INTRODUCTION
Neutrophils are polymorphonuclear leukocytes (PMNs), the main cell type of white blood cells in humans and are known for their specific segmented nucleus and their granules. They are the human body’s main cellular components of the innate immune system, having an anti-infectious and a pro-inflammatory function. Upon infection, neutrophils are the first responders of the innate immune system to migrate towards the site of inflammation. They can ingest and kill invading microorganisms intracellularly by phagocytosis and the subsequent fusion of the phagosome with lysosomes containing antimicrobial peptides, enzymes and reactive oxygen intermediates (ROI). Neutrophils can also kill microbes extracellularly by the release of antimicrobial peptides and enzymes, stored in their granules[1]. Besides its antimicrobial function, the neutrophil is able to express genes encoding inflammatory mediators such as growth factors, chemokines and cytokines[2]. The presence of fully functional neutrophils in tissues is critical for the defense against microbial infections. This importance is seen in patients with leukocyte adhesion deficiency, which has neutrophil adhesion defects, resulting in poor crossing of the neutrophil across the endothelium covering the blood vessel into the diseased tissue area. These patients suffer from several bacterial infections, which are life-threatening, due to the inability to destroy the pathogens by neutrophil phagocytosis[3]. The importance of reactive oxygen species (ROS) formation is seen in patients with chronic granulomatous disease, who have defective oxidase function and are susceptible to recurrent bacterial and fungal infections[4].
During activation not only extracellular pathogens are affected, the surrounding cells and tissues of the host are also damaged[5]. To reduce the damage on host cells, neutrophils are quickly and efficiently removed from an inflammatory site by macrophage phagocytosis after functioning[6,7]. However, in neonates[8] and in some clinical settings such as sepsis[9,10], COPD and acute coronary syndromes[11-13], neutrophils are deactivated or apoptosis is reduced.
Every day, 1011 neutrophils are produced in the bone marrow making them the most abundant white blood cells[14]. Neutrophils are thought to live only a couple of hours outside of the bone marrow after which they are phagocytosed and cleared, in the same rate as the production rate[15]. In order to produce this amount of cells, and producing them at such a high rate, the bone marrow harbors a large constantly active granulopoiesis compartment. When during infection more neutrophils are needed, the bone marrow has reserve capacity to scale up the production.
Recently, neutrophils were found to have a blood lifespan of 5.4 d[16], which is more than twenty times longer than found before[17]. Although there are some concerns expressed about these recent findings[18], this new lifespan also changes the paradigm of the neutrophil as a short living cell, produced in huge quantities only to kill microbes. Interestingly, with this significantly longer life span, new functions of a neutrophil can be foreseen. Indeed the first papers appear that describe a role for neutrophils in the shaping T-cell independent antibody responses[19,20], but also functions such as antigen presentation and interactions with T cells are reported[21-23]. Such functions demand a longer life span than the reported 5 h and could explain some recently identified roles of neutrophils in inflammatory diseases[24-28].
Here, we review the current knowledge about neutrophil production, function and clearance. We address the question if the neutrophil is just a microbe killer with a unidirectional short life span or whether the neutrophil can reverse its unidirectional fate and by doing so prolong its life span.
First a general description of the exciting life of a neutrophil from birth to death is given. Different parts of the neutrophil life cycle are discussed as well as the kinetics. Several functions of neutrophils and the consequences of these functions to their life span will be discussed. Subsequently, the clearance of neutrophils is discussed in light of recent calculations of neutrophil populations and methods used, focusing on how the calculations are performed and which assumptions are made.
GRANULOPOIESIS
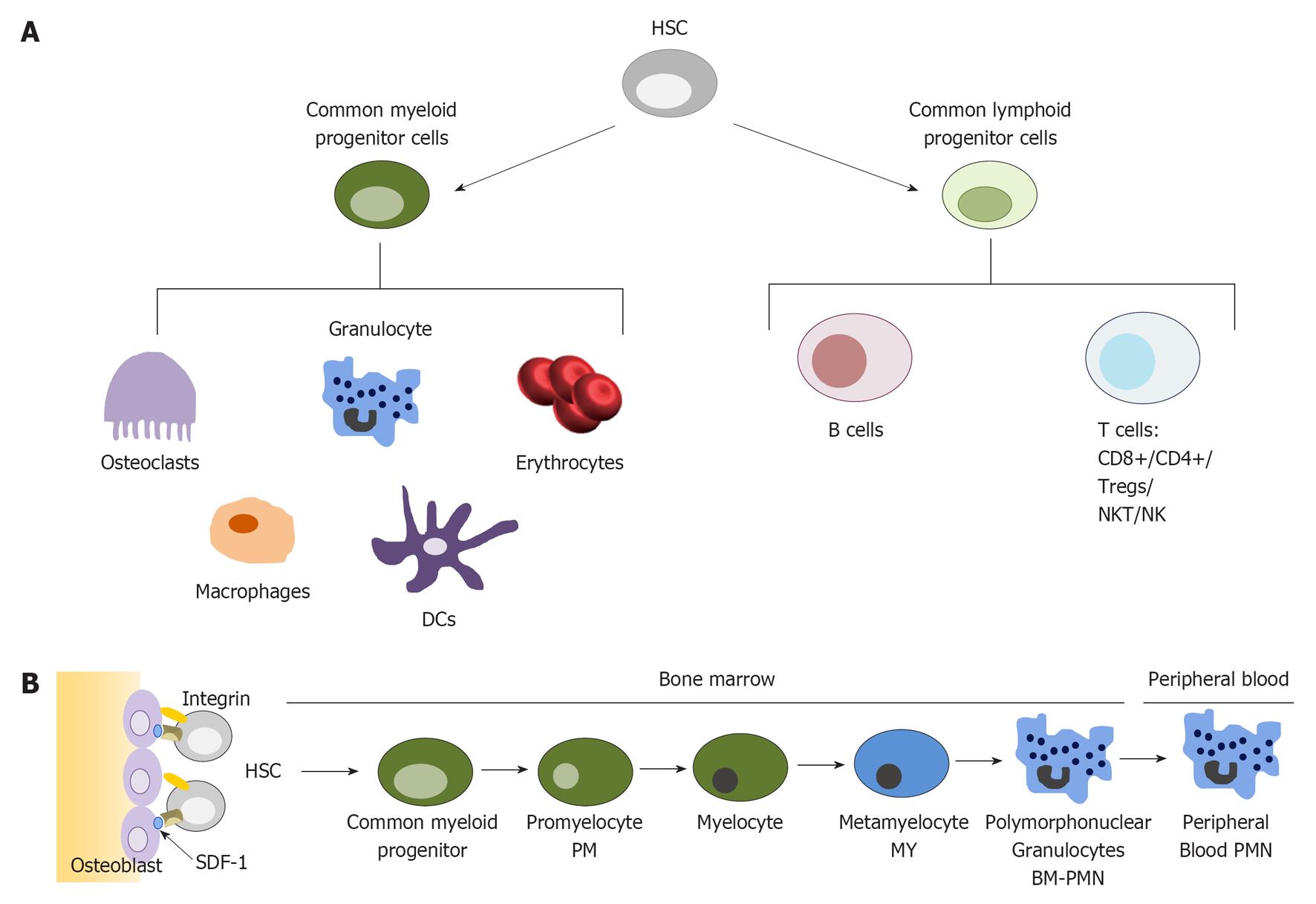
Neutrophils are produced in the bone marrow, where the blood-forming process called hematopoiesis takes place[29]. A hematopoietic stem cell (HSC) can proliferate and differentiate into a wide range of white and red blood cells (Figure 1A). Approximately two-thirds of the hematopoiesis is devoted to myelopoiesis: the formation of monocytes, megakaryocytes, red blood cells, dendritic cells and granulocytes[1]. Each day, approximately 1011 neutrophils, the largest group of the granulocytes, are produced from HSCs under normal conditions (Figure 1B) but the rate of neutrophil production is highly dynamic. Factors influencing the rate of production are the rate of neutrophil apoptosis and immunological stress conditions. In immunologically stressed conditions, granulopoiesis and thereby the formation of neutrophils, is induced due to the production of several cytokines. For example, T-helper 17 cells have been shown to secrete interleukin (IL)-17 and other cytokines during inflammation that promote granulopoiesis, neutrophil proliferation and accumulation[30]. On the other hand, during inflammation, neutrophils produce Pre-B cell colony-enhancing factor, thereby inhibiting neutrophil apoptosis and subsequently granulopoiesis[31].
