Revised: July 7, 2014
Accepted: July 25, 2014
Published online: August 6, 2014
The renin-angiotensin system (RAS) has been known for more than a century as a cascade that regulates body fluid balance and blood pressure. Angiotensin II(Ang II) has many functions in different tissues; however it is on the kidney that this peptide exerts its main functions. New enzymes, alternative routes for Ang IIformation or even active Ang II-derived peptides have now been described acting on Ang II AT1 or AT2 receptors, or in receptors which have recently been cloned, such as Mas and AT4. Another interesting observation was that old members of the RAS, such as angiotensin converting enzyme (ACE), renin and prorenin, well known by its enzymatic activity, can also activate intracellular signaling pathways, acting as an outside-in signal transduction molecule or on the renin/(Pro)renin receptor. Moreover, the endocrine RAS, now is also known to have paracrine, autocrine and intracrine action on different tissues, expressing necessary components for local Ang II formation. This in situ formation, especially in the kidney, increases Ang II levels to regulate blood pressure and renal functions. These discoveries, such as the ACE2/Ang-(1-7)/Mas axis and its antangonistic effect rather than classical deleterious Ang II effects, improves the development of new drugs for treating hypertension and cardiovascular diseases.
Core tip: Activation of the angiotensin converting enzyme (ACE)/ Angiotensin II (Ang II)/AT1 axis leads to vasoconstriction, anti-diuresis, anti-natriuresis, release of aldosterone and anti-diuretic hormone, which can result in hypertension, renal and cardiovascular diseases. Inhibition of renin and ACE or blocking AT1 receptor is the most used therapies for heart failure and hypertension. Nevertheless, the discovery of local Ang II synthesis, new Ang II metabolites, receptors and axis of this system, makes possible the development of new drugs and strategies for renal and cardiovascular diseases treatment, such as activation of ACE2/Ang-(1-7)/Mas axis, which presents opposite effects of AT1 activation by Ang II.
- Citation: Ferrão FM, Lara LS, Lowe J. Renin-angiotensin system in the kidney: What is new? World J Nephrol 2014; 3(3): 64-76
- URL: https://www.wjgnet.com/2220-6124/full/v3/i3/64.htm
- DOI: https://dx.doi.org/10.5527/wjn.v3.i3.64
The first observation that the arterial blood pressure could be regulated was in 1898 after the discovery of a soluble protein extracted from the kidney that increased blood pressure in rabbits, called “renin”[1]. Over 30 years later, Goldblatt et al[2] associated the decrease of blood pressure in kidneys with hypertension by using a silver clamp to partially constrict dogs renal arteries, resulting in reno-vascular hypertension. Using the same methodology as Goldblatt, Braun-Menendez et al[3] in 1940 isolated a vasoconstrictor substance responsible for the reno-vascular hypertension from renal venous blood of the hypertensive kidney dog, calling it “hypertensin”. Page et al[4] independently described a vasoconstrictor substance by injecting renin into cats, called “angiotonin”. The same group also described angiotensinogen, first referred to as a “renin activator”[4]. The name “angiotensin” for the vasoconstrictor substance “hypertensin” by Braun-Menendez and “angiotonin” by Page emerged in 1958 after they both agreed on this hybrid name, since these 2 substances proved to be the same potent vasoactive octapeptide. The World Health Organization and the American Heart Association in 1987 suggested the abbreviation Ang for angiotensin, numbering the amino acids residues of angiotensin I (Ang I) as a reference for all angiotensin-derived peptides[5]. The decapeptide Ang I has no physiological effect, but is hydrolyzed by angiotensin converting enzyme (ACE) generating angiotensin II (Ang II), which was considered the only peptide in renin-angiotensin system (RAS) with biological actions[6].
More than a century since the discovery of renin by Robert Tigerstedt and Bergman[1], the RAS, remains a fascinating subject for research. Although it is well known the distinct roles of RAS in different tissues, such as brain, adipose tissue, gastrointestinal tract and cardiovascular system[7-10], it is on the kidney that Ang II has its main function on regulating body fluid content and blood pressure by alterating Na+ and water homeostasis, intrarenal hemodynamics and glomerular filtration[11,12]. Ang II stimulates anti-diuretic hormone secretion in the pituitary gland with increased water reabsorption in the collecting duct, and also increases aldosterone secretion, a steroid hormone synthesized mainly by the adrenal cortex, and a downstream effector of Ang II that induces sodium reabsorption and concomitant potassium and hydrogen ion excretion by the kidney[13].
