Published online Nov 4, 2015. doi: 10.5492/wjccm.v4.i4.296
Peer-review started: May 14, 2015
First decision: June 24, 2015
Revised: July 8, 2015
Accepted: August 4, 2015
Article in press: August 7, 2015
Published online: November 4, 2015
AIM: To examine complications associated with the use of therapeutic temperature modulation (mild hypothermia and normothermia) in patients with severe traumatic brain injury (TBI).
METHODS: One hundred and fourteen charts were reviewed. Inclusion criteria were: severe TBI with Glasgow Coma Scale (GCS) < 9, intensive care unit (ICU) stay > 24 h and non-penetrating TBI. Patients were divided into two cohorts: the treatment group received therapeutic temperature modulation (TTM) with continuous surface cooling and indwelling bladder temperature probes. The control group received standard treatment with intermittent acetaminophen for fever. Information regarding complications during the time in the ICU was collected as follows: Pneumonia was identified using a combination of clinical and laboratory data. Pulmonary embolism, pneumothorax and deep venous thrombosis were identified based on imaging results. Cardiac arrhythmias and renal failure were extracted from the clinical documentation. acute respiratory distress syndrome and acute lung injury were determined based on chest imaging and arterial blood gas results. A logistic regression was conducted to predict hospital mortality and a multiple regression was used to assess number and type of clinical complications.
RESULTS: One hundred and fourteen patients were included in the analysis (mean age = 41.4, SD = 19.1, 93 males), admitted to the Jackson Memorial Hospital Neuroscience ICU and Ryder Trauma Center (mean GCS = 4.67, range 3-9), were identified and included in the analysis. Method of injury included motor vehicle accident (n = 29), motor cycle crash (n = 220), blunt head trauma (n = 212), fall (n = 229), pedestrian hit by car (n = 216), and gunshot wound to the head (n = 27). Ethnicity was primarily Caucasian (n = 260), as well as Hispanic (n = 227) and African American (n = 223); four patients had unknown ethnicity. Patients received either TTM (43) or standard therapy (71). Within the TTM group eight patients were treated with normothermia after TBI and 35 patients were treated with hypothermia. A logistic regression predicting in hospital mortality with age, GCS, and TM demonstrated that GCS (Beta = 0.572, P < 0.01) and age (Beta = -0.029) but not temperature modulation (Beta = 0.797, ns) were significant predictors of in-hospital mortality [χ2 (3) = 22.27, P < 0.01] A multiple regression predicting number of complications demonstrated that receiving TTM was the main contributor and was associated with a higher number of pulmonary complications (t = -3.425, P = 0.001).
CONCLUSION: Exposure to TTM is associated with an increase in pulmonary complications. These findings support more attention to these complications in studies of TTM in TBI patients.
Core tip: Therapeutic hypothermia and normothermia (fever control) are used in patients with traumatic brain injury. This is most commonly done for intracranial hypertension control. The potential complications associated with this therapy when it is used outside of the scope of a closely regulated clinical trial are not well known. This is a retrospective review of patients with traumatic brain injury treated with therapeutic temperature modulation carried out to quantify the non neurological complications associated with this therapy.
- Citation: O’Phelan KH, Merenda A, Denny KG, Zaila KE, Gonzalez C. Therapeutic temperature modulation is associated with pulmonary complications in patients with severe traumatic brain injury. World J Crit Care Med 2015; 4(4): 296-301
- URL: https://www.wjgnet.com/2220-3141/full/v4/i4/296.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v4.i4.296
The systemic cooling of patients with severe traumatic brain injury (TBI) has become an established second tier treatment modality for refractory intracranial hypertension (ICP)[1-5].
