Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.174
Peer-review started: June 23, 2015
First decision: August 14, 2015
Revised: November 13, 2015
Accepted: December 1, 2015
Article in press: December 2, 2015
Published online: March 24, 2016
Kidney transplantation is the best available treatment for patients with end stage renal disease. Despite the introduction of effective immunosuppressant drugs, episodes of acute allograft rejection still endanger graft survival. Since efficient treatment of acute rejection is available, rapid diagnosis of this reversible graft injury is essential. For diagnosis of rejection, invasive core needle biopsy of the graft is the “gold-standard”. However, biopsy carries the risk of significant graft injury and is not immediately feasible in patients taking anticoagulants. Therefore, a non-invasive tool assessing the whole organ for specific and fast detection of acute allograft rejection is desirable. We herein review current imaging-based state of the art approaches for non-invasive diagnostics of acute renal transplant rejection. We especially focus on new positron emission tomography-based as well as targeted ultrasound-based methods.
Core tip: Kidney transplantation is the best available treatment for patients with end stage renal disease. For diagnosis of rejection, invasive core needle biopsy of the graft is currently considered as the “gold-standard”. As biopsies carry the risk of significant graft injury, a non-invasive, specific and fast tool screening the whole graft for acute rejection is desirable. We herein review current imaging-based state of the art approaches for non-invasive diagnosis of acute kidney allograft rejection, focussing particularly on new positron emission tomography-based as well as targeted ultrasound-based methods.
- Citation: Thölking G, Schuette-Nuetgen K, Kentrup D, Pawelski H, Reuter S. Imaging-based diagnosis of acute renal allograft rejection. World J Transplant 2016; 6(1): 174-182
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/174.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.174
Kidney transplantation (KTx) is the favorable treatment for patients suffering from end stage renal disease (ESRD)[1]. Although modern immunosuppressive regimens offer good patient and graft survival rates, acute rejection (AR) after KTx remains a serious problem significantly limiting both graft and patient survival[2,3].
Therefore, early detection and treatment of AR is necessary. To date, renal biopsy is the “gold-standard” to diagnose AR, but might jeopardize allograft recipients due to its invasive character.
Thus, non-invasive techniques for detection of AR are desired. During the last decades, medical imaging techniques have improved tremendously. Novel methods do not only focus on structural details, but also visualize functional processes.
This review focuses on the current non-invasive imaging techniques to detect AR which might replace renal biopsies in the future.
Sonographic allograft examination is part of the standard care of transplanted patients. This procedure detects allograft swelling, morphological changes, abatement of corticomedullary differentiation, alterations of echogenicity and distinctive structures such as medullary pyramids; renal blood circulation can be analyzed by means of Doppler ultrasound and contrast-enhanced ultrasound examination. While the method is cost-effective and widely available, it still has considerable limitations in sensitivity and specificity for the diagnosis of AR.
New approaches might overcome these caveats. The resistive index (RI) is a noninvasive method using the vascular resistance and elastic compliance to evaluate the function of the allograft. Unfortunately, the RI measured in the allograft is influenced by systemic parameters like the vascular compliance, pulse pressure, heart rate and rhythm. Due to progressing arteriosclerotic processes of the vascular system, older recipient age is the strongest determinant for a higher RI[4]. Higher RIs are also associated with antibody-mediated rejection and acute tubular necrosis in index biopsies[4], and RIs of 0.8 or higher are associated with decreased patient survival[4,5]. However, data on the correlation between RI and allograft outcome are unequivocal[4-6].
Recently, another non-invasive index for the prediction of AR has been developed on the base of contrast-enhanced ultrasonography (CEUS). It includes CEUS factors such as rising time, time to peak and delta-time among regions of interest[7].
Acoustic radiation force impulse imaging (ARFI) assesses tissue elasticity and was utilized to identify AR in a small series of 8 patients. ARFI-values were elevated by more than 15% in patients undergoing AR, when compared to other causes of allograft damage[8]. However, the method has not been evaluated by others and is not used in clinical routine yet.
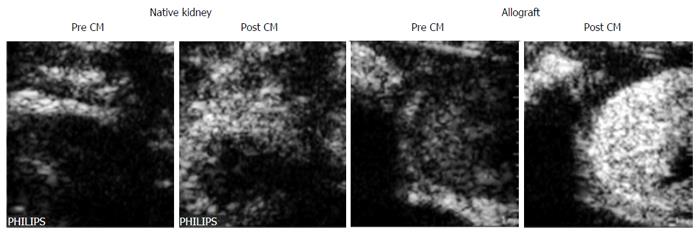
An experimental but promising procedure is the use of microbubbles targeting T-lymphocytes. The accumulation of T cells during AR can be visualized via microbubbles coupled to anti-CD3 antibodies (Figure 1)[9]. The method allows differential diagnosis of AR with high specificity.
Magnetic resonance imaging (MRI) is another non-invasive method to evaluate kidney allograft function. MRI is based on the detection of signals from hydrogen nuclei or protons changing their magnetic behaviour in response to altered magnetic fields in the MRI system, and can reveal various tissue characteristics, including intrinsic MR properties like the relaxation times T1 and T2[10]. An important advantage of MRI is the high spatiotemporal resolution, which allows the precise visualization of anatomical structures as well as functional assessment of the graft. MRI allows the detection of distinctive features of vascular and interstitial structures, there by discriminating between different mechanisms of renal allograft injury such as AR or acute tubular necrosis (ATN)[11]. In the field of nephrology, various MRI techniques can be used to visualize different pathophysiological processes[10].