Many new findings suggest new properties of this system, with new enzymes, different routes for Ang II formation, new receptors and active Ang II-derived peptides (Table 1). The classical axis ACE/Ang II/AT1 is not the only signaling pathway within RAS, since others such as angiotensin converting enzyme 2 ACE2/Ang-(1-7)/Mas receptor and Angiotensin IV/AT4 indicate new activities for this cascade[14,15]. Besides the inhibition of renin and ACE, and also angiotensin type 1 receptor (AT1) receptor blockade, activation of the ACE2/Ang-(1-7)/Mas axis is a possible alternative target for new drugs, since some protective effects on renal and cardiovascular function have been reported[14,16-18]. Ang II is not the only active peptide of the RAS, there now being physiological properties associated with many Ang II-derived peptides[14,15,19]. Ang II can be hydrolyzed by > 13 “angiotensinases”, proteolytic enzymes such as aminopeptidases, carboxipeptidases, endopeptidases, ACE2 and neprilysin, generating angiotensin III (Ang III), angiotensin IV (Ang IV), angiotensin-(1-7) [Ang-(1-7)], angiotensin-(3-4) [Ang-(3-4)], angiotensin A (Ang A), and alamandine, which can bind to specific receptors or act on the same receptors as Ang II[14,15,19-22]. Although AT1 and AT2 receptors are the most studied receptors for Ang II, two other receptors - Mas receptor for Ang-(1-7), and AT4 receptor for Ang IV - have been cloned[14,15]. Ang II-derived peptides could have similar effects to Ang II, or counteract its effects on renal function. For instance, like Ang II, Ang-(1-7) can increase intracellular Ca2+via AT1 receptor, but has the opposite effect to Ang II, since it can induce antiproliferative and protective effect through the Mas receptor[23,24]. New functions for well known members of the RAS have been found. For example, ACE, known for its catalytic action on Ang I, also functions as a signal transduction molecule, initiating a series of intracellular events when stimulated[25,26]. Besides increasing catalytic activity of renin and prorenin, the renin/(Pro)renin receptor (PRR), cloned in 2002[27], can also induce an intracellular signaling pathway generating effects in an angiotensin-independent manner[6,27].
| Classic RAS | Recent RAS | |
| Hormone process | Endocrine | Paracrine |
| Autocrine | ||
| Intracrine | ||
| Bioactive peptide | Ang II | Ang II |
| Ang III | ||
| Ang IV | ||
| Ang-(1-7) | ||
| Ang-(3-4) | ||
| Ang A | ||
| Alamandine | ||
| Receptor | AT1 | AT1a |
| AT2 | AT1b | |
| AT2 | ||
| Mas | ||
| Mrg | ||
| AT4 | ||
| PRR | ||
| ACE |
It is now considered that RAS assumes paracrine, autocrine and intracrine mechanisms of action in hormone signaling[6,28]. Many tissues and cells, including kidneys, have all the necessary RAS components to form Ang II in situ[29-31]. Renal levels of Ang II are much higher than in the plasma[32], indicating that the source of Ang II within the kidney is not only provided by filtered plasma Ang II. The kidney expresses all the major components of the RAS, such as angiotensinogen, renin and ACE[29-31]. Locally synthesized Ang II can act on cell surface, nuclear and cytoplasmic AT1 and AT2 receptors[33-35].
We will describe a novel view of the classic RAS that includes new members, routes, receptors, and new drugs and targets for the treatment of heart failure and hypertension. Due to the high Ang II concentration in different compartments of the kidney, and the importance of Ang II effects on renal function in physiological and physiopathological conditions, the focus will be on the intrarenal RAS, especially its paracrine and intracrine functions. This new aspect of RAS will improve our present understanding of RAS and the role of its new members, which should benefit the development of new treatments for hypertension and kidney diseases.