Figure 1 Granulopoiesis process.
A: The production of blood cells from a hematopoietic stem cell. Modified from[131]; B: Neutrophil maturation from the hematopoietic stem cell to mature neutrophils. Modified from[1]. HSC: Hematopoietic stem cell; NK: Natural killer; DCs: Dendritic cells; PMN: Polymorphonuclear leukocyte.
The granulopoietic compartment in the bone marrow can be divided into three pools: the stem cell pool (HSCs), the mitotic pool and the post-mitotic pool. The mitotic pool is the group of progenitor cells that are massively proliferating and differentiating. The bone marrow also comprises a reserve pool of mature neutrophils, approximately 20 times the number of neutrophils in circulation[14]. The fully differentiated mature neutrophils define the post-mitotic pool, a pool ready for on demand release. Several stages of maturation of neutrophils can be discerned (Figure 1B). As differentiation and maturation progress, cells lose their ability to proliferate[1]. In the terminally differentiated mature neutrophil state, cells can only progress unto death[32].
For maintaining homeostatic levels of peripheral neutrophils and other blood cells, proliferation and differentiation of progenitor cells is tightly regulated and controlled by several intrinsic and extrinsic factors. For example, in bone marrow niches, HSCs retain in the niches through interaction of β-integrins on their membrane with osteoblasts and with the extracellular matrix (Figure 1B). An interaction essential for homing of HSCs and mature neutrophils is the interaction of chemokine receptor (CXCR4) with the bone marrow stromal cell derived factor 1 (SDF-1)[33]. The interaction of Notch on HSCs with Jagged1 on osteoblasts is known to inhibit differentiation of HSCs in the bone marrow[34]. Soluble factors known to maintain HSCs in the bone marrow are for example IL-1, -6, and -10 and thrombopoietin[34].
One of the main regulating factors essential for tuning the production of neutrophils, is granulocyte colony stimulating factor (G-CSF)[35]. G-CSF affects hematopoietic cells, through commitment of progenitor cells to the granulocyte lineage, massive proliferation of granulocytic precursors (e.g., promyelocytes and myelocytes) and release of mature cells from the bone marrow[36]. It induces effects via the G-CSF receptor, thereby activating an intracellular signaling cascade via signal transducer and activator of transcription 3 (STAT3). Where loss of the G-CSF receptor decreases the number of circulating neutrophils, injection of G-CSF increases neutrophil numbers in circulation[37,38]. Furthermore, its production is up regulated with neutrophil apoptosis in the bone marrow and downregulated when the number of neutrophils increases. In addition, during inflammation, different cytokines induce the production of G-CSF[39] or act in synergy, like IL-1β[40,41]. Other factors regulating neutrophil maintenance are IL-3, granulocyte-macrophage CSF (GM-CSF) and lymphoid enhancer-binding factor-1, targeting genes like survivin, cyclin D1, CEBP-α and c-myc[42].
NEUTROPHIL RELEASE FROM THE BONE MARROW
After maturation in the bone marrow, neutrophils are stored, awaiting release into the circulation. To exit the bone marrow, the neutrophils have to migrate across the bone marrow endothelium that separates the marrow from the circulation. Stimulation to leave the bone marrow occurs during inflammation or infection by the presence of chemoattractant factors as leukotriene LTB4, complement factor C5a, CXCL8 and intrinsic regulation factors like G-CSF, but recent findings describe that also circadian rhythms can contribute to neutrophil recruitmen from the bone marrow[43]. Under homeostatic conditions, G-CSF is the main regulator release of neutrophils. During maturation, G-CSF receptors maintain highly expressed on the surface of neutrophils[44], as well as on bone marrow stromal cells. G-CSF inhibits stromal cell production of SDF-1, thereby inhibiting the interaction with its receptor CXCR4 on neutrophils[45]. G-CSF also functions in another bone marrow interaction with neutrophils. Bone marrow endothelial cells express vascular cell adhesion molecule 1 (VCAM-1), which interact with the integrin very late antigen-4 on neutrophils. G-CSF administration results in a loss of VCAM-1 on endothelial cells. G-CSF stimulates granule release of neutrophils, which contain proteases able to cleave VCAM-1[46]. A third effect of G-CSF is exerted on the cytokine receptor CXCR2, which is essential for neutrophil release. G-CSF stimulates the expression of CXCR2 ligands on bone marrow endothelial cells, facilitating neutrophil release[47]. In summary, G-CSF stimulates bone marrow endothelial cells in several ways to down regulate their neutrophil homing receptors and increase the expression of ligands inducing neutrophil release. After release, neutrophils can follow the gradient of chemoattractants into the tissues.
LEAVING THE CIRCULATION: HOMEOSTATIC VS INFLAMMATORY CONDITIONS
Upon infection and inflammation, several pro-inflammatory signals, like fMLP, LTB4, CXCL8, C5a, CXCL1 and CXCL5, activate the vascular endothelium causing it to present adhesion molecules and chemotactic factors on the surface[48-50]. P-selectins and E-selectins induced on endothelial cells will interact with PSGL-1, L-selectin and CD44 on neutrophils, mediating rolling and activation of the neutrophil integrins at the site of maximal chemokine concentration. These integrins then interact with ICAM-1 molecules on the endothelial cells, causing neutrophil arrest. Adhesion strengthening occurs with subsequent spreading of the neutrophil, resulting in intravascular crawling. The chemotactic process and the chemoattractant gradient both lead to a cytoskeletal rearrangement, necessary for the spreading and transmigration[51]. The leukocyte adhesion cascade is described in detail elsewhere and this information can be found in[49].
Once in the tissues, neutrophils are more prone to phagocytosis than blood neutrophils. As transmigration is partly mediated by fusion of secretory vesicles with the neutrophil membrane, several surface membrane receptors are added to the membrane as well as other functional proteins like chemoattractant and phagocytosis receptors. Upon stimulation by microbial moieties, G-CSF or GM-CSF, tumor necrosis factor-α (TNF-α) or Type I and II interferons in the inflamed tissue, neutrophils are functionally activated and start to transcribe and produce other chemokines, for example CXCL8[2,52].