Basic science evidence, anecdotal clinical reports and several low quality trials have suggested that the prophylactic application of this strategy (i.e., primary therapeutic hypothermia) may also exert neuroprotective effects in severe TBI[6]; however, these benefits have not been confirmed by high-quality human randomized controlled studies. Thus, outside of well-designed clinical trials, the implementation of therapeutic hypothermia after head injury remains justified for and largely limited to patients with uncontrolled ICP elevation. Yet, there are concerns that induced hypothermia may be associated with hemodynamic, pulmonary and infectious complications, as significant pathophysiological changes are known to occur with its induction and maintenance, especially when prolonged for more than 48 h[7]. However, overall rates of serious hypothermia-related adverse events remain poorly studied in TBI[8]. Given the knowledge that systemic, non-neurological complications are an independent contributor to morbidity and mortality after TBI[9], a more rigorous evaluation of the potential adverse effects associated with the use of hypothermia becomes of crucial importance to better determine the safety profile of this strategy in the setting of TBI. The purpose of this study is to examine types and rates of clinical complications in our severe TBI population who are treated with therapeutic temperature modulation (TTM).
The protocol was reviewed and approved by the institutional review board of our institution. This is a retrospective, observational cohort study. We carefully reviewed the charts for 114 patients with severe TBI admitted to our trauma center between 2007 and 2009. Inclusion criteria included a post resuscitation Glasgow Coma Scale (GCS) < 9, admission to the intensive care unit (ICU) > 24 h and non-penetrating TBI. Patients were divided into two cohorts: The treatment group that received TTM and the control group, which did not. Patients in the temperature modulation group received continuous surface cooling and temperature measurement via an indwelling bladder probe. This group included both therapeutic hypothermia with a target temperature of < 36 °C or induced normothermia with a target temperature of 36 °C-37 °C. The control group received intermittent acetaminophen as need to treat fever. The decision to use TTM and the degree of cooling were determined on an individual basis by the clinical team. The clinical record was reviewed to identify the following events: pneumonia, pneumothorax, acute respiratory distress syndrome (ARDS), acute lung injury (ALI), acute renal failure, cardiac arrhythmias, pulmonary embolism (PE) and deep venous thrombosis (DVT). Pneumonia was identified using a combination of the following criteria: purulent sputum, chest imaging with an infiltrate or consolidation, fever > 38 °C, leukocytosis (> 12000 wbc/mm3 or leukopenia < 4000 wbc/mm3) or worsening oxygenation. Pneumothorax and DVT and PE were identified based on imaging and ARDS and ALI were identified using a combination of imaging and arterial blood gas findings. Data on length of stay in the intensive care unit, duration of mechanical ventilation and in hospital mortality were also collected.
All data were analyzed using IBM SPSS version 21. A logistic regression was conducted to predict hospital mortality and a multiple regression was used to assess number of complications. Independent variables for both models were age, GCS on admission, and temperature modulation.
One hundred and fourteen patients with severe TBI (mean age = 41.4, SD = 19.1, 93 males), admitted to the Jackson Memorial Hospital Neuroscience ICU (mean GCS = 4.67, range 3-9), were identified and included in the analysis. Method of injury included motor vehicle accident (n = 29), motor cycle crash (n = 20), blunt head trauma (n = 12), fall (n = 29), pedestrian hit by car (n = 16), and gunshot wound to the head (n = 7). Ethnicity was primarily Caucasian (n = 60), as well as Hispanic (n = 27) and African American (n = 23); four patients had unknown ethnicity. Patients received either temperature modulation (i.e., aggressive temperature control, as detailed below) or no continuous modulation (i.e., permissive temperature management), and were monitored for number and type of complications, as well as in hospital mortality.
Forty-three patients underwent temperature modulation (TM). Specifically, eight patients were treated with induced normothermia (mean temp = 36.25 °C, SD = 0.85) and 35 with mild therapeutic hypothermia (mean temp 34.8 °C, SD = 0.75). Temperature modulation was achieved by application of antipyretic agents (acetaminophen) and surface cooling techniques.
