Published online May 25, 2021. doi: 10.5527/wjn.v10.i3.29
Peer-review started: January 9, 2021
First decision: March 1, 2021
Revised: March 21, 2021
Accepted: May 7, 2021
Article in press: May 7, 2021
Published online: May 25, 2021
Gadolinium-based contrast agents (GBCAs) used in magnetic resonance imaging are vital in providing enhanced quality images, essential for diagnosis and treatment. Nephrogenic systemic fibrosis (NSF) with GBCAs has been a deterrent for the physician and has led to avoidance of these agents in patients with impaired kidney function. NSF is a progressive debilitating multisystem condition described classically in patients with renal insufficiency exposed to gadolinium contrast media. It is characterized by an induration and hardening of the skin. NSF is described to first involve the extremities and can imperceptibly involve internal organs. Lack of therapeutic interventions to treat NSF makes it more challenging and warrants deep insight into the pathogenesis, risk factors and treatment strategies.
Core Tip: Nephrogenic systemic fibrosis is a chronic debilitating fibrosing disorder resulting in progressive hardening or induration of the skin. It mostly develops in patients with impaired kidney function. This review aims to provide insight into the pathogenesis, risk factors, clinical features and challenges in the management of nephrogenic systemic fibrosis.
- Citation: Bhargava V, Singh K, Meena P, Sanyal R. Nephrogenic systemic fibrosis: A frivolous entity. World J Nephrol 2021; 10(3): 29-36
- URL: https://www.wjgnet.com/2220-6124/full/v10/i3/29.htm
- DOI: https://dx.doi.org/10.5527/wjn.v10.i3.29
Nephrogenic systemic fibrosis (NSF) was first described in 1997 by Cowper et al[1] in a renal transplant recipient with poor graft function. He described this as a scleromyxedema-like condition. However, no data on NSF were published until 2000[1]. This later emerged as one of the greatest concerns for nephrologists. NSF was originally named nephrogenic fibrosing dermopathy due to the characteristic skin findings[2]. However, subsequent studies showed that some patients also had systemic involvement. Symptoms typically include thickening of the skin with pruritus. Symptoms and signs may progress rapidly, with some patients developing contractures and joint immobility. This may be fatal in a few patients. In this review, we attempt to explain the pathogenesis, risk factors, clinical features and therapeutic interventions in NSF.