Dynamic contrast enhanced MRI (DCE MRI) is a common MRI method involving the use of a contrast agent. DCE MRI using gadolinium-based contrast agents is also termed MR renography (MRR). The contrast agents are freely filtered at the glomeruli but are not secreted or reabsorbed in the tubules. Therefore they can optimally be used to quantify renal perfusion, glomerular filtration rate (GFR) and tubular function, which helps to distinguish between AR and ATN[11]. The assessment involves the measurement of cortical and medullary blood flow within the graft after administration of contrast agent. In contrast to normal grafts, the cortical and medullary blood flow is significantly reduced in grafts experiencing AR. The predominantly reduced medullary blood flow seems to be characteristic for AR and helps to differentiate between AR and ATN[12].
Identification of and discrimination between various mechanisms of allograft damage is also possible by using a tracer kinetic renal model which determines the mean transit time (MTT) of a tracer through the different compartments of the kidney[13]. However, although differences in the fractional MTT values between normal grafts or grafts undergoing AR or ATN have been observed, substantial overlaps among these groups and with healthy control kidneys exist. Moreover, the rare but characteristic risk of gadolinium-induced nephrogenic systemic fibrosis needs to be considered[14].
Another MRI technique which is independent from contrast agent usage is diffusion-weighted MRI (DWI MRI). DWI MRI depends on the signal decay that is induced by the relative diffusion-based displacement of water molecules, which can be quantified by calculating the so called apparent diffusion coefficient (ADC). The ADC is influenced by the tissue microstructure and does not account for directionality of molecular motion. To address this issue of anisotropic diffusion properties due to the radial orientation of main anatomic structures like vessels and tubules, the more sensitive diffusion tensor imaging (DTI) has been applied[15]. DTI allows the assessment of the fractional anisotropy (FA) of tissues, thereby considering the directionality of diffusion. Recently, the role of diffusion-weighted MRI for differentiation between AR and ATN was discussed, and new automated segmentation protocols might be helpful[16].
The differentiation between AR and ATN might also be possible by applying blood-oxygen level-dependent (BOLD) MR[17-19]. This method utilizes the paramagnetic effects of deoxyhemoglobin. Deoxyhemoglobin is increased in tissues with lower oxygen concentration and shortens the transverse relaxation time constant T2*. Inversely, the apparent relaxation rate, R2* (= 1/T2*), is elevated. Therefore, BOLD MR can serve as a non-invasive technique to evaluate the renal parenchymal oxygenation concentration. In kidneys displaying AR, a significantly lower medullary R2*, corresponding to a higher oxygenation, was observed compared to ATN[18,20].
Arterial spin labeling (ASL) MRI is another approach to assess allograft function especially for longitudinal perfusion evaluation. ASL MR utilizes arterial blood as an endogenous contrast agent. Inflowing blood is selectively labeled by altering its longitudinal magnetization to have an opposite magnetization compared to the destination tissue. The difference between a labeled image (tag) and a non-labeled image (control) can be used to determine tissue perfusion. ASL MR has successfully been applied to examine native and transplant kidneys. ASL studies using a flow sensitive alternating inversion recovery (FAIR-ASL) scheme (for details see[21]) revealed a significant lower overall or medullary perfusion in allografts when compared to healthy kidneys for subjects with eGFR > 60 mL/min per 1.73 m2 or with eGFR < 60 mL/min per 1.73 m2 respectively[22]. Also, a significant lower cortical perfusion in renal grafts with acute decrease in renal function was observed when compared to allografts with good postoperative and long-term function[23].
Given the need for non-invasive diagnosis of renal inflammation, several studies used nanoparticles to detect specific immune cells or immune proteins in the kidney (for review see[24]). In the context of renal transplantation, Hauger et al[25] and Chae et al[26] reported successful usage of super magnetic iron oxide (SPIO) particle-loaded macrophages to differentiate between various causes of graft failure. Accumulation of iron particles in the kidney during AR was shown 3 and 5 d after application, respectively. Unfortunately, non-phagocytic cells such as T-cells generally have a low labeling efficiency and poor contrast agent incorporation, which limits cellular MR imaging in vivo. Recently, Liu et al[27] reported a new synthesized class of MRI contrast agent, IOPC-NH2 particles, for labeling of T-cells in allograft rejection in a rat model of heart-lung transplantation. This technique might represent an approach for potential clinical translation of MRI-based tracking of non-phagocytic cells, such as T- and B-lymphocytes.
Various MRI techniques including BOLD, DWI and ASL have been combined in several longitudinal studies, but case numbers were low and results were contradictory[28,29]. Further longitudinal studies with larger sample sizes are needed to determine the value of the different MR techniques for the evaluation of long-term allograft function.
Positron emission tomography (PET) is an imaging procedure based on the detection of internal radiation. After administration of an intravenous radioactive tracer, gamma rays emitted by the tracer are recorded by an external detector system called gamma camera. PET enables whole body visualization with high intrinsic sensitivity and provides high specificity although only very low concentrations of the tracer are needed[30,31]. The method offers a spatial resolution of 3-5 mm and generates 3D images[32]. Metabolic and cellular processes like pH-changes, apoptosis, inflammation and infection can be visualized[33].
The use of 18F-fluordeoxyglucose (FDG) for scintigraphic detection of glucose metabolism was published in 1978[34] and became the mainly used radionuclide in PET. After injection of the tracer, 18F-FDG enters the cell using glucose transporters like GLUT1. 18F-FDG acts like a glucose analogue and correlates with the metabolic activity of the cell. After phosphorylation of 18F-FDG, it cannot be further metabolized and is entrapped in cells with a high metabolism. The biodistribution of 18F-FDG can be assessed by PET[35]. 18F-FDG-PET is a well-established method used in clinical diagnostic. However, PET with glucose-based radionuclides is not specific for a particular disease and needs to be evaluated in the clinical context. For example, the uptake of 18F-FDG depends on the presence of glucose transporters which are upregulated under several conditions, like inflammation and tumor genesis. The application field of PET has extended over the last years, and 18F-FDG-PET has successfully been used in many pathological processes like cancer[36-38], vasculitis[39], fever of unknown origin[40], asthma[41], cystic fibrosis[42], and organ transplantation[43-46].