Temperature modulation was combined for the remaining analyses (Table 1). In-hospital mortality and number of complications did not statistically differ between normothermia and hypothermia groups [pearson χ2 (4) = 4.99, ns].
| Temperature modulation | Control | |
| No. of patients | 44 | 70 |
| M:F | 1:4.5 | 1:4.4 |
| Mean GCS (SD) | 4.6 (1.9) | 4.7 (1.9) |
| Mean age (SD) | 33.3 (14.2) | 46.5 (20.2) |
| Mortality | 15.9% | 31.4% |
Initially, an unadjusted analysis of mortality suggested a lower rate of mortality in TTM group (6% vs 19%, P = 0.06). However, the mean age in the TTM group was younger (33 years, SD 14) vs 46 years (SD 20). As expected, a logistic regression predicting in hospital mortality with age, GCS on admission, and temperature modulation demonstrated that GCS (Beta = 0.572, P < 0.01) and age (Beta = -0.029) but not temperature modulation (Beta = 0.797, ns) were significant predictors of in-hospital mortality [χ2 (3) = 22.27, P < 0.01 when mortality was adjusted for age the difference between the groups was not significant.
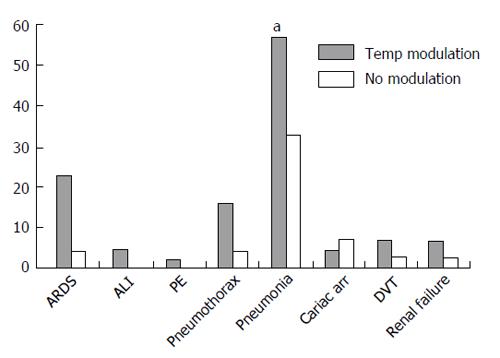
A multiple regression predicting number of complications with age, GCS on admission, and temperature modulation demonstrated that receiving temperature modulation was the main contributor and was associated with a higher number of complications [F(3) = 4.59, P < 0.005, t = -3.425, P = 0.001]. Age (t = 0.71, ns) and admission GCS (t = 1.42, ns) were not significant contributors. Temperature modulation was significantly associated with ARDS, pneumothorax, and pneumonia (Figure 1).
The present study examined rates of medical complications associated with the application of TM in patients with severe TBI. However, it was not designed to assess potential clinical benefits of TM as we did not measure long-term functional outcome.
Many physiological effects of hypothermia make its use theoretically attractive in the TBI setting. These include: (1) attenuation of neuro-excitotoxicity, via suppression of glutamate release, and ensuing stabilization of the intracellular influx of calcium (effects that ultimately reduce the magnitude of mitochondrial damage and cell demise secondary to the post-injury activation of multiple intracellular enzymatic cascades); (2) stabilization of the blood-brain barrier and blunting of the neuroinflammatory response from microglia, which may limit the development of cerebral edema and oxidative stress[10-12]; and (3) reduction in the cerebral metabolic rate of oxygen consumption (CMRO2) by approximately 7% for each degree Celsius decline in body temperature; the latter effect has the dual benefit of preserving brain oxygen stores (thereby conferring protection against cerebral hypoperfusion) and promoting cerebral vasoconstriction with ensuing decrease in ICP[13,14]. Nevertheless, despite these potential beneficial properties multiple randomized, controlled trials have failed to provide data in support of the primary application of induced hypothermia as a neuroprotective strategy aimed at improving mortality and functional outcome in TBI patients. In addition, concerns have been voiced about possible detrimental effects in trauma patients, with some evidence suggesting an increased risk for hemodynamic and pulmonary complications[7,15-17]. While data from a recent randomized controlled trial of 48-h hypothermia in TBI patients revealed “no significant differences in the percentage of patients with any individual complication or group of complications, whether critical or non-critical, between groups”[18], other clinical studies (mostly in patients with stroke and TBI) have reported a higher risk of adverse events, such as pneumonia, when cooling was carried out over longer periods of time (> 48-72 h)[19,20]. Nevertheless, the inadequate control for possible confounding influences (e.g., poor glycemic control, barbiturate use) in those studies has left uncertainty over a causative link between induced hypothermia and risk of pneumonia or other complications.