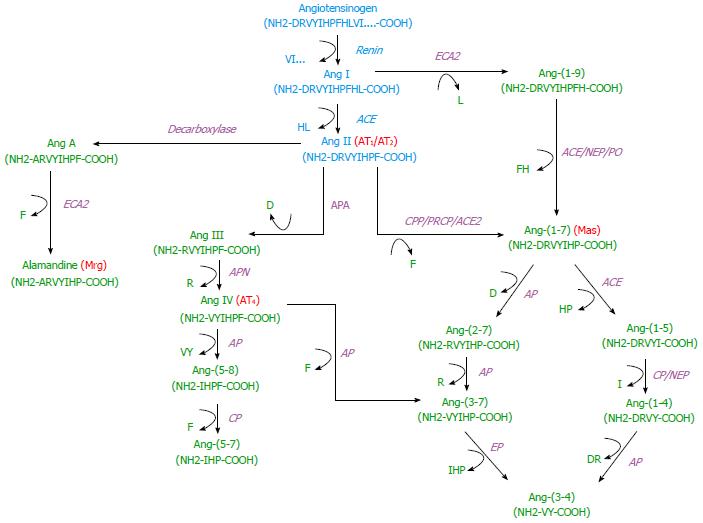
Classically, renin is secreted by juxtaglomerular cells in response to 3 stimuli: (1) decreased arterial blood pressure, detected by baroreceptors; (2) decreased sodium levels in the macula densa ultrafiltrate; and (3) increased sympathetic nervous system activity. Renin is an enzyme with only one known substrate, angiotensinogen. The reaction catalyzed by renin, generating the decapeptide Ang I, is the rate-limiting step in Ang IIformation. Ang I is then converted to Ang II by ACE, a monomeric glycoprotein that acts as an exopeptidase to cleave dipeptides from the C-terminus of Ang I -(1–10) (Asp-Arg-Val-Tyr-Ile-His-Pro-Phe-His-Leu) into the octapeptide Ang II-(1-8)[36] (Figure 1). The main Ang II effects are mediated by the AT1 receptor, such as vasoconstriction, anti-diuresis, anti-natriuresis, release of aldosterone and anti-diuretic hormone, whereas AT2 activation counterbalances these effects[19,36,37].
It is widely accepted that small peptides derived from Ang II have local physiological effects, especially in the kidney (Figure 1). ACE2 is a transmembrane glycoprotein that shares a 42% of homology with ACE and contains a single active site domain more closely to the N domain of ACE[16,38]. Unlike ACE, ACE2 is a monocarboxypeptidase, generating Ang-(1-7) by the cleavage of a single Phe residue from Ang II, and Ang-(1-9), removing the C-terminal Leu residue from Ang I [16,38]. Within the renal brush-border vesicles of the rat, Ang-(1-7) is preferentially hydrolyzed by aminopeptidases and neprylisin (NEP) after aminopeptidase blockade, generating Ang-(1-4)[39]. In the basolateral membrane, brush-border vesicles of the pig and purified preparations of renal NEP Ang I is hydrolyzed primarily to Ang-(1-7) and Ang-(1-4)[40,41]. In sheep proximal tubules, urine and serum, Ang II is converted to Ang-(1-7) by both membrane-bound and soluble forms of ACE2[38].
The physiological importance of Ang-(1-7) has become increasingly evident, especially after Santos et al[14] found a G protein-coupled receptor for Ang-(1-7), the Mas receptor, using a selective Ang-(1-7) antagonist. The Mas protooncogene was cloned and sequenced in 1986, after being detected by its tumorigenicity in mice[42]. This gene encodes a protein with 7 hydrophobic transmembrane domains, first considered as an ‘‘orphan’’ G protein-coupled receptor[43]. Ang-(1-7) exerts many effects on renal function, such as diuresis and natriureses, and it can be detected in human urine[44]. This peptide is of importance during late gestation in rats, where RAS overactivity is associated with increased kidney and urine levels of Ang-(1-7) and enhanced kidney immunostaining of Ang-(1-7) and ACE2[45].