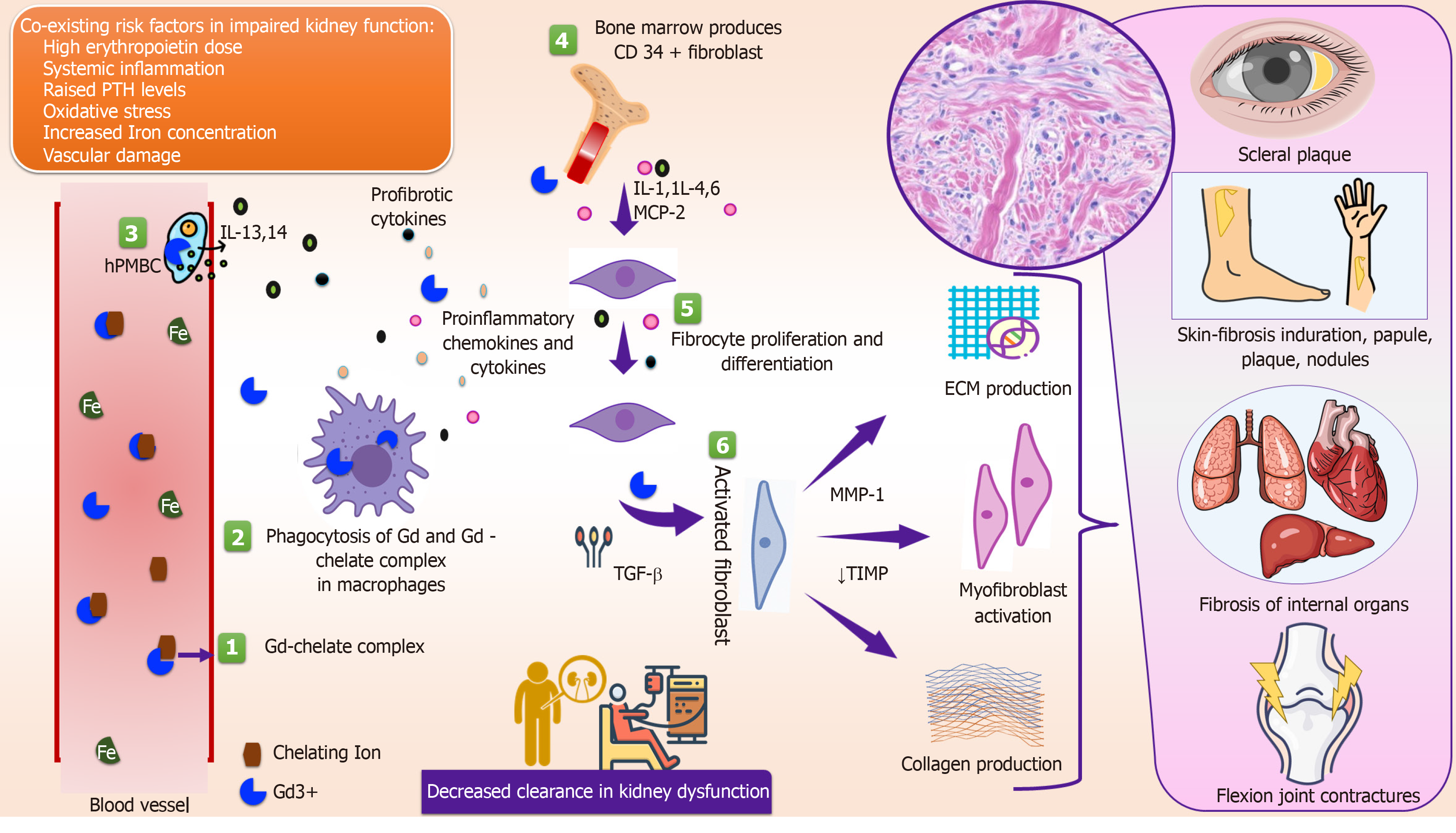
The skin lesions are predominantly erythematous papules or nodules which coalesce to form indurated plaques. The lesions are commonly distributed symmetrically and initially involve the lower extremities. They then spread proximally to involve the upper extremities. Furthermore, the trunk is usually involved, and the head is mostly spared except for a scleral plaque. During evolution, lesions are slightly edematous and erythematous associated with itching and a burning sensation. With time they become hard and “woody” sometimes assuming a “cobblestone” appearance. The timing of the appearance of skin lesions following gadolinium-based contrast agents (GBCAs) exposure is controversial. It may vary from a few months to several years (1 d to 10 years)[3]. The course of skin lesions is usually unremitting and chronic. However, a rapid progressive fulminant course has also been reported in approximately 5% of patients[4].
Several case studies have reported the involvement of subcutaneous structures such as fascia, joints, muscle and periarticular tissue and tendons[5]. Involvement of joints can be severe and compromise range of motion with loss of ambulation and confinement to a wheelchair. Deep fibrosis results in flexion contractures of joints mainly in the hands, wrists, knees and ankles. Data from Mendoza et al[6] showed fibrosis of deep visceral organs such as diaphragm muscles, the myocardium including small coronary arterioles, lungs, kidneys and testes. Another analysis by Schieren et al[7] revealed esophageal dysmotility with consecutive liver fibrosis and esophageal varices. Visceral involvement in the form of cardiomyopathy and pulmonary fibrosis was also reported by Swartz et al[8]. Yellow scleral plaques are also a frequent finding in patients of NSF, and are seen clinically in young patients. These scleral plaques differ from the typical Cogan plaques of aging (present in older age) that are due to calcium phosphate deposition, and acellular collagen in the sclera with no inflammatory response[9].