The results of our study show that temperature modulation, applied for > 48 h, in the form of normothermia or hypothermia, is not a predictor of in-hospital mortality, but is associated with a significantly increased risk for pulmonary complications (pneumonia, ARDS, and pneumothorax). We were unable to detect a difference between the patients treated with normothermia vs those treated with hypothermia because the sample size was quite small. With regard to pneumothorax, we speculate that the increased incidence of pneumothorax may reflect a more prolonged and aggressive course of mechanical ventilation, with use of higher positive end expiratory pressure levels, in patient developing severe hypoxemia secondary to pneumonia or ARDS. Thus, the major systemic complications associated with the implementation of temperature modulation in TBI patients appear to be pneumonia and ARDS.
Our finding of an increased incidence of pneumonia with temperature modulation in a purely clinical setting is consistent with the results of 5 published systematic reviews and meta-analyses of randomized controlled clinical trials on the effectiveness of hypothermia in TBI[8,17,21-23], which identified 6 trials reporting a significant higher rate of pneumonia with induced hypothermia. Similarly, a more recent meta-analysis, which included 23 randomized controlled trials involving adult patients treated with therapeutic hypothermia of various duration (from several hours to several days) and for different indications (including TBI), revealed that patients undergoing systemic cooling were more likely to develop pneumonia (risk ratios, 1.44) compared to control groups[15].
An increased susceptibility to pneumonia may result from impaired central immune suppression after acute neurological injuries, including TBI. It is also possible that TM may promote the emergence of clinically apparent pneumonia by counteracting the ability of the body to fight infection. A substantial body of evidence from animal studies supports the concept that fever plays a central role in the host response to infection. The immunological effects of temperature elevation within a physiologic febrile range are multiple and beneficial. They include stimulation of neutrophil cell motility and phagocytosis, enhanced expression of receptors involved in mediating antibody responses, promotion of lymphocyte migration to sites of infection, and reduced growth of intracellular bacteria[24]. While these potentially beneficial consequences of fever cannot be disregarded, they come at the cost of a substantial increase in cerebral metabolic rate of oxygen consumption (CMRO2), neuroinflammation, activation of calcium-mediated intracellular enzymatic cascades, all of which may promote and exacerbate secondary brain injury. Thus, in TBI patients, a balance must be struck between the benefits of suppressing the above processes with temperature modulation and the potential detrimental effects on host defence mechanisms leading to an increased risk for infection.
It has been suggested that a longer duration of cooling increases risk of infection. This is consistent with the observation, in some clinical studies (mostly in patients with stroke and TBI), of a higher risk of pneumonia when cooling was carried out over more than 48 h duration[19,20]. This might explain why Clifton’s second randomized trial, which limited the use of hypothermia to 48h, did not detect any significant difference in the rates of non-neurological organ dysfunction between hypothermic and normothermic patients. Unfortunately, this adds a layer of complexity to the management of TBI because a period longer than 48 h may be needed to sufficiently control brain edema. This longer duration may expose the patient to an increased risk of complications. This may offset the potential benefits of prolonged cooling. Severe respiratory failure as a result of ARDS and/or pneumonia may adversely affect cerebral oxygenation and brain energy metabolism, and contribute to secondary brain injury. It is unknown if regional methods of cooling using new devices such as intranasal cooling[25,26] will offer the neurological benefits of TTM with fewer systemic side effects.
Our findings demonstrate that TM is associated with an increased incidence of pulmonary complications which may restrict the neuroprotective potential of this strategy. Inclusion of protocols to prevent pneumonia in patients with TBI undergoing TM may improve the efficacy of this strategy and should be included in future study protocols.
Our study has several limitations including the retrospective design, a small sample size and no functional measure of neurologic outcome. The questions raised here will need to be demonstrated in a larger study with a prospective design.
In conclusion, our study demonstrates that in patients with TBI, exposure to temperature modulation is associated with a significant increase in pulmonary complications, specifically, pneumonia, ARDS and pneumothorax. These findings support more detailed collection of information about these complications in studies of therapeutic temperature modulation in TBI patients to determine their relevance to outcome. Prospective studies are needed to determine possible detrimental effects on functional neurological recovery that could result from hypothermia-related complications such as ARDS and pneumonia.
The benefit of hypothermia used for neuroprotection is still debated. The benefits of this therapy have not been proven in large prospective randomized trials for patients with traumatic brain injury. However, the therapy is effective for lowering intracranial pressure. Therefore it is sometimes used for this population. Therefore it is important to understand the potential complications associated with its use.
