Published online Mar 27, 2014. doi: 10.5313/wja.v3.i1.119
Revised: August 15, 2013
Accepted: August 28, 2013
Published online: March 27, 2014
AIM: Providing analgesia via peripheral nerve catheters attached to an infusion pump is an effective pain management option in children.
METHODS: Portable infusion pumps are being used with increased frequency in pediatric patients. Because these pumps are infusing potentially toxic doses of medications, the accuracy and consistency of these devices becomes very important in this patient population. This study is a comparison of the actual delivery volume of local anesthetic of three elastomeric infusion devices approved for patient use in the ambulatory setting. Three brands of disposable elastomeric infusion devices were used (Five On-Q, Five Baxter, and Five Ambu pumps). Each was filled with 200 mL of Ropivicaine 0.1% and connected to a single, end hole infusion catheter and set to infuse at 12 mL/h. The devices were run simultaneously. The fluid delivered was measured every hour with a graduated column over a tenhour period. The ambient temperature was also recorded.
RESULTS: There were statistically significant differences in the output from each elastomeric device over the 10 h infusion period when compared to the nominal rate of 12 mL/h. The output from the Ambu and Baxter pumps was less than that set on the regulator, while the output from the On-Q pump was greater than that set on the regulator. The results remained statistically significant after adjusting the nominal rate to correct for differences in temperature. The Ambu infusion device was the most consistent, while the Baxter infusion device was the most accurate. This emphasizes the importance of health care providers understanding the infusion profile of the pump being used for continuous peripheral nerve block, as these alterations in flow could result in inadequate analgesia, early reservoir exhaustion, excessive muscle weakness or potential toxicity, especially when used in pediatric patients.
CONCLUSION: This investigation demonstrates that three modern elastomeric infusion pumps have significantly different output than the nominal rate set on the regulator.
Core tip: This study demonstrates that three single use elastomeric infusion devices have rates that are significantly different from the set nominal delivery volume. These alterations in flow may be clinically significant, resulting in either inadequate analgesia, early exhaustion of the reservoir, excessive muscle weakness or the potential for toxicity, especially when used with pediatric patients. Therefore, in order to provide the best care, physicians must not only take into account the temperature at which the pump will be kept, viscosity of the solution used, and the height of the reservoir but also the infusion profile of the individual pump being used.
- Citation: LeRiger M, Bhalla T, Martin D, Bettesworth J, Tobias JD. Comparison of flow rate accuracy and consistency between the on-Q, baxter, and ambu pain infusion devices. World J Anesthesiol 2014; 3(1): 119-123
- URL: https://www.wjgnet.com/2218-6182/full/v3/i1/119.htm
- DOI: https://dx.doi.org/10.5313/wja.v3.i1.119
There are multiple benefits of postoperative perineural local anesthetic infusions have been shown, including potent analgesia, decreased opioid requirements, and improved rehabilitation[1]. Disposable elastomeric pumps with peri-neural infusions are as effective as patient controlled intravenous pumps for post-operative pain relief[2]. A prospective descriptive study of children undergoing major orthopedic surgery determined that disposable elastomeric pumps for perioperative continuous peripheral nerve block (CPNB) provided excellent postoperative analgesia with no adverse effects noted[3]. They concluded that the use of elastomeric disposable pumps for CPNB in children is an effective technique[3]. Another study confirmed that CPNB is an effective and feasible option in pediatric patients, allowing children to experience earlier ambulation and have shortened hospital stays[4]. Consequently, portable infusion pumps have been used with increasing frequency to provide peri-neural infusions of local anesthetic for pediatric patients.
Elastomeric pumps exhibit a high degree of acceptance. Patients prefer them over electronically controlled pumps because of their portability, disposability, silent operation, minimal interference with daily activities, ease of use, impact on sleep as well as the relative inexpense[5-8]. However, evaluation of recent literature shows that elastomeric pumps tend to over infuse (110%-130%) during the initial 3-8 h of infusion and within the final hours before reservoir exhaustion[8]. In addition, Chung et al[9] confirmed the notion of a non-uniform drug delivery via the elastomeric balloon infusion devices. Inaccuracy or altered flow rates could lead to decreased delivery of the local anesthetic solution with inadequate analgesia or excessive delivery with the risk of toxicity. With a very narrow therapeutic window of analgesia in the pediatric population, both accuracy and consistency of peripheral nerve infusion devices is imperative.