Various disorders may mimic the skin lesions of NSF. In patients with similar skin lesions, conditions such as lipodermatosclerosis, deep morphea, scleromyxedema, eosinophilic fasciitis, and chronic-graft-versus-host disease should also be considered.
NSF is characterized by dermal fibrosis. In the early phase, collagen bundles are found dispersed within edema and mucin. The circulating fibrocytes are interspersed between the strands of collagen. The CD34 positive dendritic processes encircling collagen bundles are present. These circulating fibrocytes are dual positive (CD34 and procollagen I) suggesting characteristics of mesenchymal stem cells of bone marrow origin. In advanced lesions there is proliferation of spindle shaped dermal fibrocytes leading to thick and fibrosed dermis. NSF skin lesion histology is typically characterized by markedly increased cellularity, persistence of thick bundled collagen, preserved clefts and CD34 positive fibrocytes in the absence of inflammation[10]. Moreover, Mendoza et al[6] found an early dermal infiltration with CD 68+/factor XIIIa+ dendritic cells by immunohistochemistry and in situ hybridization studies. Immunohistochemistry has revealed substantial upregulated expression of transforming growth factor β1 (TGFβ1) mRNA in the dermis, and affected tissue of NSF with TGFB1 upregulation could be a pathogenetic event in this entity.
Gadolinium (Gd) is a metal of the lanthanide series recognized for its high paramagnetic properties. Ionized Gd is toxic and requires chelation with a ligand for clinical use. GBCAs are categorized based on their biochemical structure (linear vs macrocyclic) and chelate charge (nonionic vs ionic). Macrocyclic chelates (for example gadobutrol, gadoterate meglumine, and gadoteridol) bind Gd more tightly than linear chelates (gadodiamide) and are more stable both in vitro and in vivo. They have a lower dissociation rate; thus, there is a reduced association with NSF[11]. The recommended dose is 0.1 mmol/kg[12]. They have minimal protein binding capacity, and a short half-life of 1.5 h in patients with normal kidney function. Elimination of GBCAs is impaired and retention takes place in those with renal insufficiency[13]. Based on the reported incidence of NSF, the American College of Radiology (ACR) Committee on Drugs and Contrast Media has classified Gd-based contrast agents into various groups[14] (Table 1).
| Group I: Agents associated with the greatest number of NSF cases: |
| Gadodiamide (Omniscan® – GE Healthcare) |
| Gadopentetate dimeglumine (Magnevist® – Bayer HealthCare Pharmaceuticals) |
| Gadoversetamide (OptiMARK® – Guerbet) |
| Group II: Agents associated with few, if any, cases of NSF: |
| Gadobenate dimeglumine (MultiHance® – Bracco Diagnostics) |
| Gadobutrol (Gadavist® – Bayer HealthCare Pharmaceuticals; Gadovist in many countries) |
| Gadoteric acid (Dotarem® – Guerbet, Clariscan – GE Healthcare) |
| Gadoteridol (ProHance® – Bracco Diagnostics) |
| Group III: Agents for which data remains limited regarding NSF risk, but for which few, if any cases of NSF have been reported: |
| Gadoxetate disodium (Eovist – Bayer HealthCare Pharmaceuticals; Primovist in many countries) |
Although the true cause of NSF is still unknown; almost all patients who develop NSF have an underlying kidney dysfunction. Approximately 90% of the patients described in the registry have end-stage kidney disease and they are either on hemodialysis or peritoneal dialysis[15]. The remaining patients have some degree of chronic kidney dysfunction or acute kidney injury (AKI). From the previously published literature, it can be estimated that one or more exposures to group 1 GBCA may pose a risk in developing NSF in patients with an estimated glomerular filtration rate (eGFR) < 30 mL/min/1.73 m2)[16].
