Published online Jun 22, 2015. doi: 10.5498/wjp.v5.i2.210
Peer-review started: December 4, 2014
First decision: January 20, 2015
Revised: February 20, 2015
Accepted: March 16, 2015
Article in press: March 18, 2015
Published online: June 22, 2015
Olfactory deficits on measures of identification, familiarity, and memory are consistently noted in patients with psychotic disorders relative to age-matched controls. Olfactory intensity ratings, however, appear to remain intact while the data on hedonics and detection threshold are inconsistent. Despite the behavioral abnormalities noted, no specific regional brain hypoactivity has been identified in psychosis patients, for any of the olfactory domains. However, an intriguing finding emerged from this review in that the amygdala and pirifom cortices were not noted to be abnormal in hedonic processing (nor was the amygdala identified abnormal in any study) in psychotic disorders. This finding is in contrast to the literature in healthy individuals, in that this brain region is strongly implicated in olfactory processing (particularly for unpleasant odorants). Secondary olfactory cortex (orbitofrontal cortices, thalamus, and insula) was abnormally activated in the studies examined, particularly for hedonic processing. Further research, using consistent methodology, is required for better understanding the neurobiology of olfactory deficits. The authors suggest taking age and sex differences into consideration and further contrasting olfactory subgroups (impaired vs intact) to better our understanding of the heterogeneity of psychotic disorders.
Core tip: Olfactory identification, familiarity, and memory deficits are consistently noted in patients with psychotic disorders relative to age-matched controls. Olfactory intensity ratings remain intact while olfactory hedonics and detection threshold show inconsistent findings. This review found no consistent differences in functional activity in amygdala and pirifom cortices in psychotic patients relative to control subjects. Secondary olfactory cortices were abnormally activated in psychosis patients, however. Further methodologically consistent research is required for better understanding the neurobiology of olfactory deficits in psychotic disorders. The authors recommend examining sex differences contrasting olfactory subgroups (impaired vs intact) in future examinations.
- Citation: Good KP, Sullivan RL. Olfactory function in psychotic disorders: Insights from neuroimaging studies. World J Psychiatr 2015; 5(2): 210-221
- URL: https://www.wjgnet.com/2220-3206/full/v5/i2/210.htm
- DOI: https://dx.doi.org/10.5498/wjp.v5.i2.210
Olfactory input, although mostly unconsciously processed and relatively ignored, colours our appreciation for food, influences our mood and warns us of impending danger (e.g., spoiled food, smoke, chemical spill etc.). It is only when olfaction is impaired that humans begin to realize how much olfactory stimulation influences decisions in everyday life. In fact, olfactory compromise has been linked to depression[1] and for patients with traumatic brain injury, post traumatic anosmia is negatively linked to return to work after injury[2,3]. For individuals with neurodegenerative disorders, such as Parkinson’s disease[4], Dementia (e.g., Alzheimer’s disease[5]), or psychotic disorders[6], olfactory decline may be one of the first symptoms subjectively noted. Consequently, formal assessment of olfactory function may allow for early identification of those at risk of developing these illnesses prior to the illness onset. Understanding the neurobiology of olfactory decline may prove useful to clinicians and allow for early detection, intervention and treatment.
In psychotic disorders, including schizophrenia, olfactory deficits are observable early in the course of illness, but progress very little, if any[7]. However, these olfactory problems may be a harbinger for poorer prognosis, both symptomatically and in terms of functional outcome[8,9]. A better understanding of olfactory compromise in these disorders is fundamental to better understanding the heterogeneity of illness course and symptoms. Further elucidating the neurobiology of olfactory deficits may provide further clues to the underlying brain abnormalities in this disorder.
The purpose of this paper, therefore, is to review the literature on olfactory function in patients with schizophrenia, paying particular attention to the neuroimaging data that has accumulated.
Small postage stamp sized regions of superior aspect of each nasal cavity and upper portion of the nasal septum contain the olfactory epithelium. Within this region, specialized G-protein coupled olfactory receptors bind to inhaled volatile chemicals that have dissolved in the olfactory mucosa. Once odorants are bound to receptors, action potentials are initiated and are transmitted to the brain via the olfactory nerves. Axons project through fenestrations within the cribriform plate of the ethmoid bone and synapse on neurons (mitral and tufted cells) within the olfactory bulb. From here, signals project predominantly through the lateral olfactory tract to the primary olfactory cortex (including the piriform and entorhinal cortices, part of the amygdala and the olfactory tubercule). Collaterals from these axons project to the anterior olfactory nucleus. A minority of fibres project via the medial olfactory tract and cross, via the anterior commissure, to the contralateral olfactory bulb. However, the vast majority of olfactory projections are ipsilateral[10].
The olfactory system is unique among the senses as it projects initially to cortical regions, rather than thalamic nuclei. Thalamic connections occur post-cortically, along with projections from piriform cortex to other limbic regions, such as the hypothalamus and hippocampus. Projections to the orbitofrontal cortex can be either direct from primary olfactory cortex, or indirect via the dorsomedial nucleus of the thalamus[11].
A further differentiating factor that separates the olfactory system from other senses is that the signals from primary sensory receptors to higher processing regions are predominantly sent ispilaterally, rather than having a contralateral representation. This fact allows for the assessment of the relative contribution of each hemisphere to the processing of olfactory stimuli. If stimulus presentation is restricted to one nostril only, the ipsilateral hemisphere will be preferentially (initially) engaged. This anatomic detail has been exploited by some research groups and lateralized findings have been reported[12,13].
Olfactory dysfunction can occur as a result of damage at any level of the olfactory system, from destruction of the olfactory receptors due to exposure to inhaled chemical toxins, to deficits in olfactory detection threshold as a result of olfactory nerve/tract damage during closed head injury, to distinct higher level olfactory deficits (e.g., olfactory agnosia) as a result of lesions to secondary olfactory cortex[14,15].
Between 1%-3% of the population suffers from non-affective psychotic disorders, with schizophrenia being the most common form[16]. These disorders are associated with a diverse range of abnormal mental phenomena, including hallucinations, delusions, unusual thought content, anhedonia, social withdrawal and alterations in cognitive domains such as memory, attention, language and executive function. The typical age of onset of these disorders is relatively early, with most individuals presenting with their first psychotic episode in late adolescence or early adulthood. For many, the course of illness is one of waxing and waning of symptomatology; few make a full recovery after one episode.
The issue of whether olfactory sensitivity (detection threshold/acuity) is impaired in schizophrenia has not yet been resolved, with some studies showing normal acuity[17,18], others impaired[19,20] and still others demonstrating enhanced sensitivity[21,22]. As well, the issue of whether olfactory hedonics are abnormal in patients with psychotic disorders continues to be debated[19,23-27]. There is evidence that pleasantness ratings differ by sex[28,29] and are likely related to symptom presentation[30,31]. More consistent findings have emerged when olfactory discrimination (same as/different) and olfactory memory/familiarity are assessed. Patients with psychotic disorders appear to be impaired on both of these functions[26,32,33]. Odour intensity ratings, however, appear to be intact in patients with psychotic disorders[26].
The most robust difference between patients with psychotic disorders and control subjects has been noted in studies examining olfactory identification ability. In these investigations, consistently abnormal olfactory identification has been shown in psychosis patients[7,9,18,30,34]. Olfactory identification deficits do not appear to be influenced by age of illness onset[17,34], smoking history[17,35], exposure to antipsychotic medications[13,17,32] or cannabis use[7]. Olfactory identification deficits, however, are thought to correlate with measures of verbal memory function[36,37], and negative (but not positive) psychotic symptoms[7,9,34].