Current research efforts focus on the potential benefits of local or regional therapies for temperature management. These studies include trans nasal evaporative cooling which has been studied in cardiac arrest and stroke patients.
This study provides data taken from patients being treated outside of clinical trials. It may be more generalizable than data from carefully controlled trials with a very specific patient population.
This study should provide support for future studied to more carefully consider and collect data on pulmonary complications in patient being treated with therapeutic temperature modulation. Additionally, these data may inform the cost benefit analysis in a larger prospective study utilizing temperature management in this population.
Therapeutic hypothermia or targeted temperature management: Is a therapy that tries to achieve and maintain a specific body temperature to mitigate tissue injury and improve outcome.
This retrospective review has issues with lack of definitions of the key outcome measures used.
P- Reviewer: Inchauspe A, Ntoumenopoulos G S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
| 1. | Bloch M. Cerebral effects of rewarming following prolonged hypothermia: significance for the management of severe cranio-cerebral injury and acute pyrexia. Brain. 1967;90:769-784. [PubMed] [Cited in This Article: ] |
| 2. | Clifton GL, Allen S, Barrodale P, Plenger P, Berry J, Koch S, Fletcher J, Hayes RL, Choi SC. A phase II study of moderate hypothermia in severe brain injury. J Neurotrauma. 1993;10:263-271; discussion 273. [PubMed] [Cited in This Article: ] |
| 3. | Marion DW, Obrist WD, Carlier PM, Penrod LE, Darby JM. The use of moderate therapeutic hypothermia for patients with severe head injuries: a preliminary report. J Neurosurg. 1993;79:354-362. [PubMed] [Cited in This Article: ] |
| 4. | Shiozaki T, Sugimoto H, Taneda M, Yoshida H, Iwai A, Yoshioka T, Sugimoto T. Effect of mild hypothermia on uncontrollable intracranial hypertension after severe head injury. J Neurosurg. 1993;79:363-368. [PubMed] [Cited in This Article: ] |
| 5. | Shapiro HM, Wyte SR, Loeser J. Barbiturate-augmented hypothermia for reduction of persistent intracranial hypertension. J Neurosurg. 1974;40:90-100. [PubMed] [Cited in This Article: ] |
| 6. | Pomeranz S, Safar P, Radovsky A, Tisherman SA, Alexander H, Stezoski W. The effect of resuscitative moderate hypothermia following epidural brain compression on cerebral damage in a canine outcome model. J Neurosurg. 1993;79:241-251. [PubMed] [Cited in This Article: ] |
| 7. | Polderman KH. Application of therapeutic hypothermia in the intensive care unit. Opportunities and pitfalls of a promising treatment modality--Part 2: Practical aspects and side effects. Intensive Care Med. 2004;30:757-769. [PubMed] [Cited in This Article: ] |
| 8. | Peterson K, Carson S, Carney N. Hypothermia treatment for traumatic brain injury: a systematic review and meta-analysis. J Neurotrauma. 2008;25:62-71. [PubMed] [Cited in This Article: ] |
| 9. | Lim HB, Smith M. Systemic complications after head injury: a clinical review. Anaesthesia. 2007;62:474-482. [PubMed] [Cited in This Article: ] |
| 10. | Schmitt KR, Diestel A, Lehnardt S, Schwartlander R, Lange PE, Berger F, Ullrich O, Abdul-Khaliq H. Hypothermia suppresses inflammation via ERK signaling pathway in stimulated microglial cells. J Neuroimmunol. 2007;189:7-16. [PubMed] [Cited in This Article: ] |
| 11. | Gibbons H, Sato TA, Dragunow M. Hypothermia suppresses inducible nitric oxide synthase and stimulates cyclooxygenase-2 in lipopolysaccharide stimulated BV-2 cells. Brain Res Mol Brain Res. 2003;110:63-75. [PubMed] [Cited in This Article: ] |