Endothelial dysfunction is common in chronic kidney disease (CKD) patients, which interferes with platelets to interact and attach to damaged endothelium and thus can be a critical risk factor. Moreover, vascular surgery related procedures, central catheter placement, right atrial clots from indwelling hemodialysis catheters, deep venous thrombosis or thrombosed vascular access are frequently observed before the occurrence of NSF[17]. Thus, it can be speculated that this endothelial dysfunction primes for a secondary event or a trigger such as Gd that eventually initiates the process of fibrosis.
AKI or acute dysfunction of the kidney can also be a risk factor for NSF. NSF has been noted in 10%-20% of patients with AKI, often superimposed upon baseline CKD. NSF has also developed in patients with AKI without underlying CKD[18].
There are case reports of NSF after a single exposure and single dose of GBCAs. Higher cumulative doses of Gd, either administered in a single dose or multiple administrations can augment the chances of developing NSF[19].
As only some patients with kidney dysfunction develop NSF following the use of Gd and other patients do not, cofactors may have a contributory role. These include metabolic acidosis; raised serum levels of iron, calcium, and phosphate; the use of high-dose erythropoietin stimulating agents, pre-existing vasculopathy, and other acute proinflammatory states[20-22]. However, the probable role of these cofactors has not yet been well-established. Therefore, routine screening prior to Gd administration is not advised.
Possible risk factors for NSF are shown in Table 2.
| Risk factors for NSF |
| Patients on dialysis (hemodialysis or peritoneal dialysis) |
| Advanced or end-stage CKD (CKD 4 or 5, eGFR < 30 mL/min/1.73 m2) without dialysis |
| Acute kidney injury |
| Proinflammatory state in a patient with impaired kidney function |
| Higher doses and multiple administrations of GBCAs, within a short period of time |
The exact mechanism of NSF is unknown. It is hypothesized that usually a trigger is needed to initiate the fibrosing process and Gd may act as a potential trigger for NSF development in patients with kidney disease. The most widely held hypothesis is that kidney dysfunction impairs the renal excretion of Gd which prolongs its half-life and increases the chance of Gd dissociation from the chelate. Kidneys are responsible for the elimination of almost all (97%) Gd contrast from the body. Reduced kidney function results in a substantial increase in its half-life from 1.5 h (normal kidney function) to 5.61 h and 9.18 h at CKD stage 4 and 5, respectively[23]. Free Gd can bind with anions such as phosphate or iron, and form insoluble precipitates that are deposited in various tissues[24]. Vascular injury and endothelial dysfunction enhance free Gd invasion into tissues, where Gd is phagocytosed by macrophages to release local profibrotic cytokines and sends signals to attract circulating fibrocytes into the tissues. These circulating fibrocytes are cardinal cells that play the interlinking role between Gd deposition and the initiation of fibrosis. In tissues, circulating fibrocytes commence the fibrosing process that is similar to the normal healing process followed by scar formation. This hypothesis is held up by the presence of excess Gd in the affected tissues of NSF patients when compared to unaffected tissues[25] (Figure 1). A few studies have shown Gd in tissues using scanning electron microscopy and energy dispersive X-ray spectroscopy[26]. However, these techniques are complicated and are not considered a pre-requisite for the diagnosis of NSF.
Since their introduction in magnetic resonance imaging (MRI), there have been many advances in GBCAs during the last 30 years. The risk of developing NSF with newer GBCAs, even in patients with impaired kidney function or on dialysis, is exceedingly small. This risk is further reduced by prescribing low standard recommended dosages and avoiding repeated use.