Recent research has shown that olfactory compromise may be a marker for poorer outcome. Good et al[8] examined symptomatic outcome in a group of patients who were antipsychotic drug naive at initial olfactory testing. Poorer negative and cognitive symptom outcome was associated with a lower baseline olfactory identification (UPSIT) test score. This same group prospectively followed a group of first episode patients for 4 years[9]. Those with poorer olfactory scores at initial assessment had worse symptom outcome and reduced functional outcome at follow up. Additionally, individuals who are at risk for developing a psychotic disorder (by virtue of having a first degree relative with the disorder and/or showing attenuated psychotic symptoms), having lower olfactory test scores appears to be related to the conversion to a true psychotic disorder[6]. Taken together, these findings suggest that not only may olfactory deficits predict transition to illness, but that subgroups may be identified, early in the course of illness, who may be at risk for a more severe illness course.
That patients with psychotic disorders have olfactory dysfunction is not surprising given the overlap between olfactory processing regions and areas of the brain that have been consistently noted as abnormal in these patients. Abnormalities in medial temporal regions, including the hippocampus, amygdala and parahippocampal gyrus have been observed in patients with psychotic disorders. For a review, see[38,39]. Moreover, orbitofrontal and dorsomedial nucleus of thalamus have also been found to be both structurally and functionally abnormal[40,41]. Left sided abnormalities are more commonly reported[42].
The olfactory system has been well described anatomically (see above). However, recent neuroimaging studies have revealed a more widespread network of interrelated olfactory brain regions required for different aspects of olfactory processing. For example, although the piriform cortex is reciprocally connected with the olfactory bulb, functional magnetic resonance imaging (FMRI) studies have reported that piriform cortex activation is most robust during active “sniffing” of the odorants, and not during passive odours presentation such as when subjects are instructed not to purposely inhale the odorants[43] but see also[44]. However, the primary olfactory cortex appears to habituate more quickly than other brain regions and thus may be a better reason why consistent activation is not found in this brain region in all studies. The insula and anterior cingulate cortex are not necessarily associated with olfaction per se; however, activation in these regions has been demonstrated relatively consistently during odour presentation, suggesting a role in higher-order olfactory processing[45] (Table 1).
| Olfactory domain | Methods by which this domain is assessed |
| Passive smelling | Odorant is presented passively to the subject. Imaging done without response or overt cognitive appreciation |
| Olfactory detection | Similar to passive smelling, however, the subject must press a button to signal the onset of the odorant perception |
| Odour “sniffing” | Subjects are given instructions to inhale the odorant during presentation |
| Olfactory intensity | Rating the odorant as to its perceived strength. Can be done by a button press (Is the odour “strong”?) |
| Intensity may be assessed by ratings of odorants (e.g., VAS) outside of the scanner and presenting odours that have been rated on opposite ends of the continuum (very strong vs very weak) | |
| Olfactory familiarity | Recollect prior exposure without the exact autobiographical context and also without naming. Button press if odour is “familiar” |
| Olfactory hedonics (valence) | The determination of pleasantness of the odorant. |
| Button press to rate whether an odorant is pleasant | |
| Pleasant/Unpleasant odorant rated (e.g., VAS) outside of the scanner and presenting odours that have been rated on opposite ends of the continuum (very unpleasant to very pleasant) | |
| Olfactory identification | Correct identification- those items that are correctly labeled vs those for which label is incorrect |
| Olfactory identification test score used to capture most impaired, compared with normal sense of smell | |
| Forced choice odour naming during scanning |
Brain regions that are activated after presentation of odours when subjects are instructed not to sniff or nor consciously process the odorants include areas associated with primary olfactory cortex [piriform cortex (however, see above), amygdala, hippocampus, entorhinal cortex] and secondary olfactory regions (thalamus, orbitofrontal cortex, cingulate and insula)[46-49]. These regions can be thought of as “core” olfactory regions. When olfactory-cognitive load is increased, other ancillary regions are also engaged.
As previously mentioned, the piriform cortex may be activated solely in the presence of active “sniffing” or nasal airflow[43,50]. A more recent positron emission tomography (PET) study, however, did not confirm these results[44].
Odour hedonics: Humans are particularly poor at naming odours de novo (see section below). However, odours can evoke very strong emotional reactions, even without conscious awareness. Consequently, the primary dimension by which humans characterize odorants is according to the odour’s pleasantness, and particularly when odorants are difficult to name[51]. Researchers have capitalized on this aspect of olfactory processing in imaging studies; but unfortunately, mixed findings have resulted. The data is clear on one point: pleasant and unpleasant odorants invoke activation in similar neural networks, (primary and secondary cortices). However, intensities and degree of engagement may differ and some unique brain regions may also be invoked[52], particularly for unpleasant/aversive odorants. Both pleasant and unpleasant odorants activate the bilateral piriform/amygdala[45,53,54] but see also[55], right[49] and bilateral insula[45,56], and orbitofrontal cortex[49,53,54,57,58]. Most contrasts between pleasant (P) and unpleasant (UP) odorants have shown a greater degree of activation in the UP-P subtraction, rather than the other way around (P-UP). To this end, aversive or unpleasant odorants appear to further engage orbitofrontal cortex[54] - but only when subjects are engaged in a hedonic estimation task, and the activation seems to be concentrated in the lateral aspect rather than medial subdivisions[58]. Moreover, the left insula[45,52], but see also[56] who showed insular activity predominantly during pleasant odour stimulation), the anterior cingulate[45,52,58], brainstem/hypothalamus[52], and piriform/amygdala[45] activation has been noted after unpleasant odor presentation over and above that demonstrated during pleasant odorant stimulation.
Odour familiarity: Determining whether an odour is familiar requires an implicit set of memory processes; the perceptual input needs to be compared with semantic odour associations[59]. The smeller must recollect prior exposure without the exact autobiographical context and also without naming. Only few studies have examined this aspect of olfactory processing. In 1999, Royet et al[60] examined, among other aspects of olfactory processing, odour familiarity in healthy subjects. In this study, they demonstrated right orbitofrontal cortex activation during odour familiarity ratings, suggesting a lateralization of secondary olfactory cortical function. In a later study by this same group, Royet et al[57] noted a larger network of olfactory brain regions associated with odour familiarity. Bilateral orbitofrontal cortex (OFC), anterior cingulate along with left superior and right middle frontal gyri were all active during a PET scan during which an odour familiarity task was compared to control activation (presentation of odorless air). When compared with odour detection, left superior and left inferior frontal gyri engagement was noted, suggesting a hierarchical process, with odour familiarity ratings at higher levels of processing. This group suggested that the lateral frontal activation may represent accessing stored representations.