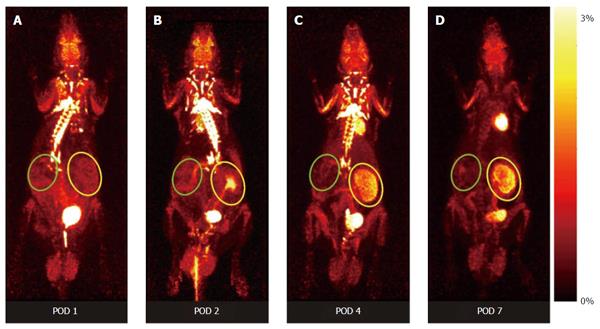
Recently, our group was able to non-invasively assess renal function by 18F-fluoride clearance and to monitor graft inflammation by 18F-FDG[43,47]. This PET method allows the visualization of molecular and cellular processes characteristic for AR, e.g., the assessment of metabolic activity of recruited leucocytes, hypoxia cell death, as well as allograft function. The pattern of the 18F-FDG-uptake during AR indicates a state of increased metabolism, driven by inflammatory cells (Figure 2). The specific distribution pattern of cell activity allows the discrimination of AR from other pathological conditions in both a rat renal transplantation model and in transplanted patients[44,48]. Despite specific signals in kidney allografts undergoing AR, the clearance of 18F-FDG has to be taken into account. 18F-FDG signals derived from urinary tracer remnants within the urinary pelvis can be avoided by extending the time between the application of the tracer and the PET procedure, or by simply using 18F-FDG labelled T-cells[44,49]. As 18F-FDG uptake by renal allografts immediately decreases after successful treatment of AR, the method might also be used to monitor treatment efficacy[43].
Single photon emission computed tomography (SPECT) is another nuclear imaging-based method for the detection of AR in kidney allografts. Similar to PET, SPECT provides functional rather than morphological data, but while PET captures an indirect signal (pairs of gamma rays resulting from annihilation of the emitted positrons with electrons) SPECT directly measures gamma radiation from the deployed radioisotopes. Although PET provides higher spatial resolution[32], better sensitivity and better quantification, SPECT is still the most commonly used technique. Beside its high availability and the wide range of adequate radionuclides, the cost-effectiveness is a noteworthy advantage of SPECT[50]. Regarding the available tracers used to visualize metabolic processes as well as cellular and molecular events, the generally longer half-lives of SPECT radionuclides are of additional advantage, as they better correspond to the duration of the investigated biological processes. Common markers in SPECT are 111In, 67Ga, 123I and 99mTc, the latter offering the broadest application spectrum because of its relatively simple production, availability and optimal decay characteristics compared to the rather unstable and short-lived PET tracers[51]. However, the more complex incorporation process of 99mTc into a molecule which is impeded by involvement of chelating moieties and possible steric hindrance needs to be mentioned. Thus, thorough definition and characterization of the respective processes to be examined is necessary in order to choose the appropriate tracer.
The broad application field of SPECT imaging in numerous diseases has continuously expanded during the last years. Existing technologies have been optimized and new, more sophisticated approaches have been evolved. Particular in oncology, lots of different strategies have been introduced facilitating SPECT-based diagnosis and therapeutic monitoring in oncological patients[52-54]. Moreover, processes like tissue injury, cell death or angiogenesis in cardiac and pulmonary diseases[55-57], as well as specific bacterial infections[58], inflammation severity in rheumatoid arthritis[59] and neurological disorders[60-62] can be detected and monitored with increasing precision.
According to the various pathophysiological mechanisms involved in AR after kidney transplantation, different markers for SPECT imaging have been developed during the last decades. The general principles of detecting the diverse pathophysiological processes and their implementation in PET-based diagnosis have already been discussed above. Many of these processes can be assessed by SPECT as well.
As early as in 1976, George et al[63] were able to visualize kidney allograft rejection using 99mTc-sulfur colloid, which accumulates in areas of fibrin thrombi in acute and chronic rejecting allografts.
As leukocyte recruitment plays a crucial role in allograft rejection, many attempts to label various cell lines ex vivo and in vivo have been made. Common markers used for radiolabelling white blood cells in SPECT are 99mTC-HMPAO or 111In-oxine[64-66]. Compared to 18F-FDG, these markers are more stable, have a longer half-life time and therefore should be used for sustained biological processes[67]. Labeling efficiency and viability of the marked cells are additional concerns. Whereas the labeling rate of 18F-FDG is only about 60%, 111In-oxine and the PET marker 64Cu exhibit are more efficient and have labeling rates of approximately 80%. Viability of the cells was shown to be comparable within the first four hours for 111In-oxine, 99mTc-HMPAO, 64Cu and 18F-FDG, while a significant decline of cell survival was observed after 24 h[68]. Regarding kidney transplantation, the use of 99mTc-HMPAO-labeled mononuclear cells has been shown to differentiate between rejection and ATN[69].
