Published online Nov 18, 2014. doi: 10.5312/wjo.v5.i5.694
Revised: March 30, 2014
Accepted: May 31, 2014
Published online: November 18, 2014
Vanishing bone disease (Gorham-Stout syndrome) is a rare entity of unknown etiology, characterized by destruction of osseous matrix and proliferation of vascular structures, resulting in destruction and absorption of bone. Despite the extensive investigation of the pathogenetic mechanisms of the disease, its etiology hasn’t been clarified and several theories exist. The syndrome can affect one or multiple bones of the patient, including the skull, the upper and lower extremities, the spine and pelvis. The clinical presentation of a patient suffering from vanishing bone disease includes, pain, functional impairment and swelling of the affected region, although asymptomatic cases have been reported, as well as cases in which the diagnosis was made after a pathologic fracture. In this short review we summarize the theories regarding the etiology as well as the clinical presentation, the diagnostic approach and treatment options of this rare disease.
Core tip: Vanishing bone disease (Gorham-Stout syndrome) is a rare entity of unknown etiology, characterized by destruction of osseous matrix and proliferation of vascular structures, resulting in destruction and absorption of bone. The syndrome can affect one or multiple bones of the patient, including the skull, the upper and lower extremities, the spine and pelvis. Physicians should be aware of the existence of this rare entity and reliably direct affected patients to right diagnosis and therapeutic approach.
- Citation: Nikolaou VS, Chytas D, Korres D, Efstathopoulos N. Vanishing bone disease (Gorham-Stout syndrome): A review of a rare entity. World J Orthop 2014; 5(5): 694-698
- URL: https://www.wjgnet.com/2218-5836/full/v5/i5/694.htm
- DOI: https://dx.doi.org/10.5312/wjo.v5.i5.694
Vanishing bone disease is a rare entity characterized by destruction of osseous matrix and proliferation of vascular structures with benign origin[1]. Despite the extensive investigation of the pathogenetic mechanisms of the disease, its etiology hasn’t been clarified. The first that described this entity was Jackson in 1838, who reported the case of a young man with a gradually vanishing humerus[2]. Moreover, in 1955, Gorham and Stout published a paper, which correlated the massive osteolysis noted in the disease with hemangiomatosis[3], that seems to have played an important role in the fact that vanishing bone disease is also called “Gorham-Stout syndrome”.
This syndrome is considered as the type IV of osteolysis, according to Hardegger et al[4], among five types: type I is hereditary multicentric osteolysis with dominant transmission, type II is hereditary multicentric osteolysis with recessive transmission, type III is nonhereditary multicentric osteolysis with nephropathy and type V is Winchester syndrome, defined as a monocentric disease of autosomal recessive inheritance[4].
Despite its previously mentioned benign character, its prognosis is unpredictable[5] and the presence of several serious complications in some cases cannot be ignored. Therefore, meticulous research has been done concerning the molecular mechanisms of the disease and possible pharmacological targets are systematically investigated.
Principally, a reference to the molecular basis of the disease has to be done. Dickson et al[6] in 1987 were the first that approached Gorham-Stout syndrome from a cytochemical point of view. Investigating the cytochemistry of alkaline and acid phosphatase, they suggested that mononuclear phagocytes, multinuclear osteoclasts and the vascular endothelium participate in bone resorption in this disease[6]. Furthermore, in 1996, Devlin et al[7] attributed this massive osteolysis to the enhanced activity of the osteoclasts, in which Interleukin-6 seems to play a critical role, since its levels in the serum of patients suffering from Gorham-Stout syndrome in early stages, were elevated. Moreover, an interesting observation of Korsić et al[8], in 1998, was that the disease appeared in a person with agenesis of C-cells of the thyroid gland and subsequent lack of calcitonin, an hormone with antiosteoclastic activity. On the other hand, Möller et al[9] in 1999 referred to the increased number of stimulated osteoclasts as a factor that is involved in the pathogenesis of vanishing bone disease, while, in 2001, Hirayama et al[10] concluded that the increased number of the circulating osteoclasts is the consequence of the increased sensitivity of their precursors to humoral factors that lead to osteoclast formation. Another important point about the histopathology of Gorham-Stout syndrome was noted by Colucci et al[11] (in 2006), who found that the cells they isolated from a patient’s lesion belonged to a monocyte-macrophage lineage and could release high amounts of osteoclastogenic and angiogenic molecules. Additionally, Hagendoorn et al[12] (in 2006) underlined the critical role that could play the signaling pathway of the PDGFR-b (receptor of the lymphangiogenic growth factor Platelet Derived Growth Factor BB) in the pathogenetic mechanism of the disease. Besides, in 2007, Bruch-Gerharz et al[5] tried to shed more light onto the pathogenesis of the syndrome, writing about lymphatic vascular malformations involving the skin and the soft tissues adjacent to the diseased bone. The character of Gorham-Stout syndrome as a disease of disordered lymphangiogenesis is also highlighted by Radhakrishnan et al[13] (in 2008), who supported that research should focus on the investigation of lymphangiogenic pathways.