Odour intensity: A further dimension of odour processing is that of rating odour intensity. Odour pleasantness ratings change as a function of an odour’s perceived intensity. For example, the rose-like odorant (phenyl ethanol) tends to be perceived as pleasant at lower concentrations, but less pleasant at higher concentrations[61]. In many studies assessing olfactory hedonics, the intensity of presented odorants was not controlled. In order to dissociate intensity and valence, Anderson et al[54] focused on the amygdala and the orbitofrontal cortex. The amygdala has been reliably shown to activate during presentation of aversive stimuli[62]. The orbitofrontal cortex, as mentioned previously, appears to be invoked in higher order processing of olfactory stimuli. By presenting two different odorants at two different concentrations (high intensity-unpleasant, high intensity-pleasant, low intensity-unpleasant, low intensity-pleasant), Anderson et al[54] noted that that the orbitofrontal cortex engaged in relation to the valence of the odorant while the amygdala was preferentially engaged according to the intensity of the odorant. No ratings were performed during the scan; rather, subjects were only instructed to “sniff” for the duration of a presented message and respond if an odour was detected. A more recent study lent some further explanation to this unexpected finding. Winston et al[63] replicated the Anderson et al[54] study, but included both high- and low-intensity neutral odours for comparison. In this study, the amygdala was engaged predominantly with both the pleasant and unpleasant high-intensity odors, but not the high intensity neutral odour (nor any of the low intensity odours either). A more parsimonious explanation was suggested by these researchers in that the amygdala is engaged when odours are encountered that may be relevant for survival. High intensity pleasant or unpleasant odours are more likely to be behaviorally salient than are neutral odours (or low intensity odours along the hedonic spectrum).
Olfactory identification: Odor naming, particularly when potential exemplars are not provided, is a difficult task for humans[64,65]. By providing a multiple-choice format, such as the University of Pennsylvania Smell Identification Test (UPSIT), performance is improved significantly[61].
In a recent study, Kjelvik et al[47] examined whether there were any differences in activation patterns when individuals were successful in naming odorants vs when they incorrectly named the odours. Odorants were presented to subjects (only women) during an FMRI scan. Post scanning, subjects were presented with these same odours and were instructed to spontaneously name them (no exemplars provided). Brain activation patterns differed between correctly identified odours vs those not correctly identified in left entorhinal cortex, bilateral temporal poles, orbitofrontal cortex (right moreso than left), right thalamus and left insula. Other, non-olfactory areas (e.g., putamen, primary visual and auditory cortices, premotor cortex, SII and cerebellum, inferior frontal, and fusiform area) were also activated more during identified vs non-identified odours. When specifically examining the medial temporal regions, left entorhinal cortex, and bilateral posterior parahippocampal gyri were engaged. The data suggested that the entorhinal cortex and the hippocampus are more attuned to identifying odours.
Sex differences: Many of the studies mentioned above (e.g.,[47,55]) examined only female subjects as women reliably outperform men on olfactory detection, memory and identification tasks[66-68]. Three different research groups have examined whether this female olfactory superiority translates into enhanced brain activity. In an early FMRI study, Yousem et al[69] noted that female brains had significantly more active voxels in the perisylvian and inferior frontal regions during odour presentation. Levy et al[53], using a ratio of pixel activated to the number of pixels in a region, also showed sex differences; However, their findings were in the opposite direction (males > females). In contrast, Bengtsson et al[70] were unable to detect any male/female differences in brain activation during passive perception of odours. This group suggested that males and females engage similar networks during low level processing of olfactory stimuli. However, due to methodological inconsistencies, the intensity of activation was not examined in this PET study, making comparisons with other papers data difficult. In contrast, Royet et al[45] found that women activated the left orbitofrontal cortex over and above what was noted in male brains. The differences again may prove to be related to methodological differences, or rather due to the processing demands placed on the subjects during olfactory scanning (for part of the study, subjects in the Royet et al[45], study were asked to make an hedonic judgment regarding the odours presented while Bengtsson et al[70] were examining olfactory processing during passive administration). Nevertheless, these data taken as a whole, suggest that sex differences should be taken into account when examining olfactory brain activations.
As the ability to perceive odours deteriorates with age, do functional alterations in brain regions associated with the sense of smell also accompany these changes A study published by Wang et al[71] noted that there were significant differences in the processing of olfactory stimuli when comparing young and older subjects. Although for both groups, the same regions were activated by odorants, lower cluster volumes and intensities were noted in the aged group when compared to the younger group. This finding suggests that age should be taken into consideration when examining neural activation to olfactory stimuli (Table 2).
| Ref. | AuthorsPassive smelling | Year | Modality | Subject group(s) | Finding |
