patient selection. Plast Reconstr Surg. 2008;122:29-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 106] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 41. | Wechselberger G, Schoeller T. The transverse myocutaneous gracilis free flap: a valuable tissue source in autologous breast reconstruction. Plast Reconstr Surg. 2004;114:69-73. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 116] [Cited by in F6Publishing: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Bodin F, Schohn T, Lutz JC, Zink S, Wilk A, Bruant Rodier C. [The transverse musculocutaneous gracilis free flap: Innovative autologous breast reconstruction]. Ann Chir Plast Esthet. 2013;58:18-27. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 43. | Schoeller T, Huemer GM, Kolehmainen M, Otto-Schoeller A, Wechselberger G. A new „Siamese“ flap for breast reconstruction: the combined infragluteal-transverse myocutaneous gracilis muscle flap. Plast Reconstr Surg. 2005;115:1110-1117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 44. | Schaverien MV, Macmillan RD, McCulley SJ. Is immediate autologous breast reconstruction with postoperative radiotherapy good practice?: a systematic review of the literature. J Plast Reconstr Aesthet Surg. 2013;66:1637-1651. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 45. | Kronowitz SJ, Robb GL. Radiation therapy and breast reconstruction: a critical review of the literature. Plast Reconstr Surg. 2009;124:395-408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 267] [Cited by in F6Publishing: 257] [Article Influence: 17.1] [Reference Citation Analysis (0)] |