| 12. | Dempsey RJ, Combs DJ, Maley ME, Cowen DE, Roy MW, Donaldson DL. Moderate hypothermia reduces postischemic edema development and leukotriene production. Neurosurgery. 1987;21:177-181. [PubMed] [Cited in This Article: ] |
| 13. | Keresztes PA, Brick K. Therapeutic hypothermia after cardiac arrest. Dimens Crit Care Nurs. 2006;25:71-76. [PubMed] [Cited in This Article: ] |
| 14. | Steen PA, Newberg L, Milde JH, Michenfelder JD. Hypothermia and barbiturates: individual and combined effects on canine cerebral oxygen consumption. Anesthesiology. 1983;58:527-532. [PubMed] [Cited in This Article: ] |
| 15. | Geurts M, Macleod MR, Kollmar R, Kremer PH, van der Worp HB. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med. 2014;42:231-242. [PubMed] [Cited in This Article: ] |
| 16. | Clifton GL, Miller ER, Choi SC, Levin HS, McCauley S, Smith KR, Muizelaar JP, Wagner FC, Marion DW, Luerssen TG. Lack of effect of induction of hypothermia after acute brain injury. N Engl J Med. 2001;344:556-563. [PubMed] [Cited in This Article: ] |
| 17. | Alderson P, Gadkary C, Signorini DF. Therapeutic hypothermia for head injury. Cochrane Database Syst Rev. 2004;CD001048. [PubMed] [Cited in This Article: ] |
| 18. | Clifton GL, Drever P, Valadka A, Zygun D, Okonkwo D. Multicenter trial of early hypothermia in severe brain injury. J Neurotrauma. 2009;26:393-397. [PubMed] [Cited in This Article: ] |
| 19. | Shiozaki T, Hayakata T, Taneda M, Nakajima Y, Hashiguchi N, Fujimi S, Nakamori Y, Tanaka H, Shimazu T, Sugimoto H. A multicenter prospective randomized controlled trial of the efficacy of mild hypothermia for severely head injured patients with low intracranial pressure. Mild Hypothermia Study Group in Japan. J Neurosurg. 2001;94:50-54. [PubMed] [Cited in This Article: ] |
| 20. | Schwab S, Georgiadis D, Berrouschot J, Schellinger PD, Graffagnino C, Mayer SA. Feasibility and safety of moderate hypothermia after massive hemispheric infarction. Stroke. 2001;32:2033-2035. [PubMed] [Cited in This Article: ] |
| 21. | Henderson WR, Dhingra VK, Chittock DR, Fenwick JC, Ronco JJ. Hypothermia in the management of traumatic brain injury. A systematic review and meta-analysis. Intensive Care Med. 2003;29:1637-1644. [PubMed] [Cited in This Article: ] |
| 22. | Sydenham E, Roberts I, Alderson P. Hypothermia for traumatic head injury. Cochrane Database Syst Rev. 2009;CD001048. [PubMed] [Cited in This Article: ] |
| 23. | Georgiou AP, Manara AR. Role of therapeutic hypothermia in improving outcome after traumatic brain injury: a systematic review. Br J Anaesth. 2013;110:357-367. [PubMed] [Cited in This Article: ] |
| 24. | Young P, Saxena M, Eastwood GM, Bellomo R, Beasley R. Fever and fever management among intensive care patients with known or suspected infection: a multicentre prospective cohort study. Crit Care Resusc. 2011;13:97-102. [PubMed] [Cited in This Article: ] |
| 25. | Abou-Chebl A, Sung G, Barbut D, Torbey M. Local brain temperature reduction through intranasal cooling with the RhinoChill device: preliminary safety data in brain-injured patients. Stroke. 2011;42:2164-2169. [PubMed] [Cited in This Article: ] |
| 26. | Castrén M, Nordberg P, Svensson L, Taccone F, Vincent JL, Desruelles D, Eichwede F, Mols P, Schwab T, Vergnion M. Intra-arrest transnasal evaporative cooling: a randomized, prehospital, multicenter study (PRINCE: Pre-ROSC IntraNasal Cooling Effectiveness). Circulation. 2010;122:729-736. [PubMed] [Cited in This Article: ] |