The syndrome can affect one or multiple bones of the patient, whose age has been reported to be from 1 mo[14] to 75 years old[15]. As a general rule, the persons that suffer from the disease are younger than 40-year-old[16], while an epidemiologic correlation with race, gender and geography does not seem to exist[9,17-19]. However, some authors noticed a clear “predilection” of the disease in males[20].
Gorham-Stout syndrome is met in a large spectrum of bones, but the majority of case reports refer to the maxillofacial region and the upper extremity[16]. Nevertheless, Hu et al[20] recently reported a case series, in which the femur was the predominant affected bone.
Radiologicaly, initial X-rays reveal changes resembling patchy osteoporosis. At a later stage bone deformity occurs with bone mass loss and concentric shrinkage in the long bones of upper and lower extremities. Eventually, near complete resorption of the bone occurs, resulting in the appearance of the so-called “vanishing bone” disease[9,21].
The clinical presentation of a patient suffering from vanishing bone disease includes, most frequently, pain, functional impairment and swelling of the affected region, although asymptomatic cases have been reported, as well as cases in which the diagnosis was made after a pathologic fracture[20]. Furthermore, the complications of the syndrome can be potentially fatal. For example, pleural effusion and chylothorax (which has been reported to be a complication of Gorham-Stout syndrome in a percentage up to 17% )[22] can dramatically influence the respiratory function. Chylothorax may occur due to the affected thoracic skeleton by the extension of lymphangiectasia into the pleural cavity or by the invasion of the thoracic duct[23]. Also, hemangiomatosous cutaneous lesions[5,24], bone infection and subsequent septic shock[24], spinal cord involvement and paraplegia due to vertebral lesion[25] and cerebrospinal fluid leakage and meningitis due to diseased bones of the skull[26] have been rarely reported.
The differential diagnosis of the disease includes hereditary multicentric osteolysis, osteolysis with nephropathy, osteomyelitis, rheumatoid arthritis[27], osteolysis due to intraosseous malignacies, hyperparathyroidism, eosinophilic granuloma and osteolysis due to diseases of central nervous system, like syringomyelia and tabes dorsalis[28].
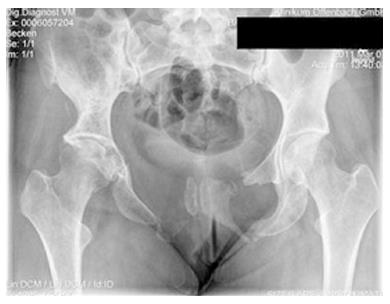
Figures 1 and 2 shows a case example of a massive Gorham-Stout syndrome of the Pelvis in a young female, treated in our institution. Only few similar cases of pelvic appearance of the disease have been described in the literature[17,29-31].
The diagnosis of the syndrome is challenging, demands a high grade of clinical suspicion and is assisted by several diagnostic examinations. Initially, blood tests are not indicative of the diagnosis, as they are usually normal, with the possible exception of alkaline phosphatase, which may be slightly elevated[23]. Additionally, a diagnostic role could be played by plain radiographs[32,33], bone scan[34], Computed tomography[35] and magnetic resonance imaging (MRI)[36]. Plain X-rays, initially, show radiolucent foci in the intramedullary or subcortical regions and, later, slowly progressive atrophy, dissolution, fracture, fragmentation and disappearance of a part of a bone, with tapering or “pointing” of the remaining osseous tissue and atrophy of soft tissues[37]. On the other hand, the results from bone scan and MRI are variable[16,38].