Priming
Activation of neutrophils is a two-step process, starting with priming by an initial exposure to mediators such as cytokines, which don’t activate the neutrophils directly, but leave them in a “primed” state. These cytokines can be early-phase cytokines like TNF-α, IL-1α and pathogen associated molecular patterns like endotoxin, as well as the earlier mentioned late-phase chemoattractants as IL-8, LTB4 and GM-CSF. Priming can be described as a resting state of a neutrophil but with a functional response (e.g., chemotaxis, ROS production) to be amplified upon another stimulus. Without priming, no maximal degranulation and activation of the NADPH oxidase can occur[53]. Priming affects the neutrophil cytoskeletal organization to reduce deformability in order to retain in capillary beds[54]. In vitro, priming (and subsequent shape change) has been shown to be reversible[55], but there is limited data on the effects of priming on neutrophil kinetics in vivo. It is suggested that as 15% of the cardiac output can pass through an inflamed site each minute, all neutrophils are exposed to the priming stimulus within min. However, in vivo studies show a maximum 60% of primed circulating neutrophils, suggestive for de-priming in vivo[56]. De-priming should protect the systemic circulation from the potentially damaging effects of primed cells, for example because of the produced H2O2, a marker of primed or activated neutrophils. Mixed venous blood (blood before the pulmonary circulation) has higher H2O2 compared with arterial blood (blood after the pulmonary circulation), suggesting the lung to be the de-priming compartment[57]. De-priming may have effects on the life span of the neutrophil, as priming can lead to neutrophil-mediated tissue damage and therefore these neutrophils are phagocytosed by macrophages early in the inflammatory response[58]. Depriming may thus give an alternative way of clearance of harmful neutrophils in inflammatory responses[55].
Functioning of activated neutrophils
Once activated in the tissues, transcriptional activity of neutrophils is up regulated, in part mediated by local G-CSF production, resulting in the production of cytokines[2]. Also, the neutrophil will start phagocytosing microorganisms, degranulate, activate the oxidative metabolism intracellularly and will finally undergo apoptosis.
Degranulation is one of the first steps of neutrophil activation and is initiated during transmigration. The components of the different granules are well known and are described elsewhere[59,60]. Not only anti-microbial proteins are stored in these compartments, but also proteases, components of the respiratory burst oxidase (described below) and a wide range of receptors, extracellular matrix proteins and soluble mediators of inflammation[61]. Soluble inflammatory factors are for example chemotactic proteins[62,63], inducers of vascular permeability changes[64] and antigen presenting cell-activators[65]. Degranulation transforms the neutrophil from passively circulating to being an effector cell of the innate immune system[60].
Upon activation of the neutrophil, also the oxidative metabolism of the cell is activated. Neutrophils are very effective at the generation of ROS, a process called the oxygen metabolism or the respiratory burst. ROS or ROI are generated by the NADPH oxidase complex on the membrane of the cell. Some components are stored in storage sites, like secondary granules, which associate with the oxidase complex after fusion of these storage sites with the membrane or with phagosomes[59]. These ROS serve as highly effective antimicrobial agents but are also highly damaging the host as the produced components are highly reactive. ROS producing neutrophils are rapidly cleared by macrophages.
An extend in the antimicrobial activity of the neutrophil is the formation of neutrophil extracellular traps (NETs)[66]. The formation of NETs is a result of nuclear swelling and dissolved chromatin. Along with the nuclear swelling, granules are also disintegrated and as a result, large strands of unpacked DNA are extruded from the cell, carrying along proteins from granules and from the cytosol. At this time, already 24 different neutrophil proteins are associated with NETs, which are primarily proteins from primary granules (such as MPO and elastase), secondary granules (e.g., lactoferrin and pentraxin 3) and tertiary granules (e.g., MMP9)[66,67]. NETs have been shown to trap microorganisms and promote interaction with the granule proteins, resulting in microbial recognition, antimicrobial activity and tissue remodeling. NET formation is a cell-death dependent process, also influencing the life span of neutrophils[66,68].
Deactivation of neutrophils
In addition to being harmful for microbes, the proteins that neutrophils secrete also damage the host tissue. Therefore it is important to control the influx of neutrophils to prevent excessive tissue damage. Neutrophil influx is controlled by several negative feedback loops at different stages of the inflammatory response. For example during the chemotactic process, it has been described that after a first encounter with CXCL8, neutrophils are desensitized to additional chemotactic signals[69,70]. Intracellularly, there are proteins recruiting phosphotyrosine phosphatases, which deactivate receptors on the surface. For example suppressor of cytokine signaling 3 down regulates G-CSF receptor signaling by blocking the phosphotyrosine on the activated receptor thereby preventing the interaction with STAT3. Extracellularly, neutrophils and macrophages partner in the termination of inflammation[71]. Neutrophils for example will express “eat-me signals” due to phospholipid asymmetry, triggering macrophages to phagocytose neutrophils[72].
The receptor Chem R23 on macrophages, DCs and endothelial cells mediates activation of macrophages that enhances the phagocytic capacity of macrophages for uptake of apoptotic neutrophils. Neutrophil apoptosis itself is a specific process with different signals triggering apoptosis via different pathways. This process is described in detail elsewhere[73,74]. Importantly, phagocytosis by macrophages reduces the risk of necrotic neutrophil death and down regulates the local G-CSF production to limit neutrophil activation[1].
Other deactivating processes are granule-proteins like LL-37 and cathepsin G that stimulate rolling monocytes to migrate into the inflamed tissue. Neutrophil-derived proteins then stimulate the extravasated monocytes to maturate into macrophages and subsequently phagocytose apoptotic neutrophils. The macrophages in turn release anti-inflammatory mediators such as IL-10, further limiting the damage neutrophils do to host tissues[71]. Importance of these deactivating signals is seen in clinical settings such as cystic fibrosis, in which neutrophils are insensitive to signals as IL-10 and corticoids[75,76].
Additional known functioning of activated neutrophils
For a long time, neutrophils were thought to only be recruited to the inflamed tissue, act as phagocytic cells, release lytic enzymes and produce ROS, after which they were cleared. However, additional functions of neutrophils in inflammatory sites have recently been described. First of all, neutrophils were shown to express genes encoding inflammatory mediators[2]. Secondly, neutrophils were found to produce anti-inflammatory molecules and factors promoting the resolution of inflammation, as described above and elsewhere[56,77] and thirdly, neutrophils were shown to engage in interactions with different cells of the immune system[20]. These new insights are very important for our understanding of inflammatory diseases, their resolution and possibility of neutrophils as targets to modulate immunity.