In a large retrospective analysis by Chrysochou et al[27] from the United Kingdom, in 74 patients with stage 5 CKD without dialysis undergoing GBCA-enhanced MRI (with gadobenate dimeglumine), no NSF events were reported. Similarly, following the administration of gadobenate dimeglumine, Altun et al[28] reported no NSF events in over 25000 patients, including at-risk patients (CKD stage 4 and 5 ) and 549 patients on dialysis. All patients were given gadobenate dimeglumine at the standard dose (0.1 mmol/kg). Various studies including, the Pro-FINEST prospective multicenter trial (255 patients), the RESCUE trial a prospective European multicenter study (70 patients), and the SECURE study (476 patients) have assessed the safety of gadoterate meglumine in patients with acute and CKD or on dialysis. No NSF was reported in any of these studies[29-31]. The ACR guidelines also cite that the risk of NSF is sufficiently low or possibly non-existent with group II agents[32]. Due to limited dose exposure, underpowering, and the retrospective nature of some studies, clinicians should be aware and not misinterpret these guidelines as newer agents having unconditional safety.
It is important to identify patients at risk of developing NSF when group I GBCAs are used. They should be screened for conditions and other factors that may be associated with renal function impairment. eGFR should be calculated for every patient using readily available Modification of Diet in Renal Disease or the Chronic Kidney Disease Epidemiology Collaboration calculators. However, in dialysis patients or known AKI cases, calculation of eGFR is not useful and is unnecessary as these patients are at high risk for NSF and group I GBCAs should be avoided. Some questionnaires that include risk factors for compromised kidney function can also be produced which can include questions on the history of kidney disease including dialysis and kidney transplantation. Although there are many other factors which may contribute to renal impairment such as multiple myeloma, systemic lupus erythematosus, urinary tract infection, medicines such as nonsteroidal anti-inflammatory drugs and aminoglycosides, the ACR Committee on Drugs and Contrast Media does not routinely recommend screening for these possible risk factors as the advantages of screening for these risk factors are considered low. The risk of NSF among patients exposed to standard doses of group II GBCAs is sufficiently low and assessment of renal function with a questionnaire or laboratory testing is optional prior to intravenous administration. The lowest recommended dose of GBCAs should be used in at-risk patients to attain the required clinical information. If multiple doses/sessions of Gd are required in these patients then group II agents should be preferred. Limited data exist on the beneficial effects of postprocedural hemodialysis.
Multiple interventions, alone or in combination have been tried but none of them have been beneficial. There is no proven efficacious therapy for the management of NSF. Due to the rare nature of this condition, most of the studies involve a small number of cases and lack long-term follow-up. Although some patients have shown improve
ECP is an immunomodulatory procedure in which a patient's leukocyte rich plasma is treated ex vivo with a photosensitizing agent and UV-A radiation followed by reinfusion of the treated blood product. Several case series have suggested the role of ECP in patients with NSF[33,34]. However, frequency and duration of ECP, other additional therapy, severity of the disease, and interpatient biological variability are some of the important factors speculated in determining the response to ECP. Common practice for using ECP is to begin with one cycle per week for 4 wk (i.e., 8 ECP sessions over 4 wk), then taper down to once every 2 wk (4-5 cycles) and then monthly. The total number of sessions is determined by response and clinical recovery in the patient[35].
Kay et al[36] reported 2 patients with NSF who responded to imatinib mesylate therapy. Improvements in skin thickening and knee joint contractures were observed. The patients received 400 mg/day imatinib mesylate orally. Imatinib mesylate acts by blocking signal transduction via both the TGFβR and the PDGF-BB receptor.
Pentoxifylline improves red blood cell flexibility to improve circulation and is known for its antifibrotic activity. As thrombosis is speculated to be an inciting event in NSF, this mechanism could explain the improvements noted clinically with pentoxifylline[19].
Sodium thiosulfate is a crystalline substance that has antioxidant and chelating properties. The TGF-β/Smad signaling cascade is one of the pathways involved in the pathogenesis of both NSF and calciphylaxis. It is already used in calciphylaxis. There is a possibility that sodium thiosulfate acts through the same mechanism of action as calciphylaxis in NSF. However, more studies are required to establish this relationship. The most common side effects are nausea and vomiting and the dosage administered is 12.5 g to 25 g post-dialysis. Yerram et al[37] reported rapid improvement in NSF following intravenous sodium thiosulfate.