Different 99mTc-, 111In- or 123I-labeled antibodies binding to cell surface markers of different immune cells, like CD3, CD4, CD20 or CD25 have been developed for in vivo imaging (for review see[31]). Detection of AR in kidney transplantation is possible by using 99mTc-OKT3, a mouse monoclonal antibody against the CD3 complex, which targets T cells, natural killer cells and natural killer T cells[70]. Side effects of this antibody due to its immunogenicity have been eliminated by using a humanized form, 99mTc-SHNH-visilizumab[71,72]. Further studies are needed to evaluate its utility in diagnosing AR.
A high-affinity radiolabelled ligand binding to FPR1, a leukocyte receptor which is involved in chemotaxis and inflammatory responses, has recently been reported as a novel method to detect leukocyte accumulation in inflammation. FPR1 is upregulated during inflammation, and the 99mTc-labeled FPR1 antagonist cFLFLFK-NH2 has been shown to bind to FPR1 without interfering with the inflammatory processes[73].
Sharif-Paghaleh et al[74] published a reporter gene mediated method of radiolabelling regulatory T cells with Techentium-99m pertechnetate (99mTcO4-) in vitro and in vivo, enabling the precise visualization of the cells as long as they are vital. This method might become a useful tool in the transplant setting as well.
Besides accumulation of immune cells, complement activation is another mechanism which plays an important role in the pathophysiology of transplantation. Recently Sharif-Paghaleh et al[75] successfully demonstrated non-invasive imaging of complement activation following ischemia-reperfusion injury (IRI) in a model of cardiac transplantation, using 99mTc-recombinant complement receptor 2 (99mTc-rCR2). As IRI and complement activation per se are involved in transplant rejection and complement inhibitors have been developed as a therapeutic option, this principle could be a useful tool to identify tissue damage after transplantation, to allow patient risk stratification and to monitor the effects of therapeutic interventions.
SPECT imaging can also be applied for monitoring of allograft function. While static imaging using 99mTc-dimercaptosuccinic acid (DMSA) can visualize functioning kidney tissue and anatomical abnormalities[76,77], dynamic imaging with 99mTc-diethylenetriaminepentaacetic (DTPA) or 99mTc-mercaptoacetyltriglycine (MAG3) further allows detection of AR and discrimination from ATN[78-81].
Although core needle biopsy of the kidney allograft is still the gold standard to discriminate causes of renal injury, imaging of immunological processes offers promising, novel and non-invasive possibilities. As perfect imaging depends on severity of rejection, imaging-based methods still suffer from low sensibility[82]. Currently, PET and SPECT are able to discriminate ATN from AR. Unfortunately, differentiation between different forms of AR, namely acute antibody mediated rejection (ABMR) and T cell-mediated rejection (TCMR), has not been tested sufficiently in preclinical imaging studies so far. As both entities are treated differently, the discrimination between both is of high clinical relevance. Identification and assessment of discriminating targets like T cells (TCMR) or C4d (ABMR) might support further differential diagnostics. The ultrasound visualization of T-cells by use of microbubbles coupled to anti-CD3 antibodies is a first approach for specific diagnostics of TCMR[9]. MRI-based assessment of IOPC-NH2 labeled T-cells is based on the same principle and has been shown to be useful for the detection of rejection of a heart-lung transplant[27]. New biomarkers, like cell free DNA, microRNA, chemokines, clusters of differentiation or tubular injury markers that correlate with AR, might provide additional information. Unfortunately, most of these markers are time-consuming, expensive and do not distinguish between subclinical tubulitis, BK virus infection and different forms of AR. Nevertheless, some of these approaches, like a combination of monitoring urinary CXCL10:creatinine ratio and donor specific antibodies, might significantly improve the noninvasive diagnosis of ABMR[83]. An approach involving the use of biomarkers as well as non-invasive imaging, might improve sensitivity as well as specificity for the detection of renal allograft AR.
Non-invasive methods for specific diagnosis of AR and surveillance monitoring of the allograft are highly desired. Advances in technology and tracer development provide new diagnostic options. At present most of the promising new imaging technologies are still used at a pre-clinical stage, but represent very useful research tools on the way into clinical use. Future studies in human allograft recipients are needed to fully support these methods for clinical routine.
The authors are grateful to Dr. Olaf Boenisch for language editing of the manuscript.