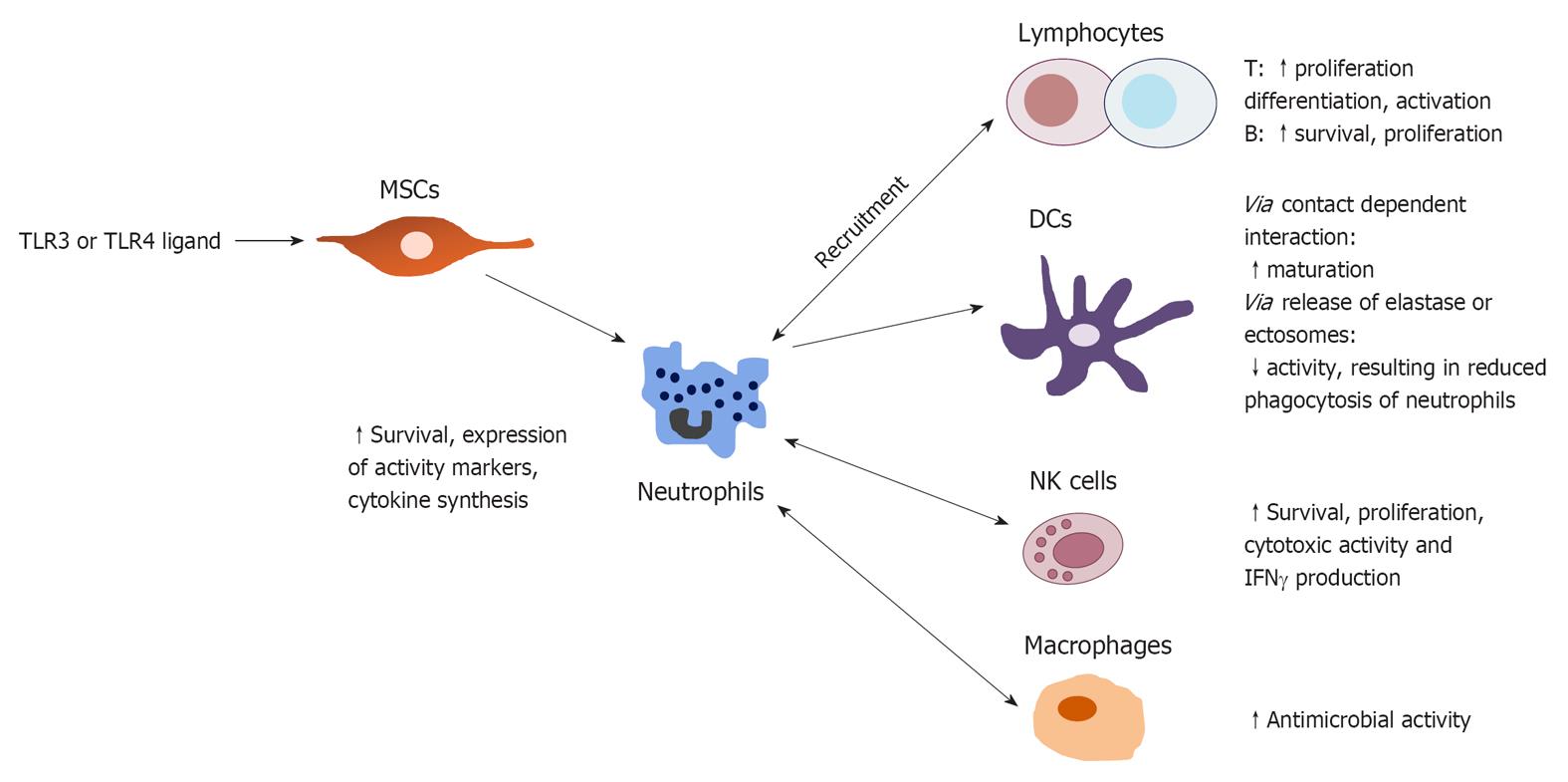
In vitro interactions with neutrophils have been shown for monocytes[78], macrophages, DCs, natural killer (NK) cells, lymphocytes and mesenchymal stem cells in the tissues and were reviewed by[79] (Figure 2). Also, crosstalk with platelets[80] and regulatory T cells are described[81]. First, neutrophils can induce the maturation of DCs in vitro through contact-dependent interactions involving CD18 and CEACAM1 on neutrophils and DC-SIGN on DCs. Subsequently, mature DCs induce T cell proliferation and polarization towards a Th1 response. However, neutrophils can also deactivate DCs via the production of elastase or ectosomes, containing transforming growth factor (TGF)-β1[79,82]. Deactivated DCs showed a reduced phagocytic activity, thereby preventing the phagocytosis of neutrophils.
Figure 2 Cellular crosstalk of neutrophils in the tissues and in the lymph nodes.
Modified from[78]. TLR: Toll-like receptor; NK: Natural killer; DCs: Dendritic cells; MSCs: Mesenchymal stem cells; IFN: Interferon.
Second, an interaction was unraveled between neutrophils and NK cells. Neutrophils are required both in the bone marrow as well as in the peripherial development of NK cells[83]. They can modulate the survival, proliferation, cytotoxic activity and interferon γ (IFNγ) production of NK cells via the generation of ROS and/or the release of granules. NK cells can in turn promote neutrophil survival, expression of activation markers, priming of ROS production and cytokine synthesis[84].
Direct cell-cell contact between neutrophils, NK cells and DCs has been shown in vitro as well, resulting in the increased release of IL-12 by DCs and an up regulated IFNγ expression by NK cells. IFNγ in turn stimulates neutrophil survival, expression of activation markers and cytokine synthesis[85]. These effects have only been described for neutrophils in vitro, so further in vivo investigation is needed, but it gives new insights in the expanding functions of inflammatory site neutrophils. The importance of these additional functions is still elusive.
A third interaction is reported for neutrophils and lymphocytes. Neutrophils and lymphocytes can modulate each other’s recruitment to the site of infection via the release of several released chemokines. Activated CD4+ and CD8+ T cells produce cytokines modulating neutrophil survival and expression of activation markers in vitro[86]. In a similar fashion, γδ T cells strongly promote neutrophil survival and activation by up regulation of CD64 and HLA-DR expression[79]. Neutrophils also play an important role in B-cell help where they can even induce class switching of B-cells, a property solely assigned to T-cells[20].
The next interaction described is the crosstalk with platelets. In transfusion-related acute lung injury, the leading cause of death after transfusion therapy, activated platelets were described to induce the formation of NETs[80]. In another study, platelet were suggested to bind to neutrophils in the lungs, with subsequent activation of neutrophils by platelet toll-like receptor (TLR)4[87].
In the interaction with monocytes, apoptotic neutrophils trigger the monocyte elicit an anti-inflammatory cytokine response through IL-10 and TGF-β, and to downregulate the production of pro-inflammatory cytokines TNF-α and IL-1β. In order to induce this response, cell-cell contact between the apoptotic neutrophil and monocytes was required[78].