NSF is a progressive, severely disabling fibrotic condition, which develops in patients with impaired kidney function exposed to GBCAs. However, the occurrence of NSF with newer contrast agents has been questioned in recent times. Determination of the putative underlying mechanisms, etiological or triggering factors necessitates further research and long-term data. Genetic predisposition may be a new revelation in future studies. Therapeutic approaches in the present literature are limited and have reduced benefit.
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: American Society of Nephrology, No. 548462.
Specialty type: Urology and nephrology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yorioka N S-Editor: Liu M L-Editor: Webster JR P-Editor: Xing YX
| 1. | Cowper SE, Robin HS, Steinberg SM, Su LD, Gupta S, LeBoit PE. Scleromyxoedema-like cutaneous diseases in renal-dialysis patients. Lancet. 2000;356:1000-1001. [PubMed] [DOI] [Cited in This Article: ] |
| 2. | Cowper SE, Bucala R, Leboit PE. Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis--setting the record straight. Semin Arthritis Rheum. 2006;35:208-210. [PubMed] [DOI] [Cited in This Article: ] |
| 3. | Waikhom R, Taraphder A. Nephrogenic systemic fibrosis: a brief review. Indian J Dermatol. 2011;56:54-58. [PubMed] [DOI] [Cited in This Article: ] |
| 4. | Attari H, Cao Y, Elmholdt TR, Zhao Y, Prince MR. A Systematic Review of 639 Patients with Biopsy-confirmed Nephrogenic Systemic Fibrosis. Radiology. 2019;292:376-386. [PubMed] [DOI] [Cited in This Article: ] |
| 5. | Cowper SE. Nephrogenic fibrosing dermopathy: the first 6 years. Curr Opin Rheumatol. 2003;15:785-790. [PubMed] [DOI] [Cited in This Article: ] |
| 6. | Mendoza FA, Artlett CM, Sandorfi N, Latinis K, Piera-Velazquez S, Jimenez SA. Description of 12 cases of nephrogenic fibrosing dermopathy and review of the literature. Semin Arthritis Rheum. 2006;35:238-249. [PubMed] [DOI] [Cited in This Article: ] |
| 7. | Schieren G, Wirtz N, Altmeyer P, Rump LC, Weiner SM, Kreuter A. Nephrogenic systemic fibrosis--a rapidly progressive disabling disease with limited therapeutic options. J Am Acad Dermatol. 2009;61:868-874. [PubMed] [DOI] [Cited in This Article: ] |
| 8. | Swartz RD, Crofford LJ, Phan SH, Ike RW, Su LD. Nephrogenic fibrosing dermopathy: a novel cutaneous fibrosing disorder in patients with renal failure. Am J Med. 2003;114:563-572. [PubMed] [DOI] [Cited in This Article: ] |
| 9. | Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663. [PubMed] [DOI] [Cited in This Article: ] |
| 10. | Cowper SE, Rabach M, Girardi M. Clinical and histological findings in nephrogenic systemic fibrosis. Eur J Radiol. 2008;66:191-199. [PubMed] [DOI] [Cited in This Article: ] |
| 11. | Aime S, Caravan P. Biodistribution of gadolinium-based contrast agents, including gadolinium deposition. J Magn Reson Imaging. 2009;30:1259-1267. [PubMed] [DOI] [Cited in This Article: ] |
| 12. | Perazella MA. Current status of gadolinium toxicity in patients with kidney disease. Clin J Am Soc Nephrol. 2009;4:461-469. [PubMed] [DOI] [Cited in This Article: ] |