This study compares the performance of three such pumps, which infuse potentially toxic medications. The three pumps compared include the On-Q pain pump, the Baxter Multirate portable elastomeric infusion system, and the Ambu elastomeric infusion device (Figure 1).These infusion pumps deliver medication at a flow rate that is determined by the pressure in the elastomeric reservoir, the flow restriction in the infusion circuit, and the viscosity of the fluid[10]. The balloon reservoir serves a sustained internal pressure as a driving force and the luer body makes a resistance as a flow control device. Consequently, the flow rate of these devices is determined by the constant pressure in the elastomeric membrane of the reservoir coupled with various flow control devices[9]. The manufacturer provided references specifies the accuracy to be ± 15%-20% from nominal for the On-Q pump, ± 10% for the Baxter pump, and ± 15% for the Ambu pump. We compare the accuracy and consistency of these three elastomeric devices approved for patient use in the ambulatory setting.
No human or animal subjects were used in these studies. Five On-Q infusion devices, 5 Baxter infusion devices, and 5 Ambu infusion devices were filled with 200 mL of 0.1% ropivicaine in normal saline according to the manufacturer’s recommendations. All pumps were previously unused. The infusion devices were then attached to a single, end hole infusion catheter and each regulator was set to infuse at 12 mL/h. The devices were placed flat, with the elastomeric reservoir and the distal end luer lock at equal height. Each device was allowed to run for 10 h total, with fluid output measured every hour using a graduated cylinder.
The ambient room temperature measured was between 18.9 °C and 20.7 °C, with an average temperature of 19.8 °C. The pumps vary in the manufacturer’s recommendations for temperature management. Baxter pumps are designed to operate at a temperature of 31.1 °C. The flow rate will decrease 2.3% per one degree Celsius and this should be accurate within plus or minus 10%. This would lead one to assume that the regulator is designed to be secured to the patient’s skin, though this is not specifically mentioned in the package insert. The On-Q pump does specify that it is designed to operate while in contact with the patient’s skin, at a temperature of 31 °C The package insert states flow rate will decrease 1.4% per 0.6 °C in temperature. This should be accurate within plus or minus 15% to 20%. Because the Ambu pump is designed to operate between 20 °C and 24 °C it must be assumed that the flow regulator need not be secured directly to the patient’s skin. The manufacturer’s state an accuracy within 5% for the Ambu pump. To adjust for the differences in operating temperature, the nominal flow rate was adjusted for the Baxter and On-Q pumps. Adjusted nominal flow rate was 8.9 mL/h for both. Because the Ambu pump is designed to operate at room temperature the nominal flow rate did not require adjustment.
The data are presented as the mean plus or minus that standard deviation as shown in Table 1. The t test was used to compare the mean value of pumps one through five for each pump, from hours one through ten, to the standard, temperature corrected value of 8.9 mL/h for the Baxter and On-Q pumps and 12 mL/h for the Ambu pump. Results for each pump are shown in Figure 2. All tests are conducted in SAS 9.20 (by SAS Institute Inc., Cary, NC, United States). A P value of less than 0.05 was considered statistically significant.