| [46] | Zatorre et al | 1992 | PET | Healthy subjects | Piri (B) and OFC (RH) |
| [53] | Levy et al | 1997 | FMRI | Healthy subjects | P and UP: piri, amyg, hippocam, cing and post inf front cortex (laterality not specified) |
| [43] | Sobel et al | 1998 | FMRI | Healthy subjects | Passive smelling, lat OFC; Sniffing-piri (B) and post OFC (B) |
| [48] | Savic et al | 2000 | PET | Healthy subjects | OFC (RH), amyg/piri (RH), thal (RH), insula (LH), ant cing |
| [49] | Gottfried et al | 2002 | FMRI | Healthy subjects | P and UP: Amyg (B), post OFC (B), post piri (B), insula (RH) |
| [45] | Royet et al | 2003 | FMRI | Healthy subjects | UP and P odorants-piri/amyg, hypothal, sup temp, insula and OFC, ant cing |
| [44] | Kareken et al | 2004 | PET | Healthy subjects | Piri (B), amyg (B), insula (B); sniffing lateral OFC (B) |
| [47] | Kjelvik et al | 2012 | FMRI | Healthy subjects | Piri (B), amyg (B), OFC (B) and thal (B) cing (RH), insula (LH) |
| [25] | Plailly et al | 2006 | PET | Psychosis patients | HC > SZ Piri (LH) and inf front (LH) |
| SZ > HC insula (RH) | |||||
| Good et al | Under review | FMRI | Psychosis patients | HC > SZ thal (RH), caud (B), ant cing, OFC (B only midly greater), mid temp gyrus (B) | |
| Hedonics (Valence) | |||||
| [55] | Zald et al | 1997 | PET | Healthy subjects | Aversive odorant- amyg and OFC; less aversive- only OFC (LH) |
| [56] | Fulbright et al | 1998 | FMRI | Healthy subjects | Pleasant- BA 46/9 (RH), 32 (LH), 8 (B) 6 (RH) and insula (B); Unpleasant- 46/9 (RH), 32 (LH), 6 (B), insula (RH) |
| [57] | Royet et al | 2000 | PET | Healthy subjects | Hedonic judgement- ant cing, OFC (LH), sup front (LH), midd front gyrus (LH), inf midd temporal (LH) |
| [49] | Gottfried et al | 2002 | FMRI | Healthy subjects | UP: insula (RH), lat hypothal (LH), amyg (RH), post OFC (L); P: piri (RH), insula (RH), post OFC (RH) |
| [45] | Royet et al | 2003 | FMRI | Healthy subjects | Hedonic valence -UP > P: piri (LH), amyg (LH); P > U: cing (LH) |
| [58] | Rolls et al | 2003 | FMRI | Healthy subjects | P: med OFC; ant cing |
| [54] | Anderson et al | 2003 | FMRI | Healthy subjects | |
| [72] | Crespo-Facorro et al | 2001 | PET | Psychosis patients | HC > SZ P vs UP: insula (LH), para hipp (LH), sup temp gyrus (LH), accumbens (RH), lingual gyrus (B), cb. vermis (LH) SZ > HC Med OFC (B), DLPFC (B), med front (LH), lat front (RH), lat front (LH), front operculum (LH), parahipp (RH), post cing (LH) |
| [25] | Plailly et al | 2006 | PET | Psychosis patients | HC > SZ Insula (LH) and Inf front (LH) |
| [27] | Schneider et al | 2007 | FMRI | Psychosis patients | HC > SZ UP: Midd temp gyrus (RH) Midd front gyrus (RH) Insula; P: thal (L) SZ > HC UP: Midd front (RH) ant cing (RH) |
| Intensity | |||||
| [54] | Anderson et al | 2003 | FMRI | Healthy subjects | Amyg (B) |
| [63] | Winsto et al | 2005 | FMRI | Healthy subjects | Amyg, (but only at the extremes of valence), OFC and piri |
| Familiarity | |||||
| [60] | Royet et al | 1999 | PET | Healthy subjects | Med frontal (RH), inf front (LH), sup front(LH) cingulate (B) |
| [57] | Royet et al | 2000 | PET | Healthy subjects | OFC (B), ant cing, sup front (LH) midd front (RH) |
| [25] | Plailly et al | 2006 | PET | Psychosis patients | HC > SZ piri (LH), sup temp gyrus(LH), gyrus rectus(RH), OFC (LH), inf OFC (LH) |
| Identification | |||||
| [47] | Kjelvik et al | 2012 | FMRI | Healthy subjects | Correctly identified entorhinal cortex (LH), Hippocam (RH), parahippocam gyrus (B), OFC and piriform cortices Incorrectly identified: OFC and piri cortices |
| [73] | Clark et al | 1991 | PET | Psychosis patients | HC > SZ front (B) parietal (B); Norm > Micro thal (RH) and basal ganglia |
| [33] | Malaspina et al | 1998 | SPECT | Psychosis patients | HC > SZ inf. Front (RH), sup temp (RH) supramarg/angular gyrus (RH) HC (but not SZ) hippocam(B), fusiform(RH) |
| Genetic Trait | |||||
| [27] | Schneider et al | 2007 | FMRI | Psychosis patients | HC > FDR UP: mid front gyrus(RH) FDR > HC P: ant cing(LH) |
Although many studies exist demonstrating olfactory deficits in patients with psychotic disorders, only few neuroimaging investigations have been published to date examining the relationship of olfactory deficits to neural abnormalities.
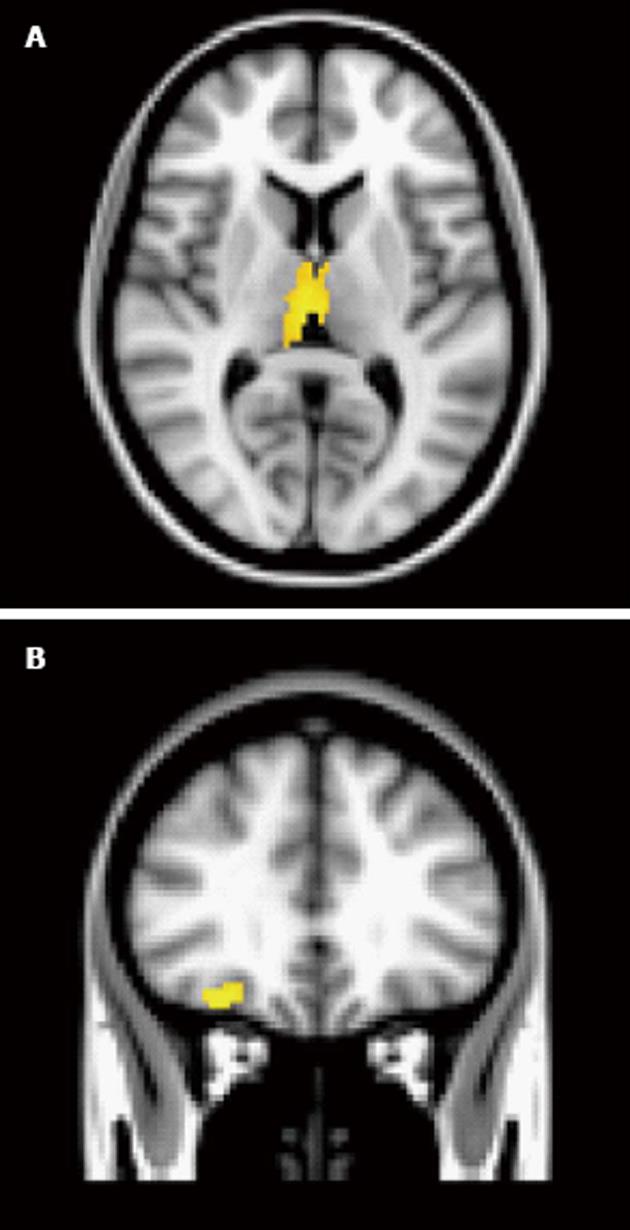
Odour activation patterns are similar between patients with psychotic disorders and control subjects during passive odour administration[25]. However, there are some differences regarding the intensity or cluster volume in these regions between patients and control subjects. Plailly et al[25] examined olfactory detection (passive odour administration), hedonics and familiarity using PET. A moderately sized sample of patients with schizophrenia and healthy controls were presented with odours during the scan and were instructed to press a button when they detected an odour (for the detection condition), if the odour was pleasant (for the hedonic condition) and if it was familiar (for the familiarity condition). For the detection condition, this group noted that the piriform cortex and the orbitofrontal cortex (inferior frontal) were activated in both groups. However, small clusters remained in these two regions when a subtraction analysis (HC-SZ) was performed. So, although both primary and secondary cortices were activated in both groups, patients with psychotic disorders appeared to show less robust activity in these two regions. Our group has recently examined passive odour administration in a group of patients with non-affective psychosis (Good et al, under review). We noted that patients and controls had similar activation patterns in amygdala, OFC and left thalamus, but the subtraction analysis (HC-SZ) showed slightly greater activation in OFC and left thalamus. In contrast, controls, but not patients, activated right thalamus, caudate (bilateral), cingulate, middle temporal gyrus and frontal pole (Figure 1).