Despite the usefulness of the previously reported diagnostic means, the disease is confirmed by the histopathological analysis of the lesions[39]; the biopsy shows nonmalignant hyperproliferation of small vessels[4,5,17]. So, Heffez et al[40] suggested the following 8 diagnostic criteria of Gorham-Stout syndrome: (1) positive biopsy findings in terms of angiomatous tissue presence; (2) absence of cellular atypia; (3) minimal or no osteoclastic response and absence of dystrophic calcifications; (4) evidence of local bone progressive resorption; (5) non-expansive, non-ulcerative lesion; (6) absence of visceral involvement; (7) osteolytic radiographic pattern; and (8) negative hereditary, metabolic, neoplastic, immunologic and infectious etiology[40]. Indeed, the diagnosis of vanishing bone disease should be suspected only after other causes of osteolysis, like infection, cancer, inflammatory and endocrine disorders are excluded[41].
Vanishing bone disease, despite the fact that is considered as benign[42] and its natural progression is characterized by spontaneous resolution[4], has an unpredictable prognosis[5] and possible serious complications. Since its etiology remains unclear, its treatment is still an object of research, although several therapeutic options have been proposed with various results. The treatment of syndrome includes three major categories: medicine therapy, radiation and surgery[20]. In the first field, biphoshonates have been successfully used for the treatment of the syndrome, showing an antiosteolytic activity[43]. Besides, other pharmacologic agents, like vitamin D, a-2b interferon, calcium, adrenal extracts and androgens have been suggested[5,13,39,44]. However, current research about the molecular mechanisms of the disease promises encouraging results, focusing on pathways of receptors of lymphangiogenic growth factors, which may be proved useful therapeutic targets[4].
In patients with large symptomatic lesions with long-standing disabling functional instability, radiation and surgical treatment are preferred[23]. The therapeutic results from the use of radiation in moderate doses seem to be satisfactory, with few long-term complications[42]. However, radiation could provoke some serious side effects, like secondary malignancy and growth restriction in children and adolescents who receive a high-dose therapy[23]. Finally, the surgical management is performed by resection of the lesion and reconstruction by use of bone grafts and/or prostheses[16].
In the case of chylothorax, several therapeutic solutions, like chest drainage[20], thoracic duct ligation, pleurodesis, pleurectomy, radiation therapy, interferon and bleomycin have been suggested[23].
In conclusion, provided that the etiopathology of vanishing bone disease has not been fully clarified, further research is needed for more effective therapeutic interventions. Physicians should be aware of the existence of this rare entity and reliably direct affected patients to correct diagnosis and therapeutic approach.
P- Reviewer: Espinosa-Gutierrez A, Mazinis E, Xu CY, Zacharia B S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Deveci M, Inan N, Corapcioglu F, Ekingen G. Gorham-stout syndrome with chylothorax in a six-year-old boy. Indian J Pediatr. 2011;78:737-739. [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Jackson J. A boneless arm. Boston Med Surg J. 1838;18:398-399. [Cited in This Article: ] |
| 3. | Gorham LW, Stout AP. Massive osteolysis (acute spontaneous absorption of bone, phantom bone, disappearing bone); its relation to hemangiomatosis. J Bone Joint Surg Am. 1955;37-A:985-1004. [PubMed] [Cited in This Article: ] |
| 4. | Hardegger F, Simpson LA, Segmueller G. The syndrome of idiopathic osteolysis. Classification, review, and case report. J Bone Joint Surg Br. 1985;67:88-93. [PubMed] [Cited in This Article: ] |
| 5. | Bruch-Gerharz D, Gerharz CD, Stege H, Krutmann J, Pohl M, Koester R, Ruzicka T. Cutaneous lymphatic malformations in disappearing bone (gorham-stout) disease: A novel clue to the pathogenesis of a rare syndrome. J AM ACAD DERMATOL. 2007;56:S21-25. [DOI] [Cited in This Article: ] [Cited by in Crossref: 53] [Cited by in F6Publishing: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Dickson GR, Mollan RA, Carr KE. Cytochemical localization of alkaline and acid phosphatase in human vanishing bone disease. Histochemistry. 1987;87:569-572. [PubMed] [Cited in This Article: ] |