| 13. | FDA Drug Safety Communication. FDA Drug Safety Communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings; 2018 [cited 4 October 2020]. In: FDA homepage [Internet] Available from: https://www.fda.gov/Drugs/DrugSafety/ucm589213.htm. [Cited in This Article: ] |
| 14. | American College of Radiology. ACR Manual on Contrast Media, Version 10.3, ACR Committee on Drugs and Contrast Media. Reston, VA, American College of Radiology. 2017: 22-28 Available from: https://xray.ufl.edu/files/2008/06/ACR-Manual-on-Contrast-Media-Version-10.3-2017.pdf. [Cited in This Article: ] |
| 15. | Shabana WM, Cohan RH, Ellis JH, Hussain HK, Francis IR, Su LD, Mukherji SK, Swartz RD. Nephrogenic systemic fibrosis: a report of 29 cases. AJR Am J Roentgenol. 2008;190:736-741. [PubMed] [DOI] [Cited in This Article: ] |
| 16. | Collidge TA, Thomson PC, Mark PB, Traynor JP, Jardine AG, Morris ST, Simpson K, Roditi GH. Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology. 2007;245:168-175. [PubMed] [DOI] [Cited in This Article: ] |
| 17. | Prince MR, Zhang H, Morris M, MacGregor JL, Grossman ME, Silberzweig J, DeLapaz RL, Lee HJ, Magro CM, Valeri AM. Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology. 2008;248:807-816. [PubMed] [DOI] [Cited in This Article: ] |
| 18. | Kalb RE, Helm TN, Sperry H, Thakral C, Abraham JL, Kanal E. Gadolinium-induced nephrogenic systemic fibrosis in a patient with an acute and transient kidney injury. Br J Dermatol. 2008;158:607-610. [PubMed] [DOI] [Cited in This Article: ] |
| 19. | Grobner T. Gadolinium--a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant. 2006;21:1104-1108. [PubMed] [DOI] [Cited in This Article: ] |
| 20. | Wahba IM, Simpson EL, White K. Gadolinium is not the only trigger for nephrogenic systemic fibrosis: insights from two cases and review of the recent literature. Am J Transplant. 2007;7:2425-2432. [PubMed] [DOI] [Cited in This Article: ] |
| 21. | Peak AS, Sheller A. Risk factors for developing gadolinium-induced nephrogenic systemic fibrosis. Ann Pharmacother. 2007;41:1481-1485. [PubMed] [DOI] [Cited in This Article: ] |
| 22. | Abu-Alfa AK. Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis. 2011;18:188-198. [PubMed] [DOI] [Cited in This Article: ] |
| 23. | Marckmann P, Skov L, Rossen K, Dupont A, Damholt MB, Heaf JG, Thomsen HS. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006;17:2359-2362. [PubMed] [DOI] [Cited in This Article: ] |
| 24. | Morcos SK, Haylor J. Pathophysiology of nephrogenic systemic fibrosis: A review of experimental data. World J Radiol. 2010;2:427-433. [PubMed] [DOI] [Cited in This Article: ] |
| 25. | Chopra T, Kandukurti K, Shah S, Ahmed R, Panesar M. Understanding nephrogenic systemic fibrosis. Int J Nephrol. 2012;2012:912189. [PubMed] [DOI] [Cited in This Article: ] |
| 26. | High WA, Ayers RA, Chandler J, Zito G, Cowper SE. Gadolinium is detectable within the tissue of patients with nephrogenic systemic fibrosis. J Am Acad Dermatol. 2007;56:21-26. [PubMed] [DOI] [Cited in This Article: ] |
| 27. | Chrysochou C, Power A, Shurrab AE, Husain S, Moser S, Lay J, Salama AD, Kalra PA. Low risk for nephrogenic systemic fibrosis in nondialysis patients who have chronic kidney disease and are investigated with gadolinium-enhanced magnetic resonance imaging. Clin J Am Soc Nephrol. 2010;5:484-489. [PubMed] [DOI] [Cited in This Article: ] |