| Hour | #1 | #2 | #3 | #4 | #5 | |
| On-Q | 1 | 19 | 19.4 | 18.6 | 17.9 | 18.4 |
| 2 | 18 | 18.4 | 18.4 | 16.8 | 18 | |
| 3 | 17.6 | 18.4 | 17.9 | 16.1 | 17.5 | |
| 4 | 17.8 | 18 | 16.7 | 16.4 | 17.5 | |
| 5 | 17.6 | 18.2 | 15.8 | 15.4 | 17.5 | |
| 6 | 17.5 | 18.2 | 14.9 | 16.4 | 16.9 | |
| 7 | 18.6 | 18.2 | 14.9 | 16.2 | 16.9 | |
| 8 | 19.3 | 19.8 | 14.7 | 16.1 | 16.8 | |
| 9 | 18.8 | 18.4 | 14.9 | 14.9 | 16.6 | |
| 10 | 18.5 | 19 | 15.1 | 14.8 | 16.6 | |
| Baxter | 1 | 10.8 | 10.4 | 10.8 | 10.4 | 9.8 |
| 2 | 10.1 | 10 | 10.6 | 10.3 | 10 | |
| 3 | 10 | 9.8 | 10.7 | 9.9 | 9.8 | |
| 4 | 9.8 | 9.9 | 10.5 | 10 | 9.7 | |
| 5 | 9.7 | 9.5 | 10.1 | 10 | 10 | |
| 6 | 9.8 | 9.8 | 10.5 | 10 | 9.8 | |
| 7 | 9.6 | 9.6 | 10.4 | 9.8 | 9.9 | |
| 8 | 10.5 | 9.8 | 10.4 | 10 | 9.8 | |
| 9 | 10 | 10.1 | 10.3 | 10 | 9.6 | |
| 10 | 10 | 10.1 | 10.7 | 10.1 | 9.6 | |
| Ambu | 1 | 9.5 | 9.2 | 10 | 8.5 | 10.2 |
| 2 | 9.8 | 9.2 | 9.8 | 8.6 | 9.9 | |
| 3 | 9.8 | 9.2 | 9.5 | 8.6 | 9.8 | |
| 4 | 9.6 | 9.2 | 9.8 | 8.8 | 10.2 | |
| 5 | 9.7 | 9.2 | 9.8 | 8.6 | 9.9 | |
| 6 | 9.6 | 9.2 | 9.8 | 8.9 | 9.9 | |
| 7 | 9.7 | 9.2 | 9.7 | 8.8 | 10.4 | |
| 8 | 9.7 | 9.2 | 9.9 | 8.8 | 10.2 | |
| 9 | 9.4 | 9.1 | 9.4 | 8.5 | 9.9 | |
| 10 | 9.6 | 9.2 | 10 | 8.7 | 9.8 |
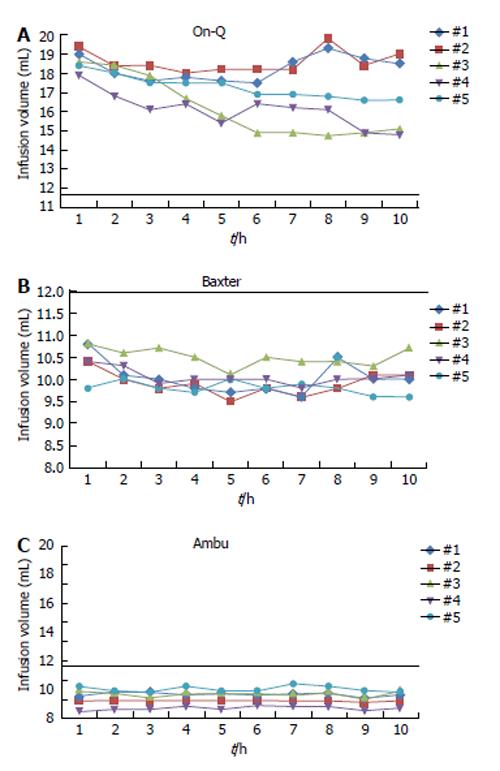
The infusion rates and infusion rate profiles for the three elastomeric pumps tested are shown in Table 1 and Figure 2. The mean output from the devices over the 10 h study period with the associated standard deviation is listed in Table 2.
| Pump | n | Mean | StdDev | Std Err | P value1 |
| On-Q | 10 | 17.3 | 0.62 | 0.19 | < 0.0001 |
| Baxter | 10 | 10.1 | 0.17 | 0.05 | < 0.0001 |
| AMBU | 10 | 9.5 | 0.09 | 0.03 | < 0.0001 |
(P < 0.0001). The mean output from each type of elastomeric infusion pump was significantly different from the standard value of 12 mL/h set on the regulators as shown in Table 1. This is consistent with previous studies on different elastomeric infusion devices[1,8,11]. The On-Q pump’s mean output of 17.3 mL/h was significantly higher than the 12 mL/h set on the regulator. This becomes even more significant when using the temperature corrected nominal rate of 8.9 mL/h. The Baxter pump’s mean output was 10.1 mL/h with a standard deviation of 0.17. This remains statistically significant after adjusting the nominal rate to 8.9 to account for differences in temperature, however under these circumstances the pump is over versus under infusing. The expected temperature corrected range was 8.0-9.8 mL/h. However, this was the most accurate of the evaluated pumps. The Ambu pump’s mean output was 9.5 mL/h with a standard deviation of 0.089, also significantly different from the standard value of 12 mL/h with a P < 0.0001.
The AMBU pump had the minimal standard deviation and standard error, and is therefore the most consistent, though not the most accurate, of the three evaluated pumps.
This study prospectively evaluated the performance of three single-uses, elastomeric infusion devices maintained at room temperature over a 10 h per time. Ideally the nominal flow should be achieved immediately and remain constant throughout the infusion. However, as previous studies have shown, we demonstrate that the infusion rate accuracy and consistency of three portable pumps used to provide CPNB is variable[1,5,9,10].