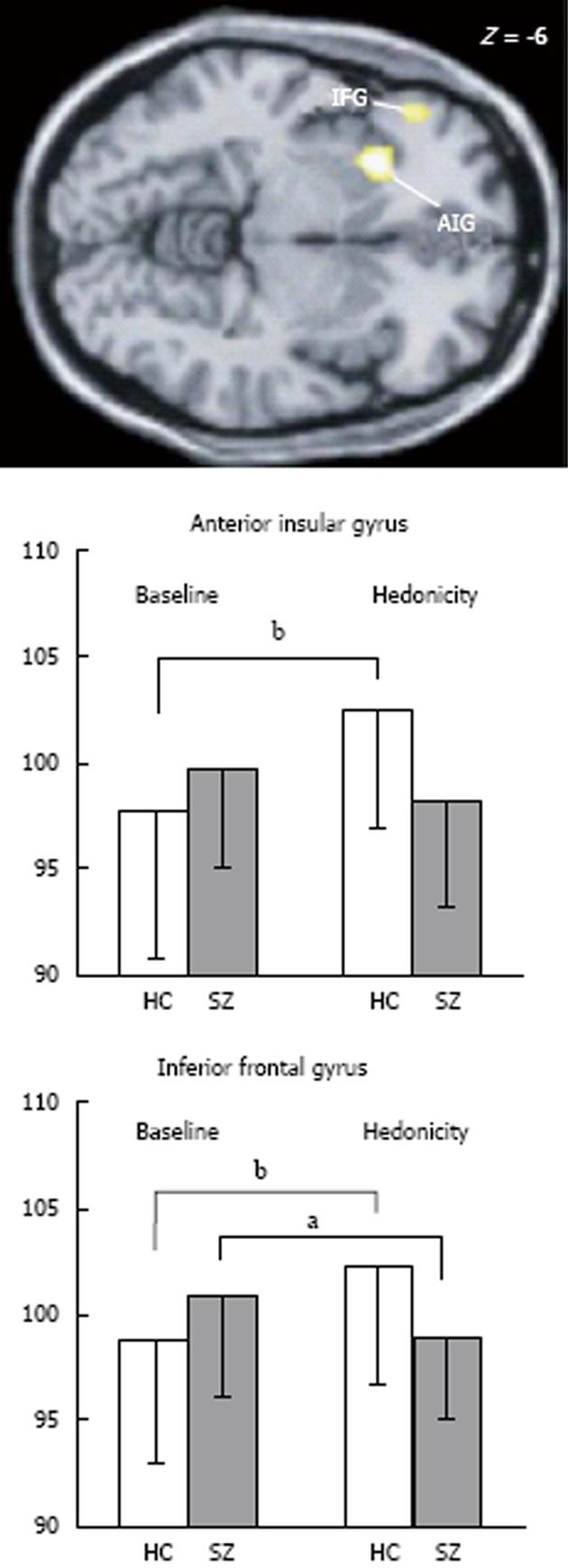
In contrast to passive odour administration, other aspects of olfactory processing appear to engage different brain regions between patients and control subjects. As previously mentioned, rating the pleasantness of odorants is an automatic process and may have survival significance, particularly if the odour is intense. As described above, in patients with psychotic disorders, their ability to rate odour pleasantness may be abnormal. In imaging studies, significant patient/control differences have emerged and show some contrasting findings. For instance, in the PET study published by Crespo-Facorro et al[72], patients were impaired on rating the pleasantness of pleasant odours, but no differences were noted between patients and control in neural processing of these same odours. In contrast, patients did not differ from control subjects on ratings of unpleasant odours; however, neural activation did differ. Control subjects engaged the left anterior insula, left parahippocampal gyrus, left superior temporal gyrus, lingual gyrus (bilateral) and left cerebellar vermis along with right accumbens when contrasted with unpleasant vs pleasant odorants, while patients did not. Interestingly, patients demonstrated higher metabolic rates in other brain regions relative to the controls, suggesting that patients invoked compensatory structures during processing. These regions included a number of right hemisphere regions (dorsolateral, lateral and parahippocampal gyrus) and left hemisphere activations (dorsolateral, medial and lateral frontal along with posterior cingulate). In a more recent PET study[25], where both positively and negatively valenced odorants were compared together, greater regional cerebral blood flow (rCBF) was noted in the left insula and left inferior orbitofrontal regions in control subjects relative to patients (Figure 2).
In an FMRI study, hypoactivity of right middle temporal and right middle frontal (BA 46) cortices in patients with psychotic disorders relative to controls during the presentation of a negatively valenced odorant; a relative hyperactivity was also seen in the middle frontal gyrus (in BA 9) and right anterior cingulate in patients. During presentation with a pleasant odorant, left thalamic hypoactivation was noted in patients relative to controls. In the presentation of both types of odorants, insular hypoactivation just missed statistical significance. Unfortunately, unpleasant and pleasant odours were never compared to each other in this study[27].
Taken together, despite different methodology and analytic techniques, the data presented suggest that dysfunction in secondary olfactory cortices and perhaps the insula may account for the olfactory hedonics dysfunction in patients with psychotic disorders. As no group noted differences in amygdala and piriform cortex activations suggest that primary olfactory cortices may be normally activated in these patients.
The neural underpinnings of processing odour intensity have not been contrasted between patients and control subjects. But as mentioned previously, no differences between these two groups have been noted on odour intensity ratings. Nevertheless, future research should examine this dimension of olfactory processing.
Determining whether an odorant has been experienced before appears to further engage frontal brain regions (see above). When patients with psychotic disorders were compared with control subjects on a familiarity task, hypoactivity of piriform cortex, orbitofrontal and superior temporal gyrus (all on the left) was observed[25]. This study also noted that patients rated odours as less familiar than did healthy controls. It was suggested that impaired familiarity ratings may be accounted for by temporolimbic/orbitofrontal dysfunction.
The neural underpinnings of olfactory identification have not been adequately examined in patients with psychotic disorders, despite the fact that olfactory identification deficits are the most robust finding in this patient group. Malaspina et al[33] were the first to examine olfactory identification using SPECT. In this study, a small sample of patients with psychotic disorders and controls were scanned while engaging in a forced-choice odour identification test and a comparator picture matching test. Differences in rCBF between the two tasks included hypometabolism in patients in inferior frontal, superior temporal and supramarginal/angular gyri (on the right). Controls, but not patients engaged right fusiform gyrus and bilateral hippocampi during the odour activation task relative to the control task. The right-sided deficits are consistent with lesion data showing more severe deficits in odour identification after rights than left sided lesions. However, the data is not entirely consistent with the majority of neuropathological studies of schizophrenia showing a predominance of left sided structural abnormalities. Moreover, there was no examination of the differences in odour activation according to the correctness of identification of the odorants.
As it appears that only a proportion of patients demonstrate abnormalities on olfactory identification testing, dividing the groups of patients into those who are impaired vs those who have normal olfactory function, may have merit. Clark et al[73] examined glucose metabolism in three groups of subjects: a group of patients with olfactory agnosia (impaired olfactory identification ability and normal olfactory detection), a group of patients with normal sense of smell (normosmic) and healthy controls (age matched). PET scanning occurred in the absence of any olfactory stimulation (resting state). In all brain regions, the agnosia patient group had the lowest metabolic rates compared to the other two groups, followed by the normosmic patients and then controls. Controls had higher rCBF than both patient groups in bilateral frontal and parietal regions. The two patient groups differed from each other on right thalamus and basal ganglia metabolism (olfactory agnosia group lower than normal olfactory group). The results of this study are consistent with earlier reports of structural and functional abnormalities in thalamic and basal ganglia. As no olfactory task was presented, these data suggest an underlying hypofunction in regions of the brain that subserve olfactory processing, rather than a processing abnormality. Nevertheless, the heterogeneity of schizophrenia should be taken into account, or at least acknowledged, when examining the neural underpinnings of olfactory function in patients with psychotic disorders. Further investigation is required.
Not only do patients with psychotic disorders exhibit abnormalities in olfactory processing, but their unaffected first-degree relatives also demonstrate mildly abnormal brain activation patterns when contrasted to non-related controls[27]. When presented with an unpleasant odour, patients’ unaffected brothers demonstrated reduced frontal activation (relative to the healthy control group) and increased activation in anterior cingulate, but during the presentation of a pleasant odorant, no differences were noted. This group suggested that hypofrontality may be a genetic trait that is expressed to a lesser degree in the non-affected brothers.
Despite age being considered an important factor to consider in olfactory neuroimaging research[71], to date, no studies have examined the difference in olfactory neural processing between young and older patients with psychotic disorders. In all studies presented in this literature review, the age of the patients and that of control subject have been well matched and also relatively young (Mean ages < 35 years). Further research may be informative in this regard.