| 7. | Devlin RD, Bone HG, 3rd , Roodman GD. Interleukin-6: A potential mediator of the massive osteolysis in patients with gorham-stout disease. J CLIN ENDOCR METAB. 1996;81:1893-1897. [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Korsić M, Jelasić D, Potocki K, Giljević Z, Aganović I. Massive osteolysis in a girl with agenesis of thyroid C cells. Skeletal Radiol. 1998;27:525-528. [PubMed] [Cited in This Article: ] |
| 9. | Möller G, Priemel M, Amling M, Werner M, Kuhlmey AS, Delling G. The Gorham-Stout syndrome (Gorham’s massive osteolysis). A report of six cases with histopathological findings. J Bone Joint Surg Br. 1999;81:501-506. [PubMed] [Cited in This Article: ] |
| 10. | Hirayama T, Sabokbar A, Itonaga I, Watt-Smith S, Athanasou NA. Cellular and humoral mechanisms of osteoclast formation and bone resorption in gorham-stout disease. J PATHOL. 2001;195:624-630. [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 103] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 11. | Colucci S, Taraboletti G, Primo L, Viale A, Roca C, Valdembri D, Geuna M, Pagano M, Grano M, Pogrel AM. Gorham-stout syndrome: A monocyte-mediated cytokine propelled disease. J BONE MINER RES. 2006;21:207-218. [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Hagendoorn J, Padera TP, Yock TI, Nielsen GP, di Tomaso E, Duda DG, Delaney TF, Gaissert HA, Pearce J, Rosenberg AE. Platelet-derived growth factor receptor-beta in Gorham's disease. Nat Clin Pract Oncol. 2006;3:693-697. [PubMed] [Cited in This Article: ] |
| 13. | Radhakrishnan K, Rockson SG. Gorham’s disease: An osseous disease of lymphangiogenesis? ANN NY ACAD SCI. 2008;1131:203-205. [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 3.3] [Reference Citation Analysis (1)] |
| 14. | Vinée P, Tanyü MO, Hauenstein KH, Sigmund G, Stöver B, Adler CP. CT and MRI of Gorham syndrome. J Comput Assist Tomogr. 1994;18:985-989. [PubMed] [Cited in This Article: ] |
| 15. | Szabo C, Habre W. Gorham syndrome: anaesthetic management. Anaesthesia. 2000;55:157-159. [PubMed] [Cited in This Article: ] |
| 16. | Kiran DN, Anupama A. Vanishing bone disease: A review. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons 2011; 199-203. [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Boyer P, Bourgeois P, Boyer O, Catonné Y, Saillant G. Massive Gorham-Stout syndrome of the pelvis. Clin Rheumatol. 2005;24:551-555. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 18. | Lee S, Finn L, Sze RW, Perkins JA, Sie KC. Gorham Stout syndrome (disappearing bone disease): two additional case reports and a review of the literature. Arch Otolaryngol Head Neck Surg. 2003;129:1340-1343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 57] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Mendez AA, Keret D, Robertson W, MacEwen GD. Massive osteolysis of the femur (Gorham’s disease): a case report and review of the literature. J Pediatr Orthop. 1989;9:604-608. [PubMed] [Cited in This Article: ] |
| 20. | Hu P, Yuan XG, Hu XY, Shen FR, Wang JA. Gorham-Stout syndrome in mainland China: a case series of 67 patients and review of the literature. J Zhejiang Univ Sci B. 2013;14:729-735. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 21. | Ross JL, Schinella R, Shenkman L. Massive osteolysis. An unusual cause of bone destruction. Am J Med. 1978;65:367-372. [PubMed] [Cited in This Article: ] |
| 22. | Tie ML, Poland GA, Rosenow EC. Chylothorax in Gorham’s syndrome. A common complication of a rare disease. Chest. 1994;105:208-213. [PubMed] [Cited in This Article: ] |
| 23. | Patel DV. Gorham’s disease or massive osteolysis. Clin Med Res. 2005;3:65-74. [PubMed] [Cited in This Article: ] |
| 24. | Kery L, Wouters HW. Massive osteolysis. Report of two cases. J Bone Joint Surg Br. 1970;52:452-459. [PubMed] [Cited in This Article: ] |