THE MARGINATED POOL
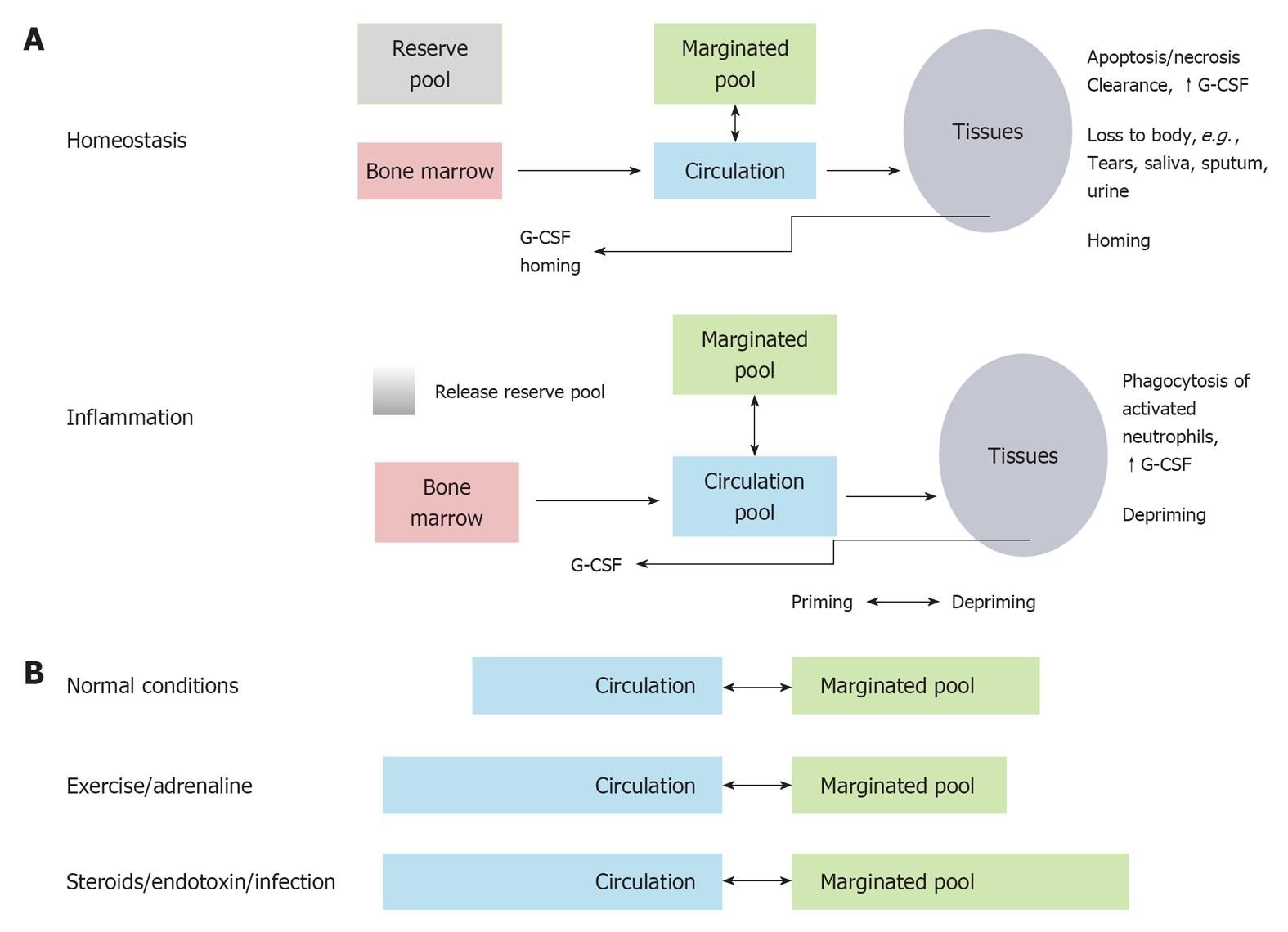
After leaving the bone marrow, the neutrophil becomes part of one of the two compartments found in blood: the circulating pool and the marginated pool. The circulating pool consists of neutrophils flowing freely through vascular spaces and the marginated pool consists of neutrophils adhered to the endothelium of capillaries and post capillary venules, often in the lung, liver and spleen[15]. Already in 1867, Cohnheim observed cells in a marginal position along venule walls. Almost 50% of labeled granulocytes injected into healthy volunteers disappear rapidly from the circulation[17]. This gave rise to the hypothesis that a marginated pool should exist. Next, it was found that leukocytes circulate freely in the blood, then adhere to the vascular endothelium, especially in sites where the blood flow is slow and then re-enter the circulation in a continuously exchanging process[88]. The relative size of the marginated and circulating pool however, can be affected during exercise or induced by adrenaline or drugs (Figure 3). It has been suggested that during infection the marginated pool is minimized, while the freely circulating pool becomes larger[89]. The marginated pool consists of neutrophils adhered to the endothelium of capillaries and postcapillary venules, often in the lung, liver and spleen. The bone marrow has also been suggested as a margination site[90]. Margination means a prolonged transit through these specific organs, resulting in an intravascular neutrophil pool. The lung has been a controversial margination site. Some data suggest that the lung is the predominant site of margination[91], but this has been called into question by others[92]. Interestingly, different neutrophil types localized in different organs[93]. Suratt et al[93] showed that mature peripheral blood neutrophils localize to the liver, bone marrow and to a lesser extent to the spleen. Younger marrow-derived neutrophils prefer to home back to the bone marrow, a process that will be described below, and inflammatory peritoneal neutrophils prefer the liver and the lungs. The biodistribution of inflammatory neutrophils might be non-comparable with homeostatic conditions as these neutrophils are different in surface expression of receptors and in functioning.
Figure 3 The marginated pool.
A: Neutrophil pools under homeostatic and inflammatory conditions. All pools remain present, but the pool sizes change significantly; B: The size of the marginated and circulating granulocyte pool can be affected due to exercise or endotoxins or steroids. Modified from[88]. G-CSF: Granulocyte colony stimulating factor.
HOMING
Apoptotic neutrophils are not detected in normal circulation, so the need for an efficient removal system is evident, as 1011 neutrophils are believed to be produced and removed every day.
Surface receptor expression is highly dynamic upon infection, but receptor expression also changes upon aging. As neutrophils become senescent, expression of a receptor for chemotaxis, CXCR2, decreases, while the expression of a chemokine receptor, CXCR4, increases[77,94]. Interestingly, the responsiveness to SDF-1α, the ligand of CXCR4, increases in coincidence, resulting in homing of senescent neutrophils to the bone marrow. CXCR4 thus is not only a signal to retain neutrophils in the bone marrow, but is also acting on homing senescent cells to the marrow for destruction.
CXCR4 expression is up regulated just before apoptosis and after homing to the bone marrow, the neutrophils will undergo apoptosis and are subsequently phagocytosed by stromal macrophages, which are present in the hematopoietic cords[73,95]. Furze et al[96] showed that in mice, about one third of 111In-labeled neutrophils were cleared via bone marrow stromal macrophages. Before, stromal macrophages were only known for the removal of cellular debris and non-productive B cells[97]. Interestingly, if the labeled neutrophils were pretreated with pertussis toxin that inhibits the chemokine receptors, neutrophil clearance via the bone marrow was inhibited for 75%, which is consistent with a role for chemokines, as clearance by the liver was unaffected by pertussis toxin treatment[96].
Homing neutrophils must actively migrate through the bone marrow endothelium, a process that is not possible for apoptotic neutrophils. Neutrophils also home back to the bone marrow while the liver and spleen also remove circulating neutrophils. Furze et al[96] showed that phagocytosis of neutrophils in the bone marrow stimulates G-CSF production which in turn induces neutrophil production in the bone marrow. Interestingly, when apoptotic neutrophils are phagocytosed by reticular endothelial macrophages in the spleen and liver or by macrophages on a site of infection, the production of G-CSF is suppressed to limit the inflammation[98]. This way, via the up regulation of G-CSF production directly in the bone marrow, the production of new neutrophils can be tightly regulated. So if neutrophils are already apoptotic in circulation, the spleen and liver will clear them. On the other hand, senescent neutrophils can migrate back into the bone marrow and will be cleared there, as a positive feedback loop for neutrophil production.
To determine whether homing neutrophils can return to circulation, isolated neutrophils from the bone marrow and peripheral blood of mice were labeled and injected back into the mice[92]. About 20 percent of labeled mature bone marrow neutrophils remobilized during an inflammatory response. However, homed bone marrow peripheral neutrophils could not be remobilized in response to inflammation. Therefore, the bone marrow could be seen as a site for clearance. In addition, this study also showed that infused marrow neutrophils may be remobilized. Other experiments indicated that 10% of labeled injected HSCs could leave the bone marrow, enter the blood, re-enter the bone marrow and still mature into granulocytes[99]. It would be very interesting to further investigate the recirculating potential of mature neutrophils, as this can greatly influence our understanding of neutrophil kinetics.
KINETICS
The kinetics of neutrophil production, the amount of cells that are produced each day, is measured as a rate of turnover of neutrophils in the blood. Blood neutrophil turnover has been determined by labeling neutrophils with [32P] DFP (di-isopropyl fluorophosphate) and has been described to be about 1.5 × 109 cells/kg per day[100,101].