While many olfactory neuroimaging studies of healthy subjects have recruited and assessed only female subjects, the data on patients with psychotic disorders has been strongly biased towards male patient data. Therefore, what is thought of as “normal” may be only normal for women and the comparison with male patients with psychotic disorders may not be appropriate. For example, only male patients were examined in the studies published by Schneider et al[27], Clark et al[73], and Plailly et al[25] and investigations by Crespo-Facorro et al[72] and Malaspina et al[33] contained samples that were predominantly male. Only Turetsky et al[20] (not described) and Good et al (under review) examined almost equal numbers of males and female subjects. The data on sex differences in olfactory processing is not fully developed; however, in patients with psychotic disorders, the overwhelming number of sex differences is a very important aspect of the disorder and is likely overlooked in neuroimaging studies of this population.
Not all patients with psychotic disorder are impaired on olfactory testing. Some of the data provided herein suggest that may be subgroups within the heterogenous diagnostic category “schizophrenia”. Not only are there potential differences in brain activation, but there are within diagnostic group differences in symptomatology and outcome[8,9]. Further investigations are needed to tease out the differences within schizophrenia.
A further limitation on the data that were presented is that patients and controls differ on many variables that are related to brain activation patterns. Most patients are medicated with antipsychotic medications. Very few studies were upfront about how much this variable may have affected their results. Clark et al[73] showed hypoactivation in almost all brain regions across the three groups examined (olfactory agnosia-patient, normal sense of smell-patient, healthy controls), but pointed out that cerebellar metabolism did not differ among the groups. They argued that this evidence suggested that state variables may not be responsible. Moreover, since the two patient groups differed in metabolism in specific olfactory brain regions also argues against antipsychotic medication being a causative factor. No imaging studies have examined antipsychotic naïve or withdrawn patients. The effects of antipsychotic medications on neuroimaging outcomes continue to be unknown. Moreover, patients with psychotic disorders are also more likely to be heavy consumers of tobacco and caffeine. Both of these substances are found to impact on activation patterns. Very few studies have controlled for smoking status (and pack-years) and none have examined caffeine use (particularly prior to scanning). These two variables should be examined more closely to uncover any effects on resultant activation maps between patients and controls. Finally, examining the effects of age in this population may be worthwhile and may provide further insights into changes in brain activity in olfactory regions with aging.
Finally the unique and varied protocols used make for a more challenging task to understand the contribution to olfactory dysfunction in patients with psychotic disorders. Whether resting state activation abnormalities are similar to that noted when patients are engaged in different olfactory tasks (e.g., hedonic ratings vs passive presentation of pleasant and unpleasant odorants; PET vs FMRI vs SPECT) is unknown. Further, more extensive research is needed to parse out the effects of each type of odour presentation/protocol.
Psychosis is linked to observable olfactory deficits in many different olfactory domains. Olfactory identification deficits tends to be most commonly reported, likely because of the availability of standardized tests. Olfactory familiarity and memory are also robustly affected in this disorder. Olfactory intensity ratings are not noticeably impaired in these patients. Olfactory hedonics and detection threshold, however, are more troublesome as the methods by which researchers have examined these domains differ markedly. It is not surprising, therefore, that conflicting results have been published. Nevertheless, the noted deficits do not appear to be state-related and may be predictive of poorer outcome.
Olfactory pathways are overlapping with areas of the brain that are found to be abnormal in schizophrenia/psychosis an assessing this sense may provide a good indicator of the integrity of different brain regions. Adding in neuroimaging techniques, we may be better able to disentangle the varied neurobiological substrates of olfactory deficits. Moreover, olfactory assessment, along with neuroimaging methods may provide a way to further our understanding of the heterogeneity of psychotic disorders
The differing methodologies employed by neuroimaging studies hampers the ability to make firm conclusions about which brain regions are impaired in patients with psychotic disorders, particularly when presented with olfactory processing demands. Hypoactivation of various brain regions is a robust finding when patients with psychotic disorders are compared with healthy, age-matched controls. What regions are hyper/hypofunctional when faced with olfactory stimuli and cognitive assessment of these inputs is still under examination. One thing is clear: patients activate the same regions as controls when faced with olfactory processing demands. However, the degree of activation may be reduced in patients with psychotic disorders. Furthermore, if compensatory regions are invoked in patients above and beyond control subject activation, this finding requires replication as it has only been examined in one study[72]. One interesting finding emerged, however. In olfactory imaging studies of healthy controls, particularly when subjects are presented with unpleasant odorants, the amygdala is typically engaged. In neuroimaging studies examining odorant processing in patients with psychotic disorders, the amygdala was not once identified as being abnormal. Piriform cortex, as well, was not noted to be abnormally activated in patients during hedonic processing. Secondary olfactory cortex (orbitofrontal cortex, thalamus and insula), on the other hand, was noted to be abnormally activated in the neuroimaging studies examined. The lack of amygdala abnormalities may have been as a result of an inability to discern the amygdala from piriform cortex. However, masking templates for use in neuroimaging research have reliably delineated this region. Moreover, given that piriform cortices have been noted to habituate more quickly than other brain regions, this fact may be responsible for the lack of abnormalities in piriform/amygdala activation (i.e., no activity may be as a result of neurobiological constraints rather than a lack of difference between groups). However, given that this review encompassed data collection using many different imaging modalities and unique protocols (some with odor presentations in the range of 2 s), this explanation is unlikely. This interesting avenue of research will undoubtedly grow and provide more insights as imaging techniques continue to improve.