| 25. | Halliday DR, Dahlin DC, Pugh DG, Young HH. Massive osteolysis and angiomatosis. Radiology. 1964;82:637-644. [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 77] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Iyer GV. Cerebrospinal fluid rhinorrhoea from massive osteolysis of the skull. J Neurol Neurosurg Psychiatry. 1979;42:767-769. [PubMed] [Cited in This Article: ] |
| 27. | Ozbayrak M, Yilmaz MH, Kantarci F, Ozer H, Harmanci K, Babacan M, Dervisoglu S. A case of an idiopathic massive osteolysis with skip lesions. Korean J Radiol. 2013;14:946-950. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Motamedi MH, Homauni SM, Behnia H. Massive osteolysis of the mandible: a case report. J Oral Maxillofac Surg. 2003;61:957-963. [PubMed] [Cited in This Article: ] |
| 29. | Leite I, Hernández-Martín A, Colmenero I, López-Gutiérrez JC, Torrelo A. Invasive lymphatic malformation (gorham-stout) of the pelvis with prominent skin involvement. Pediatr Dermatol. 2013;30:374-378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Lehmann G, Pfeil A, Böttcher J, Kaiser WA, Füller J, Hein G, Wolf G. Benefit of a 17-year long-term bisphosphonate therapy in a patient with Gorham-Stout syndrome. Arch Orthop Trauma Surg. 2009;129:967-972. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 31. | Stöve J, Reichelt A. Massive osteolysis of the pelvis, femur and sacral bone with a Gorham-Stout syndrome. Arch Orthop Trauma Surg. 1995;114:207-210. [PubMed] [Cited in This Article: ] |
| 32. | Torg JS, Steel HH. Sequential roentgenographic changes occurring in massive osteolysis. J Bone Joint Surg Am. 1969;51:1649-1655. [PubMed] [Cited in This Article: ] |
| 33. | Abrahams J, Ganick D, Gilbert E, Wolfson J. Massive osteolysis in an infant. AM J ROENTGENOL. 1980;135:1084-1086. [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 34. | Marymont JV. Comparative imaging. Massive osteolysis (Gorham’s syndrome, disappearing bone disease). Clin Nucl Med. 1987;12:153-154. [PubMed] [Cited in This Article: ] |
| 35. | Mitchell CS, Parisi MT, Osborn RE. Gorham’s disease involving the thoracic skeleton. Plain films and CT in two cases. Pediatr Radiol. 1993;23:543-544. [PubMed] [Cited in This Article: ] |
| 36. | Assoun J, Richardi G, Railhac JJ, Le Guennec P, Caulier M, Dromer C, Sixou L, Fournie B, Mansat M, Durroux D. CT and MRI of massive osteolysis of Gorham. J Comput Assist Tomogr. 1994;18:981-984. [PubMed] [Cited in This Article: ] |
| 37. | Resnick D. Osteolysis and chondrolysis. Philadelphia, PA: Saunders 2002; 4920-4944. [Cited in This Article: ] |
| 38. | Spieth ME, Greenspan A, Forrester DM, Ansari AN, Kimura RL, Gleason-Jordan I. Gorham’s disease of the radius: radiographic, scintigraphic, and MRI findings with pathologic correlation. A case report and review of the literature. Skeletal Radiol. 1997;26:659-663. [PubMed] [Cited in This Article: ] |
| 39. | Binder H, Weber PC, Siess W. Separation of inositol phosphates and glycerophosphoinositol phosphates by high-performance liquid chromatography. Anal Biochem. 1985;148:220-227. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Heffez L, Doku HC, Carter BL, Feeney JE. Perspectives on massive osteolysis. Report of a case and review of the literature. Oral Surg Oral Med Oral Pathol. 1983;55:331-343. [PubMed] [Cited in This Article: ] |
| 41. | Raghuveer HP, Jayalekshmy R. Gorham’s massive osteolysis of the mandible - a progressive radiographic presentation. Dentomaxillofac Radiol. 2009;38:292-295. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Dunbar SF, Rosenberg A, Mankin H, Rosenthal D, Suit HD. Gorham’s massive osteolysis: the role of radiation therapy and a review of the literature. Int J Radiat Oncol Biol Phys. 1993;26:491-497. [PubMed] [Cited in This Article: ] |
| 43. | Hammer F, Kenn W, Wesselmann U, Hofbauer LC, Delling G, Allolio B, Arlt W. Gorham-stout disease--stabilization during bisphosphonate treatment. J BONE MINER RES. 2005;20:350-353. [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 83] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Aizawa T, Sato T, Kokubun S. Gorham disease of the spine: a case report and treatment strategies for this enigmatic bone disease. Tohoku J Exp Med. 2005;205:187-196. [PubMed] [Cited in This Article: ] |