P- Reviewer: Badiee P, Rydzewski A S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725-1730. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3684] [Cited by in F6Publishing: 3624] [Article Influence: 145.0] [Reference Citation Analysis (0)] |
| 2. | Elbadri A, Traynor C, Veitch JT, O’Kelly P, Magee C, Denton M, O’Sheaghdha C, Conlon PJ. Factors affecting eGFR 5-year post-deceased donor renal transplant: analysis and predictive model. Ren Fail. 2015;37:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 3. | Jalalzadeh M, Mousavinasab N, Peyrovi S, Ghadiani MH. The impact of acute rejection in kidney transplantation on long-term allograft and patient outcome. Nephrourol Mon. 2015;7:e24439. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 4. | Naesens M, Heylen L, Lerut E, Claes K, De Wever L, Claus F, Oyen R, Kuypers D, Evenepoel P, Bammens B. Intrarenal resistive index after renal transplantation. N Engl J Med. 2013;369:1797-1806. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 145] [Cited by in F6Publishing: 135] [Article Influence: 12.3] [Reference Citation Analysis (2)] |
| 5. | Radermacher J, Mengel M, Ellis S, Stuht S, Hiss M, Schwarz A, Eisenberger U, Burg M, Luft FC, Gwinner W. The renal arterial resistance index and renal allograft survival. N Engl J Med. 2003;349:115-124. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 291] [Cited by in F6Publishing: 298] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 6. | Kramann R, Frank D, Brandenburg VM, Heussen N, Takahama J, Krüger T, Riehl J, Floege J. Prognostic impact of renal arterial resistance index upon renal allograft survival: the time point matters. Nephrol Dial Transplant. 2012;27:3958-3963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Jin Y, Yang C, Wu S, Zhou S, Ji Z, Zhu T, He W. A novel simple noninvasive index to predict renal transplant acute rejection by contrast-enhanced ultrasonography. Transplantation. 2015;99:636-641. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Stock KF, Klein BS, Cong MT, Regenbogen C, Kemmner S, Büttner M, Wagenpfeil S, Matevossian E, Renders L, Heemann U. ARFI-based tissue elasticity quantification and kidney graft dysfunction: first clinical experiences. Clin Hemorheol Microcirc. 2011;49:527-535. [PubMed] [Cited in This Article: ] |
| 9. | Grabner A, Kentrup D, Muhlmeister M, Pawelski H, Biermann C, Bettinger T, Pavenstadt H, Schlatter E, Tiemann K, Reuter S. Noninvasive Imaging of Acute Renal Allograft Rejection by Ultrasound Detection of Microbubbles Targeted to T-lymphocytes in Rats. Ultraschall Med. 2016;37:82-91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Zhang JL, Morrell G, Rusinek H, Sigmund EE, Chandarana H, Lerman LO, Prasad PV, Niles D, Artz N, Fain S. New magnetic resonance imaging methods in nephrology. Kidney Int. 2014;85:768-778. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 11. | Kalb B, Martin DR, Salman K, Sharma P, Votaw J, Larsen C. Kidney transplantation: structural and functional evaluation using MR Nephro-Urography. J Magn Reson Imaging. 2008;28:805-822. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Wentland AL, Sadowski EA, Djamali A, Grist TM, Becker BN, Fain SB. Quantitative MR measures of intrarenal perfusion in the assessment of transplanted kidneys: initial experience. Acad Radiol. 2009;16:1077-1085. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Yamamoto A, Zhang JL, Rusinek H, Chandarana H, Vivier PH, Babb JS, Diflo T, John DG, Benstein JA, Barisoni L. Quantitative evaluation of acute renal transplant dysfunction with low-dose three-dimensional MR renography. Radiology. 2011;260:781-789. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Daftari Besheli L, Aran S, Shaqdan K, Kay J, Abujudeh H. Current status of nephrogenic systemic fibrosis. Clin Radiol. 2014;69:661-668. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 78] [Cited by in F6Publishing: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 15. | Lanzman RS, Ljimani A, Pentang G, Zgoura P, Zenginli H, Kröpil P, Heusch P, Schek J, Miese FR, Blondin D. Kidney transplant: functional assessment with diffusion-tensor MR imaging at 3T. Radiology. 2013;266:218-225. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 83] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 16. | Abou-El-Ghar ME, El-Diasty TA, El-Assmy AM, Refaie HF, Refaie AF, Ghoneim MA. Role of diffusion-weighted MRI in diagnosis of acute renal allograft dysfunction: a prospective preliminary study. Br J Radiol. 2012;85:e206-e211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Park SY, Kim CK, Park BK, Huh W, Kim SJ, Kim B. Evaluation of transplanted kidneys using blood oxygenation level-dependent MRI at 3 T: a preliminary study. AJR Am J Roentgenol. 2012;198:1108-1114. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Sadowski EA, Djamali A, Wentland AL, Muehrer R, Becker BN, Grist TM, Fain SB. Blood oxygen level-dependent and perfusion magnetic resonance imaging: detecting differences in oxygen bioavailability and blood flow in transplanted kidneys. Magn Reson Imaging. 2010;28:56-64. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Xiao W, Xu J, Wang Q, Xu Y, Zhang M. Functional evaluation of transplanted kidneys in normal function and acute rejection using BOLD MR imaging. Eur J Radiol. 2012;81:838-845. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Liu G, Han F, Xiao W, Wang Q, Xu Y, Chen J. Detection of renal allograft rejection using blood oxygen level-dependent and diffusion weighted magnetic resonance imaging: a retrospective study. BMC Nephrol. 2014;15:158. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688-701. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 262] [Cited by in F6Publishing: 244] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 22. | Artz NS, Sadowski EA, Wentland AL, Grist TM, Seo S, Djamali A, Fain SB. Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging. 2011;29:74-82. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 23. | Lanzman RS, Wittsack HJ, Martirosian P, Zgoura P, Bilk P, Kröpil P, Schick F, Voiculescu A, Blondin D. Quantification of renal allograft perfusion using arterial spin labeling MRI: initial results. Eur Radiol. 2010;20:1485-1491. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 24. | Thurman JM, Serkova NJ. Nanosized contrast agents to noninvasively detect kidney inflammation by magnetic resonance imaging. Adv Chronic Kidney Dis. 2013;20:488-499. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 25. | Hauger O, Grenier N, Deminère C, Lasseur C, Delmas Y, Merville P, Combe C. USPIO-enhanced MR imaging of macrophage infiltration in native and transplanted kidneys: initial results in humans. Eur Radiol. 2007;17:2898-2907. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 55] [Cited by in F6Publishing: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Chae EY, Song EJ, Sohn JY, Kim ST, Woo CW, Gong G, Kang HJ, Lee JS. Allogeneic renal graft rejection in a rat model: in vivo MR imaging of the homing trait of macrophages. Radiology. 2010;256:847-854. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Liu L, Ye Q, Wu Y, Hsieh WY, Chen CL, Shen HH, Wang SJ, Zhang H, Hitchens TK, Ho C. Tracking T-cells in vivo with a new nano-sized MRI contrast agent. Nanomedicine. 2012;8:1345-1354. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Vermathen P, Binser T, Boesch C, Eisenberger U, Thoeny HC. Three-year follow-up of human transplanted kidneys by diffusion-weighted MRI and blood oxygenation level-dependent imaging. J Magn Reson Imaging. 2012;35:1133-1138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 29. | Malvezzi P, Bricault I, Terrier N, Bayle F. Evaluation of intrarenal oxygenation by blood oxygen level-dependent magnetic resonance imaging in living kidney donors and their recipients: preliminary results. Transplant Proc. 2009;41:641-644. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hall LT, Struck AF, Perlman SB. Clinical molecular imaging with PET agents other than 18F-FDG. Curr Pharm Biotechnol. 2010;11:545-554. [PubMed] [Cited in This Article: ] |
| 31. | Signore A, Mather SJ, Piaggio G, Malviya G, Dierckx RA. Molecular imaging of inflammation/infection: nuclear medicine and optical imaging agents and methods. Chem Rev. 2010;110:3112-3145. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 101] [Cited by in F6Publishing: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 32. | Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937-1949. [PubMed] [Cited in This Article: ] |
| 33. | Skotland T. Molecular imaging: challenges of bringing imaging of intracellular targets into common clinical use. Contrast Media Mol Imaging. 2012;7:1-6. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Gallagher BM, Fowler JS, Gutterson NI, MacGregor RR, Wan CN, Wolf AP. Metabolic trapping as a principle of oradiopharmaceutical design: some factors resposible for the biodistribution of [18F] 2-deoxy-2-fluoro-D-glucose. J Nucl Med. 1978;19:1154-1161. [PubMed] [Cited in This Article: ] |
| 35. | Pauwels EK, Ribeiro MJ, Stoot JH, McCready VR, Bourguignon M, Mazière B. FDG accumulation and tumor biology. Nucl Med Biol. 1998;25:317-322. [PubMed] [Cited in This Article: ] |
| 36. | Noda Y, Kanematsu M, Goshima S, Suzui N, Hirose Y, Matsunaga K, Nishibori H, Kondo H, Watanabe H, Kawada H. 18-F fluorodeoxyglucose uptake in positron emission tomography as a pathological grade predictor for renal clear cell carcinomas. Eur Radiol. 2015;25:3009-3016. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Gallamini A, Zwarthoed C, Borra A. Positron Emission Tomography (PET) in Oncology. Cancers (Basel). 2014;6:1821-1889. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 215] [Cited by in F6Publishing: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 38. | Reuter S, Vrachimis A, Huss S, Wardelmann E, Weckesser M, Pavenstädt H. A challenging case of rapid progressive Kaposi sarcoma after renal transplantation: diagnostics by FDG PET/CT. Medicine (Baltimore). 2014;93:e67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 7] [Cited by in F6Publishing: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 39. | Lensen KD, Comans EF, Voskuyl AE, van der Laken CJ, Brouwer E, Zwijnenburg AT, Pereira Arias-Bouda LM, Glaudemans AW, Slart RH, Smulders YM. Large-vessel vasculitis: interobserver agreement and diagnostic accuracy of 18F-FDG-PET/CT. Biomed Res Int. 2015;2015:914692. [PubMed] [Cited in This Article: ] |
| 40. | Balink H, Collins J, Bruyn GA, Gemmel F. F-18 FDG PET/CT in the diagnosis of fever of unknown origin. Clin Nucl Med. 2009;34:862-868. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 41. | Harris RS, Venegas JG, Wongviriyawong C, Winkler T, Kone M, Musch G, Vidal Melo MF, de Prost N, Hamilos DL, Afshar R. 18F-FDG uptake rate is a biomarker of eosinophilic inflammation and airway response in asthma. J Nucl Med. 2011;52:1713-1720. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 42. | Chen DL, Atkinson JJ, Ferkol TW. FDG PET imaging in cystic fibrosis. Semin Nucl Med. 2013;43:412-419. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 43. | Reuter S, Schnöckel U, Edemir B, Schröter R, Kentrup D, Pavenstädt H, Schober O, Schlatter E, Gabriëls G, Schäfers M. Potential of noninvasive serial assessment of acute renal allograft rejection by 18F-FDG PET to monitor treatment efficiency. J Nucl Med. 2010;51:1644-1652. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 21] [Cited by in F6Publishing: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 44. | Reuter S, Schnöckel U, Schröter R, Schober O, Pavenstädt H, Schäfers M, Gabriëls G, Schlatter E. Non-invasive imaging of acute renal allograft rejection in rats using small animal F-FDG-PET. PLoS One. 2009;4:e5296. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 45. | Chen DL, Wang X, Yamamoto S, Carpenter D, Engle JT, Li W, Lin X, Kreisel D, Krupnick AS, Huang HJ. Increased T cell glucose uptake reflects acute rejection in lung grafts. Am J Transplant. 2013;13:2540-2549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 46. | Braun RK, Molitor-Dart M, Wigfield C, Xiang Z, Fain SB, Jankowska-Gan E, Seroogy CM, Burlingham WJ, Wilkes DS, Brand DD. Transfer of tolerance to collagen type V suppresses T-helper-cell-17 lymphocyte-mediated acute lung transplant rejection. Transplantation. 2009;88:1341-1348. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Schnöckel U, Reuter S, Stegger L, Schlatter E, Schäfers KP, Hermann S, Schober O, Gabriëls G, Schäfers M. Dynamic 18F-fluoride small animal PET to noninvasively assess renal function in rats. Eur J Nucl Med Mol Imaging. 2008;35:2267-2274. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 25] [Cited by in F6Publishing: 25] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 48. | Lovinfosse P, Weekers L, Bonvoisin C, Bovy C, Grosch S, Krzesinski JM, Hustinx R, Jouret F. Fluorodeoxyglucose F(18) Positron Emission Tomography Coupled With Computed Tomography in Suspected Acute Renal Allograft Rejection. Am J Transplant. 2016;16:310-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 49. | Grabner A, Kentrup D, Edemir B, Sirin Y, Pavenstädt H, Schlatter E, Schober O, Schäfers M, Schnöckel U, Reuter S. PET with 18F-FDG-labeled T lymphocytes for diagnosis of acute rat renal allograft rejection. J Nucl Med. 2013;54:1147-1153. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 50. | Müller C, Schibli R. Single photon emission computed tomography tracer. Recent Results Cancer Res. 2013;187:65-105. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 51. | Pimlott SL, Sutherland A. Molecular tracers for the PET and SPECT imaging of disease. Chem Soc Rev. 2011;40:149-162. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 225] [Cited by in F6Publishing: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 52. | Li F, Cheng T, Dong Q, Wei R, Zhang Z, Luo D, Ma X, Wang S, Gao Q, Ma D. Evaluation of (99m)Tc-HYNIC-TMTP1 as a tumor-homing imaging agent targeting metastasis with SPECT. Nucl Med Biol. 2015;42:256-262. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 53. | Xu B, Shokeen M, Sudlow GP, Harpstrite SE, Liang K, Cheney PP, Edwards WB, Sharma V, Laforest R, Akers WJ. Utilizing the Multiradionuclide Resolving Power of SPECT and Dual Radiolabeled Single Molecules to Assess Treatment Response of Tumors. Mol Imaging Biol. 2015;17:671-679. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 54. | Rowe SP, Gorin MA, Gordetsky J, Ball MW, Pierorazio PM, Higuchi T, Epstein JI, Allaf ME, Javadi MS. Initial experience using 99mTc-MIBI SPECT/CT for the differentiation of oncocytoma from renal cell carcinoma. Clin Nucl Med. 2015;40:309-313. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 58] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 55. | Wang L, Wang F, Fang W, Johnson SE, Audi S, Zimmer M, Holly TA, Lee DC, Zhu B, Zhu H. The feasibility of imaging myocardial ischemic/reperfusion injury using (99m)Tc-labeled duramycin in a porcine model. Nucl Med Biol. 2015;42:198-204. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 28] [Cited by in F6Publishing: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Hendrikx G, De Saint-Hubert M, Dijkgraaf I, Bauwens M, Douma K, Wierts R, Pooters I, Van den Akker NM, Hackeng TM, Post MJ. Molecular imaging of angiogenesis after myocardial infarction by (111)In-DTPA-cNGR and (99m)Tc-sestamibi dual-isotope myocardial SPECT. EJNMMI Res. 2015;5:2. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 57. | Audi SH, Jacobs ER, Zhao M, Roerig DL, Haworth ST, Clough AV. In vivo detection of hyperoxia-induced pulmonary endothelial cell death using (99m)Tc-duramycin. Nucl Med Biol. 2015;42:46-52. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Welling MM, Bunschoten A, Kuil J, Nelissen RG, Beekman FJ, Buckle T, van Leeuwen FW. Development of a Hybrid Tracer for SPECT and Optical Imaging of Bacterial Infections. Bioconjug Chem. 2015;26:839-849. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 43] [Cited by in F6Publishing: 45] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 59. | Laverman P, van der Geest T, Terry SY, Gerrits D, Walgreen B, Helsen MM, Nayak TK, Freimoser-Grundschober A, Waldhauer I, Hosse RJ. Immuno-PET and Immuno-SPECT of Rheumatoid Arthritis with Radiolabeled Anti-Fibroblast Activation Protein Antibody Correlates with Severity of Arthritis. J Nucl Med. 2015;56:778-783. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 60. | Booth TC, Nathan M, Waldman AD, Quigley AM, Schapira AH, Buscombe J. The role of functional dopamine-transporter SPECT imaging in parkinsonian syndromes, part 1. AJNR Am J Neuroradiol. 2015;36:229-235. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 61. | Walker Z, Moreno E, Thomas A, Inglis F, Tabet N, Rainer M, Pizzolato G, Padovani A. Clinical usefulness of dopamine transporter SPECT imaging with 123I-FP-CIT in patients with possible dementia with Lewy bodies: randomised study. Br J Psychiatry. 2015;206:145-152. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Spehl TS, Frings L, Hellwig S, Weiller C, Hüll M, Meyer PT, Amtage F. Role of semiquantitative assessment of regional binding potential in 123I-FP-CIT SPECT for the differentiation of frontotemporal dementia, dementia with Lewy bodies, and Alzheimer’s dementia. Clin Nucl Med. 2015;40:e27-e33. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | George EA, Codd JE, Newton WT, Haibach H, Donati RM. Comparative evaluation of renal transplant rejection with radioiodinated fibrinogen 99mTc-sulfur collid, and 67Ga-citrate. J Nucl Med. 1976;17:175-180. [PubMed] [Cited in This Article: ] |