P- Reviewer: Assadi M S- Editor: Ji FF L- Editor: A E- Editor: Yan JL
| 1. | Deems DA, Doty RL, Settle RG, Moore-Gillon V, Shaman P, Mester AF, Kimmelman CP, Brightman VJ, Snow JB. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519-528. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 670] [Cited by in F6Publishing: 612] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 2. | Varney NR. Prognostic significance of anosmia in patients with closed-head trauma. J Clin Exp Neuropsychol. 1988;10:250-254. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 58] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 3. | Drummond M, Douglas J, Olver J. ‘If I haven’t got any smell … I’m out of work’: consequences of olfactory impairment following traumatic brain injury. Brain Inj. 2013;27:332-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Doty RL, Stern MB, Pfeiffer C, Gollomp SM, Hurtig HI. Bilateral olfactory dysfunction in early stage treated and untreated idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1992;55:138-142. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 228] [Cited by in F6Publishing: 235] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Devanand DP, Tabert MH, Cuasay K, Manly JJ, Schupf N, Brickman AM, Andrews H, Brown TR, DeCarli C, Mayeux R. Olfactory identification deficits and MCI in a multi-ethnic elderly community sample. Neurobiol Aging. 2010;31:1593-1600. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Brewer WJ, Wood SJ, McGorry PD, Francey SM, Phillips LJ, Yung AR, Anderson V, Copolov DL, Singh B, Velakoulis D. Impairment of olfactory identification ability in individuals at ultra-high risk for psychosis who later develop schizophrenia. Am J Psychiatry. 2003;160:1790-1794. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 146] [Cited by in F6Publishing: 154] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 7. | Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 109] [Cited by in F6Publishing: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 8. | Good KP, Whitehorn D, Rui Q, Milliken H, Kopala LC. Olfactory identification deficits in first-episode psychosis may predict patients at risk for persistent negative and disorganized or cognitive symptoms. Am J Psychiatry. 2006;163:932-933. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 12] [Cited by in F6Publishing: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Good KP, Tibbo P, Milliken H, Whitehorn D, Alexiadis M, Robertson N, Kopala LC. An investigation of a possible relationship between olfactory identification deficits at first episode and four-year outcomes in patients with psychosis. Schizophr Res. 2010;124:60-65. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Gottfried JA. Smell: central nervous processing. Adv Otorhinolaryngol. 2006;63:44-69. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Patel RM, Pinto JM. Olfaction: anatomy, physiology, and disease. Clin Anat. 2014;27:54-60. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 12. | Good KP, Martzke JS, Milliken HI, Honer WG, Kopala LC. Unirhinal olfactory identification deficits in young male patients with schizophrenia and related disorders: association with impaired memory function. Schizophr Res. 2002;56:211-223. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Szeszko PR, Bates J, Robinson D, Kane J, Bilder RM. Investigation of unirhinal olfactory identification in antipsychotic-free patients experiencing a first-episode schizophrenia. Schizophr Res. 2004;67:219-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Yousem DM, Geckle RJ, Bilker WB, McKeown DA, Doty RL. Posttraumatic olfactory dysfunction: MR and clinical evaluation. AJNR Am J Neuroradiol. 1996;17:1171-1179. [PubMed] [Cited in This Article: ] |
| 15. | Doty RL, Yousem DM, Pham LT, Kreshak AA, Geckle R, Lee WW. Olfactory dysfunction in patients with head trauma. Arch Neurol. 1997;54:1131-1140. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 174] [Cited by in F6Publishing: 158] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Perälä J, Suvisaari J, Saarni SI, Kuoppasalmi K, Isometsä E, Pirkola S, Partonen T, Tuulio-Henriksson A, Hintikka J, Kieseppä T. Lifetime prevalence of psychotic and bipolar I disorders in a general population. Arch Gen Psychiatry. 2007;64:19-28. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 868] [Cited by in F6Publishing: 808] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 17. | Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1993;8:245-250. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 91] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Moberg PJ, Arnold SE, Doty RL, Gur RE, Balderston CC, Roalf DR, Gur RC, Kohler CG, Kanes SJ, Siegel SJ. Olfactory functioning in schizophrenia: relationship to clinical, neuropsychological, and volumetric MRI measures. J Clin Exp Neuropsychol. 2006;28:1444-1461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Rupp CI, Fleischhacker WW, Kemmler G, Kremser C, Bilder RM, Mechtcheriakov S, Szeszko PR, Walch T, Scholtz AW, Klimbacher M. Olfactory functions and volumetric measures of orbitofrontal and limbic regions in schizophrenia. Schizophr Res. 2005;74:149-161. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 57] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Turetsky BI, Moberg PJ, Owzar K, Johnson SC, Doty RL, Gur RE. Physiologic impairment of olfactory stimulus processing in schizophrenia. Biol Psychiatry. 2003;53:403-411. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 21. | Bradley EA. Olfactory acuity to a pheromonal substance and psychotic illness. Biol Psychiatry. 1984;19:899-905. [PubMed] [Cited in This Article: ] |
| 22. | Sirota P, Davidson B, Mosheva T, Benhatov R, Zohar J, Gross-Isseroff R. Increased olfactory sensitivity in first episode psychosis and the effect of neuroleptic treatment on olfactory sensitivity in schizophrenia. Psychiatry Res. 1999;86:143-153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Doop ML, Park S. On knowing and judging smells: identification and hedonic judgment of odors in schizophrenia. Schizophr Res. 2006;81:317-319. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 30] [Cited by in F6Publishing: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Kamath V, Moberg PJ, Kohler CG, Gur RE, Turetsky BI. Odor hedonic capacity and anhedonia in schizophrenia and unaffected first-degree relatives of schizophrenia patients. Schizophr Bull. 2013;39:59-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Plailly J, d’Amato T, Saoud M, Royet JP. Left temporo-limbic and orbital dysfunction in schizophrenia during odor familiarity and hedonicity judgments. Neuroimage. 2006;29:302-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Hudry J, Saoud M, D’Amato T, Daléry J, Royet JP. Ratings of different olfactory judgements in schizophrenia. Chem Senses. 2002;27:407-416. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 27. | Schneider F, Habel U, Reske M, Toni I, Falkai P, Shah NJ. Neural substrates of olfactory processing in schizophrenia patients and their healthy relatives. Psychiatry Res. 2007;155:103-112. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Moberg PJ, Arnold SE, Doty RL, Kohler C, Kanes S, Seigel S, Gur RE, Turetsky BI. Impairment of odor hedonics in men with schizophrenia. Am J Psychiatry. 2003;160:1784-1789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 63] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 29. | Kamath V, Turetsky BI, Moberg PJ. Identification of pleasant, neutral, and unpleasant odors in schizophrenia. Psychiatry Res. 2011;187:30-35. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 30. | Strauss GP, Allen DN, Ross SA, Duke LA, Schwartz J. Olfactory hedonic judgment in patients with deficit syndrome schizophrenia. Schizophr Bull. 2010;36:860-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 53] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Clepce M, Reich K, Gossler A, Kornhuber J, Thuerauf N. Olfactory perception in schizophrenia: the rating range for hedonic judgements is increased during acute episodes. Psychiatry Res. 2013;208:81-83. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Wu J, Buchsbaum MS, Moy K, Denlea N, Kesslak P, Tseng H, Plosnaj D, Hetu M, Potkin S, Bracha S. Olfactory memory in unmedicated schizophrenics. Schizophr Res. 1993;9:41-47. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 62] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Malaspina D, Perera GM, Lignelli A, Marshall RS, Esser PD, Storer S, Furman V, Wray AD, Coleman E, Gorman JM. SPECT imaging of odor identification in schizophrenia. Psychiatry Res. 1998;82:53-61. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 49] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 34. | Corcoran C, Whitaker A, Coleman E, Fried J, Feldman J, Goudsmit N, Malaspina D. Olfactory deficits, cognition and negative symptoms in early onset psychosis. Schizophr Res. 2005;80:283-293. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 69] [Cited by in F6Publishing: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Brewer WJ, Edwards J, Anderson V, Robinson T, Pantelis C. Neuropsychological, olfactory, and hygiene deficits in men with negative symptom schizophrenia. Biol Psychiatry. 1996;40:1021-1031. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 100] [Cited by in F6Publishing: 98] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Good KP, Martzke JS, Honer WG, Kopala LC. Left nostril olfactory identification impairment in a subgroup of male patients with schizophrenia. Schizophr Res. 1998;33:35-43. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 23] [Cited by in F6Publishing: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Compton MT, McKenzie Mack L, Esterberg ML, Bercu Z, Kryda AD, Quintero L, Weiss PS, Walker EF. Associations between olfactory identification and verbal memory in patients with schizophrenia, first-degree relatives, and non-psychiatric controls. Schizophr Res. 2006;86:154-166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Shenton ME, Frumin M, McCarley RW, Maier SE, Westin C, Fischer IA, Dickey C, Kikinis R. Morphometric magnetic resonance imaging studies: Findings in schizophrenia. ). Psychiatric neuroimaging research: contemporary strategies. Arlington: American Psychiatric Press Inc 2001; 1-60. [Cited in This Article: ] |