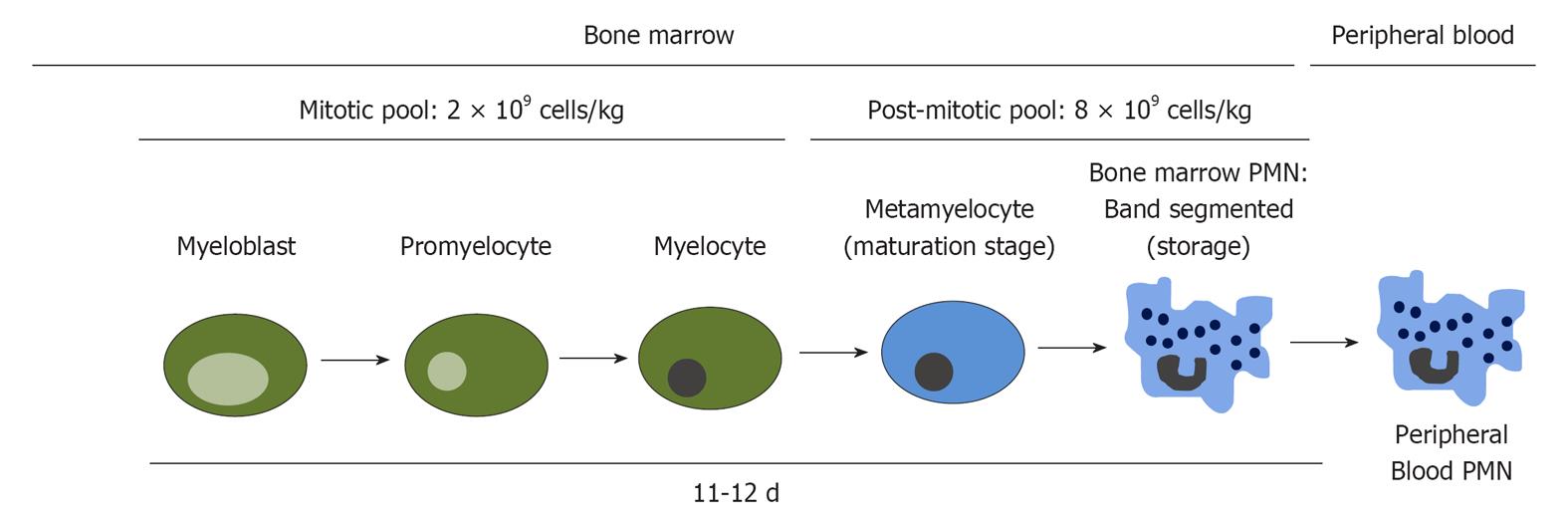
Marrow neutrophil production has been determined from the number of neutrophils in the post mitotic pool, divided by their transit time (the appearance in circulating neutrophils of injected 3H-thymidine) (Figure 4). The post mitotic pool consists of about 5.5 × 109 neutrophils/kg body weight and the transit time was about 6.6 d. The marrow neutrophil production has therefore been calculated to be 0.85 × 109 cells/kg per day. This amount corresponds to the calculated neutrophil turnover in blood. However, when cells were labeled with di-isopropylfluorophosphate-32P, a larger turnover of neutrophils was found. Care should thus be taken with calculations and amounts, as they depend on the method to label cells[14].
Figure 4 Neutrophil kinetics of the differentiation towards neutrophils in the bone marrow.
Modified from[106]. PMN: Polymorphonuclear leukocyte.
The different maturation stages all have different kinetics, which are studied in vivo and in vitro using radioisotopic labeling. These studies indicate that between the myeloblast and the myelocyte stages, approximately five cell divisions occur[102,103]. Myelocytes probably undergo about three cell divisions, indicating the major expansion of the neutrophil pool to be at the myelocyte stage. The mitotic pool of neutrophils contains about 2 × 109 cells/kg[14], whereas the post mitotic pool contains about four times as much. These radionuclide studies suggest that the transit time from myeloblast to myelocyte takes about 135 h, divided over the different myelocyte stages (Figure 2). The transition from myelocyte to blood neutrophil takes about 131-158 h, indicating a total time of approximately 12 d from precursor to mature neutrophil[102]. During infection, transition time from myelocyte to blood neutrophil can be shortened to 48 h.
Following production, mature post mitotic neutrophils (approximately 1011 cells) will remain in the bone marrow for 4-6 d[14,104]. In response to infection, the storage pool in the bone marrow will be used as source of neutrophils for blood neutrophilia[105]. In conclusion, before a neutrophil leaves the bone marrow, it takes 17 d to be produced and maturated[106].
The kinetics of neutrophils leaving the vascular compartment and their take-over by new neutrophils can easily be measured by labeling neutrophils and measure the transit time through the vascular compartment. When healthy individuals are injected with neutrophils, they leave the vascular compartment with a 7 h half life time[17,107]. Using radiolabelled neutrophils and other analytical techniques, the neutrophil intravascular transit time has been measured for the liver, spleen and bone marrow, being respectively 2 and 10 min. The intravascular transit time can be seen as the mean time taken for neutrophils to pass through the capillary bed of a specific organ. The influence of the marginated pool, homing back to the bone marrow and the kinetics in the spleen and liver on this transit time is unknown.
As the regulation of neutrophil production and clearance is an important homeostatic mechanism and also involved in the development of systemic inflammatory states, it is of great importance that the kinetics of circulation and clearance are clear. Now we know that not only the liver and spleen, but also the bone marrow clears neutrophils, and that the different organs clear different types of neutrophils[92]. But the function of neutrophils leaving the vascular compartment is largely unknown.
As described before, inflammatory neutrophils were found to have many more functions then only clearance of microbes. Possibly, neutrophils in marginated sites outside the vascular compartment, also have additional functions. There is growing evidence that to a certain extend neutrophils influence the adaptive immune response, either through pathogen shuttling to the lymph nodes[108], through antigen presentation[109], and through modulation of T helper responses[110]. However, these described functions have still not been shown in vivo and also, are they neutrophil specific or do they occur as side effects of the functioning as a microbe-killer
Methodology used for obtaining kinetic data: the effects of radioactive labeling
Without signs of infection, neutrophils do not get activated and have no need to go into the tissues. They also do not exocytose their granules, meaning that they are not as harmful for the host as activated neutrophils. The fate of these unactivated neutrophils is hard to investigate. Labeling neutrophils has revealed some of their fate, but labeling can also cause changes in the neutrophil (e.g., prime or activate), which makes it a non-optimal technique for measuring unprimed circulating neutrophils. However, the studies which labeled neutrophils and followed their route through the human body are still very useful in this context.
In studies measuring neutrophil kinetics, different types of radioactive labeling have been used. 32P-diisopropylfluorophospate (DFP32) is a potent and irreversible esterase inhibitor, which binds to granulocytes without modifying the viability of the cells and without being reused after degradation of neutrophils. Furthermore, the label is only slightly or not at all attached to lymphocytes or monocytes[111]. Other radioactive labels are In-111 oxine, Tc-99m sulfur colloid, Ga-67 labeling and Na251CrO4 or SnCl2-reduced 99mTcO4-. The effects of these radioactive labels on neutrophils have been studied by several authors, for example the effects on chemotactic responsiveness[112]. Some labels are no longer in use, for example Na251CrO4 and SnCl2-reduced 99mTcO4-, which showed less optimal results in the chemotactic responsiveness studies. Other labels are still used, for example 32DFP or 3H-thymidine.