| 28. | Altun E, Semelka RC, Cakit C. Nephrogenic systemic fibrosis and management of high-risk patients. Acad Radiol. 2009;16:897-905. [PubMed] [DOI] [Cited in This Article: ] |
| 29. | Amet S, Launay-Vacher V, Clément O, Frances C, Tricotel A, Stengel B, Gauvrit JY, Grenier N, Reinhardt G, Janus N, Choukroun G, Laville M, Deray G. Incidence of nephrogenic systemic fibrosis in patients undergoing dialysis after contrast-enhanced magnetic resonance imaging with gadolinium-based contrast agents: the Prospective Fibrose Nephrogénique Systémique study. Invest Radiol. 2014;49:109-115. [PubMed] [DOI] [Cited in This Article: ] |
| 30. | Deray G, Rouviere O, Bacigalupo L, Maes B, Hannedouche T, Vrtovsnik F, Rigothier C, Billiouw JM, Campioni P, Ferreiros J, Devos D, Alison D, Glowacki F, Boffa JJ, Marti-Bonmati L. Safety of meglumine gadoterate (Gd-DOTA)-enhanced MRI compared to unenhanced MRI in patients with chronic kidney disease (RESCUE study). Eur Radiol. 2013;23:1250-1259. [PubMed] [DOI] [Cited in This Article: ] |
| 31. | Soyer P, Dohan A, Patkar D, Gottschalk A. Observational study on the safety profile of gadoterate meglumine in 35,499 patients: The SECURE study. J Magn Reson Imaging. 2017;45:988-997. [PubMed] [DOI] [Cited in This Article: ] |
| 32. | Isaka Y, Hayashi H, Aonuma K, Horio M, Terada Y, Doi K, Fujigaki Y, Yasuda H, Sato T, Fujikura T, Kuwatsuru R, Toei H, Murakami R, Saito Y, Hirayama A, Murohara T, Sato A, Ishii H, Takayama T, Watanabe M, Awai K, Oda S, Murakami T, Yagyu Y, Joki N, Komatsu Y, Miyauchi T, Ito Y, Miyazawa R, Kanno Y, Ogawa T, Koshi E, Kosugi T, Yasuda Y; Japanese Society of Nephrology; Japan Radiological Society; and Japanese Circulation Society Joint Working Group. Guideline on the use of iodinated contrast media in patients with kidney disease 2018. Clin Exp Nephrol. 2020;24:1-44. [PubMed] [DOI] [Cited in This Article: ] |
| 33. | Richmond H, Zwerner J, Kim Y, Fiorentino D. Nephrogenic systemic fibrosis: relationship to gadolinium and response to photopheresis. Arch Dermatol. 2007;143:1025-1030. [PubMed] [DOI] [Cited in This Article: ] |
| 34. | Kafi R, Fisher GJ, Quan T, Shao Y, Wang R, Voorhees JJ, Kang S. UV-A1 phototherapy improves nephrogenic fibrosing dermopathy. Arch Dermatol. 2004;140:1322-1324. [PubMed] [DOI] [Cited in This Article: ] |
| 35. | Zhang R, Rose WN. Photopheresis Provides Significant Long-Lasting Benefit in Nephrogenic Systemic Fibrosis. Case Rep Dermatol Med. 2017;2017:3240287. [PubMed] [DOI] [Cited in This Article: ] |
| 36. | Kay J, High WA. Imatinib mesylate treatment of nephrogenic systemic fibrosis. Arthritis Rheum. 2008;58:2543-2548. [PubMed] [DOI] [Cited in This Article: ] |
| 37. | Yerram P, Saab G, Karuparthi PR, Hayden MR, Khanna R. Nephrogenic systemic fibrosis: a mysterious disease in patients with renal failure--role of gadolinium-based contrast media in causation and the beneficial effect of intravenous sodium thiosulfate. Clin J Am Soc Nephrol. 2007;2:258-263. [PubMed] [DOI] [Cited in This Article: ] |