Diuretic/natriuretic effects of Ang-(1-7) may also be due to the regulation of Na+ reabsorption within the proximal tubule. In vivo and in vitro studies showed that Ang-(1-7) is a potent inhibitor of Na+ reabsorption in this nephron segment, acting on different receptors[46-49]. Ang-(1-7) can bind to distinct receptors and induces different cellular responses depending on the cell type. For instance, in distal tubule cell (MDCK), Ang-(1-7) inhibits (Na+ + K+)-ATPase activity through the AT1 receptor to stimulate the PI-PLC/PKC signaling pathway[47], whereas in the proximal tubule, it inhibits Na+-ATPase via the AT2/G(i/o) protein/cGMP/PKG pathway[48]. Moreover, at different concentrations of Ang-(1-7) (10-12, 10-9, or 10-6 mol/L) used in intratubular perfusion in the absence or presence of the Mas receptor antagonist (A779) of rat isolated proximal tubules, it was shown that Ang-(1-7) has a biphasic dose-dependent effect on the Na+/H+ exchanger mediated by Mas receptor and gave a moderate increase in intracellular Ca2+ levels ([Ca2+]i)[49]. Increased [Ca2+]i stimulated by Ang-(1-7) also occurred in MDCK cells, but through the AT1 receptor, which in turn stimulated Ca2+ release from endoplasmic reticulum via the PLC pathway and Ca2+ influx through PLA2-dependent store-operated Ca2+ entry[24]. In this way, ACE2/Ang-(1-7)/Mas axis can counteract most of the deleterious effects of ACE/Ang II/AT1. It has been corroborated that acute intravenous infusion of Ang-(1-7) induces diuresis, natriuresis and renal vasodilatation[50].
Like to Ang-(1-7), there is another heptapeptide derived from Ang II having the opposite effect to Ang II, namely Ang-(2-8), also known as Ang III. Ang II can be hydrolyzed by aminopeptidase A, generating Ang III[51] (Figure 1). Heretofore there has been no evidence of a specific receptor for Ang III, and Ang III normally binds to AT1 with greater affinity than to the AT2 receptor inducing natriuresis on rats[52,53]. Intrarenal Ang III induces natriuresis via the AT2 receptor in the proximal tubule by a cGMP-dependent mechanism[51].
Ang III can be hydrolyzed by aminopeptidase N generating Ang-(3-8), also called Ang IV, which can be also generated directly from Ang II by D-aminopeptidase[20,54]. The receptor for Ang IV, AT4, was initially detected in the guinea pig hippocampus[15]. Protein purification and peptide sequencing showed that the AT4 receptor is an insulin-regulated aminopeptidase[54]. AT4 receptor is also found in the kidney, where this angiotensin-derived fragment can elicit many responses[55]. Aminopeptidases A and N are abundant in the kidney, especially in proximal nephron, and Ang IV is formed in the glomerulus[56,57]. Ang IV increases blood flow in the kidney and decreases in Na+ transport in proximal tubules[55]. Ang IV induces Ca2+ mobilization in human proximal tubule cells[58] through the AT1 receptor. In AT4 knockout (-/-) mice, Ang IV mediated its renal vasoconstrictor effects through AT1a receptors[59].
Ang II can also be hydrolyzed to dipeptides that are biological active, and we have found an alternative pathway for Ang-(1-7) formation from Ang II by carboxypeptidase N, and posterior generation of Ang-(3-4) with Ang-(1-5) and Ang-(1-4) as intermediate peptides[20] (Figure 1), using isolated basolateral membranes from sheep proximal tubules and different peptidase inhibitors. Ang-(3-4) could counteract inhibition of plasma membrane Ca2+-ATPase promoted by nanomolar concentrations of Ang II through conformational changes in the AT2 receptor and the cAMP/PKA pathway[19,20,37].
Ang (3-4) is remarkably stable in human blood serum and has antihypertensive effects in spontaneously hypertensive rats (SHR)[60,61]. Dias et al[62] showed that oral administration of Ang-(3-4) inhibited Na+-ATPase activity in membranes of SHR and blocked the stimulation of Na+-ATPase induced by Ang II in normotensive rats via the AT2 receptor and the PKA signaling pathway. This effect leads to increased urinary Na+ concentration, and simultaneous decrease in systolic arterial blood pressure in SHR, but not in normotensive rats[62].
The presence of another angiotensin derived fragment, known as Ang A (Ala-Arg-Val-Tyr-Ile-His-Pro-Phe), occurs in the plasma of healthy humans and in high levels in end-stage patients with renal failure[21,63]. Decarboxylation of Asp1 of Ang II, in the presence of mononuclear leukocytes leads to Ang A generation, which has higher affinity for AT2 than Ang II and the same affinity for the AT1 receptor[21,63]. As the other Ang II-derived peptides, Ang A exerts its effects on the kidney, inducing renal vasoconstrictor responses in normotensive and hypertensive rats, and also in genetically modified mice[64]. Ang A can also be hydrolyzed by ECA2 in rats, mice and humans generating the heptapeptide alamandine (Ala-Arg-Val-Tyr-Ile-His-Pro), a novel peptide of the RAS[22]. Alamandine has long-term antihypertensive effect in SHRs and antifibrotic effects in isoproterenol-treated rats via the Mas-related G-protein-coupled receptor, member D (MrgD), and independent of Mas and AT2 receptor, the known vasodilator receptors of the RAS, since it is blocked by D-Pro7-angiotensin-(1-7) and PD123319, but not by the Mas antagonist A-779[22]. Most members of Mas-related G-protein-coupled receptor (Mrg), a novel class of RAS-related receptor, are orphan, with no identified endogenous ligand, but MrgD has been identified as a binding site for alamandine[22].