| 64. | Datz FL. Indium-111-labeled leukocytes for the detection of infection: current status. Semin Nucl Med. 1994;24:92-109. [PubMed] [Cited in This Article: ] |
| 65. | Peters AM, Danpure HJ, Osman S, Hawker RJ, Henderson BL, Hodgson HJ, Kelly JD, Neirinckx RD, Lavender JP. Clinical experience with 99mTc-hexamethylpropylene-amineoxime for labelling leucocytes and imaging inflammation. Lancet. 1986;2:946-949. [PubMed] [Cited in This Article: ] |
| 66. | McAfee JG, Thakur ML. Survey of radioactive agents for in vitro labeling of phagocytic leukocytes. I. Soluble agents. J Nucl Med. 1976;17:480-487. [PubMed] [Cited in This Article: ] |
| 67. | Forstrom LA, Dunn WL, Mullan BP, Hung JC, Lowe VJ, Thorson LM. Biodistribution and dosimetry of [(18)F]fluorodeoxyglucose labelled leukocytes in normal human subjects. Nucl Med Commun. 2002;23:721-725. [PubMed] [Cited in This Article: ] |
| 68. | Bhargava KK, Gupta RK, Nichols KJ, Palestro CJ. In vitro human leukocyte labeling with (64)Cu: an intraindividual comparison with (111)In-oxine and (18)F-FDG. Nucl Med Biol. 2009;36:545-549. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 69. | Lopes de Souza SA, Barbosa da Fonseca LM, Torres Gonçalves R, Salomão Pontes D, Holzer TJ, Proença Martins FP, Gutfilen B. Diagnosis of renal allograft rejection and acute tubular necrosis by 99mTc-mononuclear leukocyte imaging. Transplant Proc. 2004;36:2997-3001. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 70. | Martins FP, Souza SA, Gonçalves RT, Fonseca LM, Gutfilen B. Preliminary results of [99mTc]OKT3 scintigraphy to evaluate acute rejection in renal transplants. Transplant Proc. 2004;36:2664-2667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Cole MS, Stellrecht KE, Shi JD, Homola M, Hsu DH, Anasetti C, Vasquez M, Tso JY. HuM291, a humanized anti-CD3 antibody, is immunosuppressive to T cells while exhibiting reduced mitogenicity in vitro. Transplantation. 1999;68:563-571. [PubMed] [Cited in This Article: ] |
| 72. | Shan L. 99mTc-Labeled succinimidyl-6-hydrazinonicotinate hydrochloride (SHNH)-conjugated visilizumab. Bethesda, MD: Molecular Imaging and Contrast Agent Database (MICAD) 2004; . [Cited in This Article: ] |
| 73. | Stasiuk GJ, Holloway PM, Rivas C, Trigg W, Luthra SK, Morisson Iveson V, Gavins FN, Long NJ. (99m)Tc SPECT imaging agent based on cFLFLFK for the detection of FPR1 in inflammation. Dalton Trans. 2015;44:4986-4993. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 74. | Sharif-Paghaleh E, Sunassee K, Tavaré R, Ratnasothy K, Koers A, Ali N, Alhabbab R, Blower PJ, Lechler RI, Smyth LA. In vivo SPECT reporter gene imaging of regulatory T cells. PLoS One. 2011;6:e25857. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 75. | Sharif-Paghaleh E, Yap ML, Meader LL, Chuamsaamarkkee K, Kampmeier F, Badar A, Smith RA, Sacks S, Mullen GE. Noninvasive Imaging of Activated Complement in Ischemia-Reperfusion Injury Post-Cardiac Transplant. Am J Transplant. 2015;15:2483-2490. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 76. | Budihna NV, Milcinski M, Kajtna-Koselj M, Malovrh M. Relevance of Tc-99m DMSA scintigraphy in renal transplant parenchymal imaging. Clin Nucl Med. 1994;19:782-784. [PubMed] [Cited in This Article: ] |
| 77. | Even-Sapir E, Gutman M, Lerman H, Kaplan E, Ravid A, Livshitz G, Nakache R. Kidney allografts and remaining contralateral donor kidneys before and after transplantation: assessment by quantitative (99m)Tc-DMSA SPECT. J Nucl Med. 2002;43:584-588. [PubMed] [Cited in This Article: ] |
| 78. | Sfakianakis GN, Sfakianaki E, Georgiou M, Serafini A, Ezuddin S, Kuker R, Zilleruelo G, Strauss J, Abitbol C, Chandar J. A renal protocol for all ages and all indications: mercapto-acetyl-triglycine (MAG3) with simultaneous injection of furosemide (MAG3-F0): a 17-year experience. Semin Nucl Med. 2009;39:156-173. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 22] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 79. | Sfakianaki E, Sfakianakis GN, Georgiou M, Hsiao B. Renal scintigraphy in the acute care setting. Semin Nucl Med. 2013;43:114-128. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 80. | Dubovsky EV, Russell CD, Bischof-Delaloye A, Bubeck B, Chaiwatanarat T, Hilson AJ, Rutland M, Oei HY, Sfakianakis GN, Taylor A. Report of the Radionuclides in Nephrourology Committee for evaluation of transplanted kidney (review of techniques). Semin Nucl Med. 1999;29:175-188. [PubMed] [Cited in This Article: ] |
| 81. | Sundaraiya S, Mendichovszky I, Biassoni L, Sebire N, Trompeter RS, Gordon I. Tc-99m DTPA renography in children following renal transplantation: its value in the evaluation of rejection. Pediatr Transplant. 2007;11:771-776. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 4] [Cited by in F6Publishing: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 82. | Bertoni E, Di Maria L, Piperno R, Zanazzi M, Rosati A, Moscarelli L, Salvadori M. Limits of clinical signs and non-invasive techniques in detecting severe acute rejection. J Nephrol. 1999;12:100-103. [PubMed] [Cited in This Article: ] |
| 83. | Rabant M, Amrouche L, Lebreton X, Aulagnon F, Benon A, Sauvaget V, Bonifay R, Morin L, Scemla A, Delville M. Urinary C-X-C Motif Chemokine 10 Independently Improves the Noninvasive Diagnosis of Antibody-Mediated Kidney Allograft Rejection. J Am Soc Nephrol. 2015;26:2840-2851. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 96] [Article Influence: 10.7] [Reference Citation Analysis (0)] |