| 39. | Weinberger DR, Wagner RL, Wyatt RJ. Neuropathological studies of schizophrenia: a selective review. Schizophr Bull. 1983;9:193-212. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 152] [Cited by in F6Publishing: 152] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 40. | Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281-288. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 956] [Cited by in F6Publishing: 878] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 41. | Pakkenberg B. Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry. 1990;47:1023-1028. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 387] [Cited by in F6Publishing: 397] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 42. | Shenton ME, Kikinis R, Jolesz FA, Pollak SD, LeMay M, Wible CG, Hokama H, Martin J, Metcalf D, Coleman M. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604-612. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 770] [Cited by in F6Publishing: 688] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 43. | Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature. 1998;392:282-286. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 337] [Cited by in F6Publishing: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 44. | Kareken DA, Sabri M, Radnovich AJ, Claus E, Foresman B, Hector D, Hutchins GD. Olfactory system activation from sniffing: effects in piriform and orbitofrontal cortex. Neuroimage. 2004;22:456-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 45. | Royet JP, Plailly J, Delon-Martin C, Kareken DA, Segebarth C. fMRI of emotional responses to odors: influence of hedonic valence and judgment, handedness, and gender. Neuroimage. 2003;20:713-728. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 247] [Cited by in F6Publishing: 240] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 46. | Zatorre RJ, Jones-Gotman M, Evans AC, Meyer E. Functional localization and lateralization of human olfactory cortex. Nature. 1992;360:339-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 476] [Cited by in F6Publishing: 486] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 47. | Kjelvik G, Evensmoen HR, Brezova V, Håberg AK. The human brain representation of odor identification. J Neurophysiol. 2012;108:645-657. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 48. | Savic I, Gulyas B, Larsson M, Roland P. Olfactory functions are mediated by parallel and hierarchical processing. Neuron. 2000;26:735-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 268] [Cited by in F6Publishing: 262] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 49. | Gottfried JA, Deichmann R, Winston JS, Dolan RJ. Functional heterogeneity in human olfactory cortex: an event-related functional magnetic resonance imaging study. J Neurosci. 2002;22:10819-10828. [PubMed] [Cited in This Article: ] |
| 50. | Sobel N, Prabhakaran V, Zhao Z, Desmond JE, Glover GH, Sullivan EV, Gabrieli JD. Time course of odorant-induced activation in the human primary olfactory cortex. J Neurophysiol. 2000;83:537-551. [PubMed] [Cited in This Article: ] |
| 51. | Schiffman SS. Physicochemical correlates of olfactory quality. Science. 1974;185:112-117. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 168] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 52. | Savic I. Imaging of brain activation by odorants in humans. Curr Opin Neurobiol. 2002;12:455-461. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 96] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 53. | Levy LM, Henkin RI, Hutter A, Lin CS, Martins D, Schellinger D. Functional MRI of human olfaction. J Comput Assist Tomogr. 1997;21:849-856. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 98] [Cited by in F6Publishing: 97] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 54. | Anderson AK, Christoff K, Stappen I, Panitz D, Ghahremani DG, Glover G, Gabrieli JD, Sobel N. Dissociated neural representations of intensity and valence in human olfaction. Nat Neurosci. 2003;6:196-202. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 755] [Cited by in F6Publishing: 709] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 55. | Zald DH, Pardo JV. Emotion, olfaction, and the human amygdala: amygdala activation during aversive olfactory stimulation. Proc Natl Acad Sci USA. 1997;94:4119-4124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 508] [Cited by in F6Publishing: 535] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 56. | Fulbright RK, Skudlarski P, Lacadie CM, Warrenburg S, Bowers AA, Gore JC, Wexler BE. Functional MR imaging of regional brain responses to pleasant and unpleasant odors. AJNR Am J Neuroradiol. 1998;19:1721-1726. [PubMed] [Cited in This Article: ] |
| 57. | Royet JP, Hudry J, Zald DH, Godinot D, Grégoire MC, Lavenne F, Costes N, Holley A. Functional neuroanatomy of different olfactory judgments. Neuroimage. 2001;13:506-519. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 150] [Cited by in F6Publishing: 135] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 58. | Rolls ET, Kringelbach ML, de Araujo IE. Different representations of pleasant and unpleasant odours in the human brain. Eur J Neurosci. 2003;18:695-703. [PubMed] [DOI] [Cited in This Article: ] [Cited by in F6Publishing: 1] [Reference Citation Analysis (0)] |
| 59. | Royet JP, Plailly J. Lateralization of olfactory processes. Chem Senses. 2004;29:731-745. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 129] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 60. | Royet JP, Koenig O, Gregoire MC, Cinotti L, Lavenne F, Le Bars D, Costes N, Vigouroux M, Farget V, Sicard G. Functional anatomy of perceptual and semantic processing for odors. J Cogn Neurosci. 1999;11:94-109. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 180] [Cited by in F6Publishing: 190] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 61. | Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32:489-502. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1206] [Cited by in F6Publishing: 1159] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 62. | Hayes DJ, Duncan NW, Xu J, Northoff G. A comparison of neural responses to appetitive and aversive stimuli in humans and other mammals. Neurosci Biobehav Rev. 2014;45:350-368. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 71] [Cited by in F6Publishing: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 63. | Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. J Neurosci. 2005;25:8903-8907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 210] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 64. | Olofsson JK, Rogalski E, Harrison T, Mesulam MM, Gottfried JA. A cortical pathway to olfactory naming: evidence from primary progressive aphasia. Brain. 2013;136:1245-1259. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 65. | Cain WS. To know with the nose: keys to odor identification. Science. 1979;203:467-470. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 283] [Cited by in F6Publishing: 291] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 66. | Doty RL, Applebaum S, Zusho H, Settle RG. Sex differences in odor identification ability: a cross-cultural analysis. Neuropsychologia. 1985;23:667-672. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 220] [Cited by in F6Publishing: 181] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 67. | Choudhury ES, Moberg P, Doty RL. Influences of age and sex on a microencapsulated odor memory test. Chem Senses. 2003;28:799-805. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Good KP, Leslie RA, McGlone J, Milliken HI, Kopala LC. Sex differences in olfactory function in young patients with psychotic disorders. Schizophr Res. 2007;97:97-102. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 69. | Yousem DM, Maldjian JA, Siddiqi F, Hummel T, Alsop DC, Geckle RJ, Bilker WB, Doty RL. Gender effects on odor-stimulated functional magnetic resonance imaging. Brain Res. 1999;818:480-487. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 70. | Bengtsson S, Berglund H, Gulyas B, Cohen E, Savic I. Brain activation during odor perception in males and females. Neuroreport. 2001;12:2027-2033. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Wang J, Eslinger PJ, Smith MB, Yang QX. Functional magnetic resonance imaging study of human olfaction and normal aging. J Gerontol A Biol Sci Med Sci. 2005;60:510-514. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 104] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 72. | Crespo-Facorro B, Paradiso S, Andreasen NC, O’Leary DS, Watkins GL, Ponto LL, Hichwa RD. Neural mechanisms of anhedonia in schizophrenia: a PET study of response to unpleasant and pleasant odors. JAMA. 2001;286:427-435. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 188] [Cited by in F6Publishing: 204] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 73. | Clark C, Kopala L, Hurwitz T, Li D. Regional metabolism in microsmic patients with schizophrenia. Can J Psychiatry. 1991;36:645-650. [PubMed] [Cited in This Article: ] |