The ideal radioactive agent should have the following properties: only label cells in vivo, only label neutrophils, do not elute from cells after labeling or being reused after degradation of the neutrophil, cause no radiation damage to the cells, emit γ radiation suitable for external detection and have a long enough half-life for studies without radioactive decay but short enough to limit patient-suffering[113]. For a long time, only in vitro labeling was possible, where neutrophils were isolated from a blood sample, which could easily stimulate the neutrophils. Upon stimulation, neutrophils release their granules and are altered in surface receptor expression, and although the labeling experiments have been improved hardly any research was done to assess the activation of neutrophils or the change in surface receptor expression due to labeling[114]. Some authors claim that there is no difference in neutrophil activation, without showing the data. But as neutrophils are quick responders to differences in their homeostatic environment, in vitro, in vivo or in situ labeling can have tremendous effects on the cell, affecting the outcome of a study as well. In mice, neutrophils were shown to have a half-life of 8 to 10 h when labeled in vivo[115]. But when neutrophils were labeled ex vivo, 90% were cleared after 4 h, resulting in a half-life of only 1.5 h[92]. This shows that the methods for labeling can have an devastating effect on the outcome of the study. But unfortunately, extrapolation of mice experiments is often very difficult. In mice, neutrophils are not the main circulating white blood cell-type, they do not express the same receptors as human neutrophils (for example there is a lack CXCR1) and also chemoattractant CXCL-8 does not exist in mice. Therefore, care should be taken when mice are used for calculating neutrophil life spans.
Most experiments done with in vitro labeling have not been repeated with in vivo labeling, meaning that some knowledge needs to be adjusted. Recently, Pillay et al[16] used 2H2O, a new labeling method for labeling neutrophil pools in vivo, to calculate the rate of division of the mitotic pool in the bone marrow, the transit time of new neutrophils through the post mitotic pool and the delay in mobilization of neutrophils from the post mitotic pool to the blood. They recalculated the life-span of neutrophils and found an average circulatory neutrophil lifespan of 5.4 d, which is 10 times longer than previously reported[14]. However, there are also doubts concerning this report, as the previously used radioactive labels (e.g., 32DFP, H3-Th, Cr-51, In-111 and Tc-99m) all showed a lifespan of approximately 10 h. The new model is thought to lack the right temporal resolution to make these conclusions, as the mean value of the total life span of a neutrophil is in line with the previously described total life span[102]. Also, the authors did not show that the deuterium was not reutilized in newly dividing neutrophil precursor, thereby possibly influencing the results[18]. Furthermore, if a concentration of 3 × 106 neutrophils/mL blood is maintained, the disappearance from the blood should be 5 h, considering the production rate of 1 × 109 cells/kg body weight[88]. Either one of these two numbers should be reconsidered.
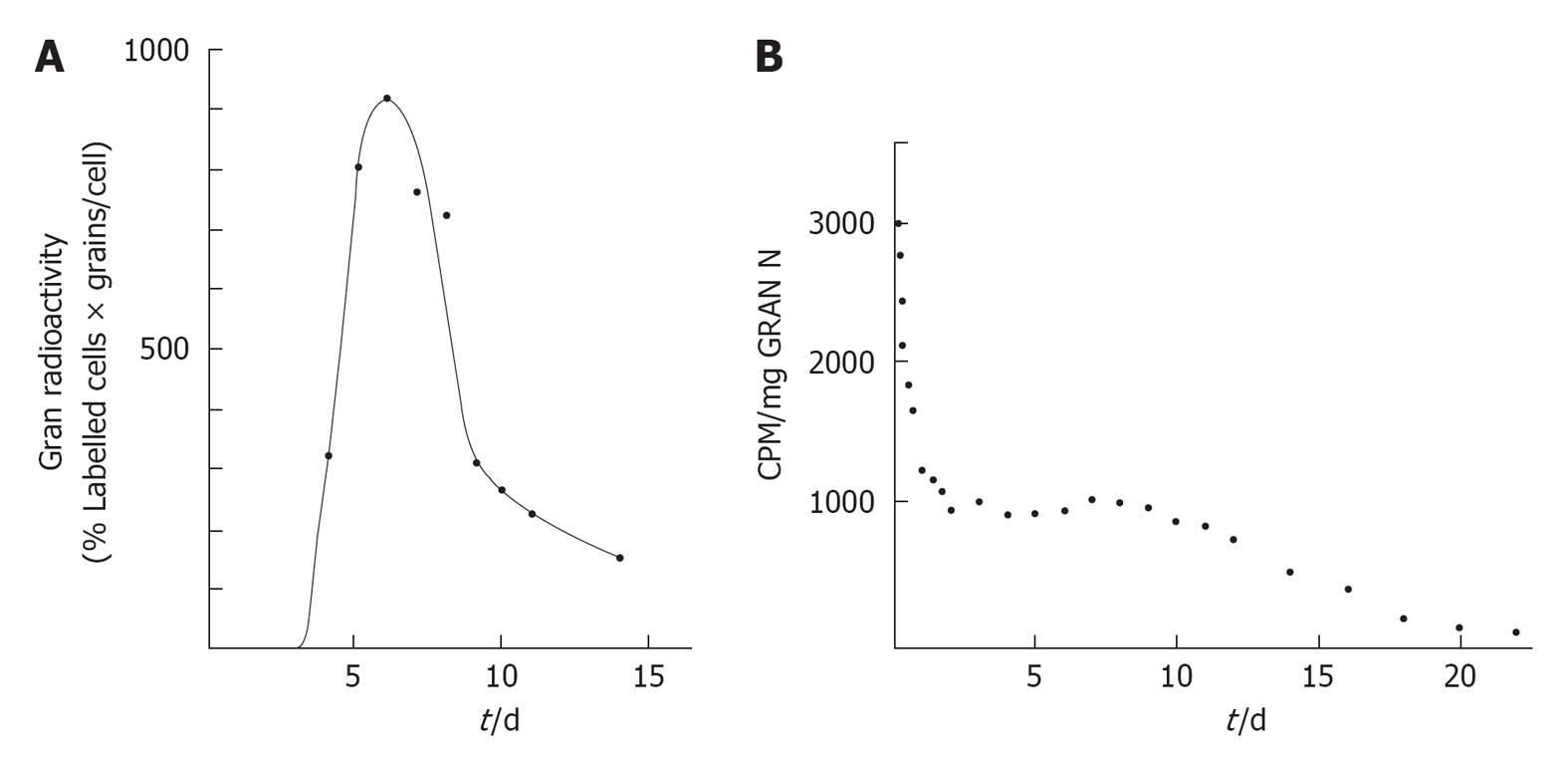
Interestingly, different maturation states of neutrophils are labeled by different radioactive labels. Warner and Athens compared the three most common radioactive labels in vitro until 1964, 3H-thymidine, 32P-labeled sodium phosphate and 32DFP, in their kinetics regarding the blood granulocyte radioactivity curves measured after administration[103]. 3H-thymidine, a compound built in the DNA of newly formed neutrophils, showed a labeling of myelocytes and more immature forms, but neither PMN neutrophils in the blood nor PMNs and metamyelocytes in the bone marrow were labeled. 32P-labeled phosphate was found in the same subsets of neutrophils, as it is also incorporated in DNA[103]. 32DFP labels granulocytes intracellularly and therefore, PMNs are directly labeled in the blood. The component(s) in the granulocytes to which DFP binds is unknown as DFP binds many different esterases and proteolytic enzymes[111]. When the blood kinetics of all three populations is compared, they are all three totally different: 32DFP levels start high, where after the labeled neutrophils disappear in marginated pools and the level of 32DFP declines. 3H-thymidine labeled neutrophils appear later in the blood, after proliferation and differentiation and then the level declines (Figure 5).