The On-Q pump infused faster than the set rate throughout the duration of the infusion, even when corrected for temperature. Per the manufacturers this discrepancy is greatest during the first one to two hours of the infusion, following which they state the rate should decrease and become accurate within ± 15%-20% of the nominal rate. In our study, the infusion rate remained greater than 20% above the nominal rate over the entire 10 h period. This increase in flow rate by the On-Q pump may have implications for patient care when applied to continuous regional anesthesia. The reservoir could exhaust earlier than that calculated based on the set rate and expected infusion duration. If the internal reservoir is exhausted early it would require either ending the duration of the infusion sooner than planned or obtaining an additional unit, as refilling the pumps is currently not approved for the majority of devices. In addition, a supra-therapeutic infusion could result in an insensate extremity, muscle weakness, or potential toxicity, especially if used in small pediatric patients.
The Ambu pump, designed to operate at room temperature, under-infused throughout the duration of the study. With an average flow of 9.5 mL/h it never reached the set nominal rate. The Baxter pump was the most accurate, running at an average of 10.1 mL/h, which is just outside the temperature corrected range of 8.0-9.8 mL/h. Both proved to be more accurate over time than the On-Q pump and did not show the variability during the initial two hours of infusion. As the infusion was only measured for a duration of ten hours, conclusions cannot be drawn about the accuracy at the completion of the infusion.
The flow rate of elastomeric pumps is affected by the temperature, the viscosity of the solution and the height of the reservoir. During our study the room temperature was between 18.8 °C and 20.7 °C with an average of 19.8 °C. As described, each pump is designed to operate at a specific temperature, with flow rate increasing as temperature increases and vice-versa. According to the manufacturer, Baxter pumps are designed to operate at 31.3 °C, which leads to the assumption that the device should be secured to the patients skin. These instructions are not specifically mentioned in the package insert. The On-Q pump is designed to operate at 31 °C and in this case the package insert clearly states that the flow regulator should be in direct contact with the patients skin. The Ambu pump is designed to operate between 20 °C and 24 °C, implying room temperature. There may be clinically significant changes in output based on the external temperature[10]. Therefore in this study the nominal flow rate was adjusted to account for the difference in temperature. The variations in operating instructions could be a potential source of confusion to the user and emphasizes the importance of knowing the specific instructions for the pump in use.
When considering viscosity, in addition to changes in temperature, the solution being used must be considered. For example, Baxter pumps are designed to operate at the nominal rate using 5% Dextrose. When 10% Sodium Chloride (NS) is used manufacturers state there will be an approximate 10% increase in flow rate. This study used 0.1% ropivicaine in NS and the infusion rate remained greater than 10% from the temperature corrected nominal rate. We controlled for the height of the pump, as the reservoirs were positioned at the same level as the luer locks.
As previously described, the On-Q pump has a flow discrepancy that is greatest during the first one to two hours of the infusion, following which the manufacturer’s state the rate should decrease and become accurate within ±15%-20% of the nominal rate. This trend was replicated in this study, where the infusion did prove to be greatest over the first two hours, following by a decreased rate. Whether inconsistencies would arise at the end of the infusion duration was not evaluated in this particular study, but this could have implications as well. The Ambu pump did not show the variability in flow during the initial two hours as seen with the On-Q pump, nor did the Baxter pump. The Ambu was the most consistent pump (Table 1), having the lowest standard deviation (0.089 mL/h). Prior studies have also found inaccuracies in elastomeric infusion pumps. A study by Chung et al[9] determined that the flow rate of two elastomeric infusors was not sustained uniformly during the entire delivery period, but rather was in proportion to the internal pressure of the infusor[9]. The infusion rates of the Accufuser Plus, a single-use, elastomeric pump was affected by the volume in the reservoir[10]. Overfilling the infusion pump by 100 mL resulted in a decreased overall infusion rate throughout a 10 h infusion period[10]. In this study each balloon was filled with a 200 mL volume. Whether this impacted our results would require another study evaluating infusion rates from different starting reservoir volumes.
This study demonstrates that three modern, single use elastomeric infusion devices have infusion rates that are significantly different from the set nominal delivery volume. This remained true even after corrected the nominal infusion rate for differences in temperature. These alterations in flow may be clinically significant, resulting in either inadequate analgesia, early exhaustion of the reservoir, excessive muscle weakness or the potential for toxicity, especially when used with small pediatric patients. Therefore, in order to provide the best care, physicians must not only take into account the temperature at which the pump will be kept, viscosity of the solution used, and the height of the reservoir but also the infusion profile of the individual pump being used for CPNB.