Figure 5 The kinetics of neutrophil production, the amount of cells that are produced each day, is measured as a rate of turnover of neutrophils in the blood.
A: Decay of radioactivity of blood granulocytes after intravenous injection of 3H-thymidine; B: Decay of radioactivity of blood granulocytes after intravenous injection of 32Di-Isopropylfluorophosphate. Modified from[103].
In our opinion in vivo labeling is the better method. Isolating blood cells, processing and inject them again in the recipient can have dramatic effects on their life span. When leukemia patients are transfused with donated red blood cells after bone marrow transplantation, the half life on the donated red blood cells is dramatically reduced, leading to massive clearance of red blood cells. The released iron due to this enhanced turnover is a well known complication of red cell transfusion[116]. This indicates that even careful isolation of blood cells without any labeling has an impressive effect on their life span.
A proper understanding of the lifespan and distribution of the neutrophil is very important, as the neutrophil can vary in phenotype and function with a longer lifespan, and the lifespan determines the need for influencing the neutrophil function in inflammatory diseases. Further investigation of these different labeling techniques, their influence on neutrophil life span and the actual life span of a neutrophil are needed.
BEHAVIOR OF TISSUE NEUTROPHILS IN COMPARISON TO BLOOD NEUTROPHILS
Besides the effects of labeling neutrophils, the behavior of blood neutrophils compared to tissue neutrophils should also be taken into account. During in vitro culture, neutrophils are able to spontaneously enter apoptosis, a process which initiation can be accelerated or delayed by several factors. Danger signals such as TLR ligands are potently anti-apoptotic[117] while pro-inflammatory cytokine GM-CSF[118] and signaling from death receptor Fas can induce cell death[119]. Furthermore, culturing neutrophils in hypoxia reduces apoptosis, which improves the lifespan in vitro[120]. Interestingly, neutrophil apoptosis and the regulation of death processes are almost all studied in blood neutrophils, while the bulk of neutrophil apoptosis takes place in the tissues, as well as the clearance. This results in a lack in information about tissue neutrophil apoptosis. Tissue neutrophils can be obtained in vitro by experiments with so-called aseptic skin chamber techniques[121]. These transmigrated neutrophils have different gene transcription and behave differently than peripheral blood neutrophils[122], as transmigration induces mobilization of certain intracellular granules[123]. Interestingly, these transmigrated neutrophils also differ in responsiveness to stimulating agents. A wide variety of anti-apoptotic factors (the earlier mentioned TLR ligands and GM-CSF) were unable to delay apoptosis in transmigrated neutrophils[124]. This way, Christenson et al[124] showed functional differences in transmigrated tissue neutrophils compared to blood neutrophils. As tissue neutrophils differ from blood neutrophils in surface receptor expression and respond differently to certain stimulation, maybe earlier made conclusions regarding apoptosis pathways and life spans based on studies on blood neutrophils are only particularly true for tissue neutrophils, and this has to be further investigated.
TRANSMIGRATION OF TISSUE NEUTROPHILS
Neutrophils are thought to have little functional plasticity after differentiation, in comparison to monocytes and macrophages[125]. After recruitment into the tissue, they fulfill their immune function and die by apoptosis and phagocytosis by macrophages. Studies with rats have suggested that neutrophils can emigrate out of inflamed tissue and return to the circulation[126]. Recently, a study in humans showed neutrophils that emigrate out of the tissues in vitro via the lymphatics[127], in a manner similar to that described for monocytes[128]. These reverse transmigrated neutrophils are phenotypically and functionally different from circulating neutrophils and are found in vivo in the blood of healthy persons. Interestingly, these neutrophils are also found at significantly higher levels in patients with chronic active inflammatory disease, suggesting a role for these neutrophils in the persistence of inflammations in humans. This new perspective on the possibility of neutrophils to reverse transmigrate gives new insight in chronic inflammation, but also in the kinetics of neutrophil clearance in the tissues. The clearance of neutrophils in tissues is no longer only subscribed to apoptosis and phagocytosis, but also to reverse transmigration back into the circulation. The in vivo kinetics of transmigration is therefore a much needed future study.
CONTAMINATION
When using sensitive quantitative studies such as RT-PCR or measuring cytokine production, there is a big risk of contamination by monocytes and lymphocytes. Depending on the cytokine, neutrophils possess 10-20 fold lower RNA levels per cell than monocytes or lymphocytes. This means that neutrophils synthesize 10-300 times less cytokines than monocytes individually and causes a 1%-2% monocyte contamination to influence the RNA yield with 20%-30%[2]. It is thus important when measuring cytokine levels, to keep the level of contamination with monocytes and lymphocytes very low (< 0.5%). The neutrophil-specific surface marker CD66b could be used to determine purity of samples[129].
As earlier mentioned the exclusion of prestimulation of neutrophils is important. Every reagent, solution or lab ware with small levels of endotoxin can stimulate neutrophils. Inappropriate methods of erythrocyte lysis can lead to stimulation as well. To exclude stimulated neutrophils, the cells should be checked for CD62L, a membrane bound antigen, rapidly released upon neutrophil stimulation[130].
With this in mind, it will be interesting to investigate some of the current papers about neutrophil kinetics. For example in the paper of Suratt et al[92], the neutrophils are only tested for viability using Trypan blue dye exclusion. No control experiment for CD62L expression and thus activation was performed. The authors did obtain neutrophils with a modified method, to ensure depletion of contaminating monocytes.
Other studies compared tissue neutrophils with blood neutrophils, but no investigation into the functionality of neutrophils after collecting them was done[124]. Care should be taken when drawing conclusions from papers without proper controls for monocyte contamination or neutrophil activation. Also the type of labeling is important and the type of neutrophil used for different studies, as tissue neutrophils differ from blood neutrophils.
CONCLUDING REMARKS
The neutrophil has more functionality than just killing microbes, it also has a role in signaling to both the innate and adaptive immunity, the resolution of inflammation and cellular signaling with DCs and T cells. The mechanism of clearance of neutrophils and homing to the bone marrow is of great importance to the balance of cellular homeostasis. Clearance in the bone marrow leads to new neutrophil production; clearance in the spleen, liver and tissues reduces damage. This way, our body is able to continue the cellular homeostasis and the levels of circulating neutrophils. The neutrophil is important, but its clearance too. Because neutrophils are readily activated in experiments, a proper in vivo experiment is difficult to set up. More investigation is needed to elucidate the role of the different types of neutrophils in immunity. Tissue neutrophils differ from blood neutrophils, as well as the marginated pool differs from the circulating pool.
There are numerous studies done to the kinetics and life span of the neutrophil. The calculated blood circulation time varies from 10 h to over 5 d, each life span having tremendous effects on the functions of neutrophils. Further investigation to the lifespan and production rate is necessary, as current calculations are all based on different labeling techniques with different disadvantages and no clear conclusions can be drawn. In analogy to red blood cells, it is to be expected that after collection of cells, the life span decreases tremendously. In vivo labeling of neutrophil can prevent such effects on life time of a neutrophil. Also, the different pools that are present have to be taken into account when assessing the lifetime of neutrophils. Many tools for investigating the function of neutrophils in mice in vivo are now available. Although understanding the role of the neutrophil in vivo in man is much more difficult, it is of great importance for the potential role of neutrophils as targets in inflammatory diseases.