The elastomeric infusion devices used in this study were provided by the manufacturer: (1) On-Q pain pump, I-Flow Corporation, Lake Forest, CA; (2) Ambu A/S (Smart Catheter), Denmark; and (3) Baxter MultirateInfusor, Deerfield, IL.
Portable elastomeric infusion pain pumps are being used with increased frequency in pediatric patients. Because these pumps are infusing potentially toxic doses of lcal anesthetic medications, the accuracy and consistency of these devices becomes very important in this patient population. This study is a comparison of the actual delivery volume of local anesthetic of three elastomeric infusion devices approved for patient use in the ambulatory setting with regional anesthesia.
Ultrasound guided pediatric regional anesthesia has become more popular over the past 5 years. The frontier of this utility includes reduction of narcotic consumption potentially leading to decreased hospital stays and improved and efficient postanesthesia care unit discharges. Also, this may help to reduce the general anesthetic requirements in babies who may be exposed to potential neuroapoptotic agents.
The main breakthrough for this discipline has been the utility of ultrasound for safer and more effective placement of regional anesthesia. In addition the known pharmacokinetics and pharmacodynamics of local anesthetics in children is imperative to avoid toxicity.
The results suggest that utilizing peripheral nerve catheters and related pain pumps should be done with caution. There is great variability in manufacturing as well as external factors.
This is a valuable investigation about three modern, single use elastomeric infusion devices. The finding of significant alterations in flow rate than their set nominal delivery volume among the three devices may provide clinical reference for physicians.
P- Reviewers: Amr YM, Allegaert K, Li JF S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Ilfeld BM, Morey TE, Enneking FK. Portable infusion pumps used for continuous regional analgesia: delivery rate accuracy and consistency. Reg Anesth Pain Med. 2003;28:424-432. [PubMed] [Cited in This Article: ] |
| 2. | Capdevila X, Macaire P, Aknin P, Dadure C, Bernard N, Lopez S. Patient-controlled perineural analgesia after ambulatory orthopedic surgery: a comparison of electronic versus elastomeric pumps. Anesth Analg. 2003;96:414-417, table of contents. [PubMed] [Cited in This Article: ] |
| 3. | Dadure C, Pirat P, Raux O, Troncin R, Rochette A, Ricard C, Capdevila X. Perioperative continuous peripheral nerve blocks with disposable infusion pumps in children: a prospective descriptive study. Anesth Analg. 2003;97:687-690. [PubMed] [Cited in This Article: ] |
| 4. | Ludot H, Berger J, Pichenot V, Belouadah M, Madi K, Malinovsky JM. Continuous peripheral nerve block for postoperative pain control at home: a prospective feasibility study in children. Reg Anesth Pain Med. 2008;33:52-56. [PubMed] [Cited in This Article: ] |
| 5. | Ackermann M, Maier S, Ing H, Bonnabry P. Evaluation of the design and reliability of three elastomeric and one mechanical infusers. J Oncol Pharm Pract. 2007;13:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Ackermann M, Maier S, Ing H, Bonnabry P. Evaluation of the design and reliability of three elastomeric and one mechanical infusers. J Oncol Pharm Pract. 2007;13:77-84. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Zahnd D, Aebi S, Rusterholz S, Fey MF, Borner MM. A randomized crossover trial assessing patient preference for two different types of portable infusion-pump devices. Ann Oncol. 1999;10:727-729. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Ilfeld BM. Continuous peripheral nerve blocks: a review of the published evidence. Anesth Analg. 2011;113:904-925. [PubMed] [Cited in This Article: ] |
| 9. | Chung IS, Cho HS, Kim JA, Lee KH. The flow rate of the elastomeric balloon infusor is influenced by the internal pressure of the infusor. J Korean Med Sci. 2001;16:702-706. [PubMed] [Cited in This Article: ] |
| 10. | Burnett T, Bhalla T, Sawardekar A, Tobias JD. Performance of the On-Q pain infusion device during changes in environmental temperature. Paediatr Anaesth. 2011;21:1231-1233. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Ilfeld BM, Morey TE, Enneking FK. New portable infusion pumps: real advantages or just more of the same in a different package? Reg Anesth Pain Med. 2004;29:371-376. [PubMed] [Cited in This Article: ] |