Classically, there are 2 well described Ang II receptors, AT1 and AT2 receptors. However, newer work on RAS and its effects shows that there are novel members of this system.
Besides the newly described Ang II-derived peptides and their corresponding receptors, there are enzyme members of RAS whose actions depend on interaction with receptors. Nguyen et al[27] in 2002 cloned the PRR, which contains a specific binding site for renin and its inactive precursor, prorenin; this interaction stimulates their catalytic activity, increasing RAS activation. Prorenin has a “handle” region that binds to the receptor with a 3-4 fold higher affinity than renin and is important in enzymatic activation of prorenin[65].
After binding, renin and prorenin can also act as agonists to its receptor, generating effects in an Ang II-independent manner. In the human kidney, PRR is expressed in glomerular mesangial cells, the subendothelium of renal arteries[27], in the distal nephron[66], collecting ducts, and mostly at the apical surface of intercalated cells, where, due to its high expression it stimulates cyclooxygenase-2 (COX-2)-derived prostaglandins to attenuate the anti-natriuretic and vasopressor effects of Ang II[67].
However, activation of PRR in kidney is also associated with many pathological conditions. Activation of human PRR and MAPK through an Ang II-independent mechanism contributes to the development of nephropathy in prorenin/renin transgenic rats overexpressing the human receptor[68]. PRR is important through the same signaling pathway in diabetic nephropathy by its activation of glomerular ERK. These studies used an AT1a receptor-deficient mice[69] and db/db mice to show that the receptor-bound prorenin leads to the development of nephropathy in type 2 diabetes[70]. In HEK cells, renin and prorenin activate its receptor to promote fibrosis in an Ang II-independent manner[71].
Kohlstedt et al[25] in 2004 revealed another unexpected function of the RAS enzymes. Human ACE, usually known by its catalytic action on Ang I in generating Ang II, could also function as an outside-in signal transduction molecule. Binding of ACE substrates or inhibitors to this enzyme can stimulate intracellular signaling pathways: ACE inhibitors (perindoprilat and ramiprilat), like the ACE substrate (bradykinin), could also increase COX-2 expression, ACE phosphorylation at Ser1270 and activation of JNK in endothelial cells[25]. The modulation of gene expression in endothelial cell by ACE inhibitors and JNK/c-Jun pathway requires ACE dimerization through the C domain of the enzyme[26]. This indicates that, although ACE is not a cell surface receptor, it is involved in cell functions. Nevertheless, whether ACE works only as a catalytic enzyme or as a signaling molecule in the kidney remains to be elucidated.
A newly recognized view of RAS assumes that Ang IIacts beyond cell surface receptors, with endocrine and paracrine action of RAS. Ang II also acts through intracellular receptors. Local RAS was first described within the kidney over 20 years ago[29-32], where the levels of Ang II are much higher than in plasma[32,72]. Intrarenal Ang II levels and local formation in the kidney have been reported by Navar and colleagues[11,32,73-76].
In addition to Ang II synthesis in the kidney, there are other well-described mechanisms that play a critical role in high renal Ang II levels, and these occur after Ang II endocytosis with the AT1 receptor[77,78]. Since AT1 receptors are expressed in different parts of the kidney, such as in the mesangial cells, afferent and efferent arterioles, glomerular podocytes, macula densa and both basolateral and luminal membranes of different nephron segments[79,80], intracellular Ang II accumulation by coupled-receptor internalization is one of main sources of renal Ang II accumulation.
In Ang II-dependent hypertension several groups have shown that Ang II can positively amplify it, leading to its high intrarenal levels. Zhuo et al[77] showed increased intracellular Ang II levels in cortical endosomes, and Ang II-infused hypertensive rats mediated by AT1 receptors. Ang II-infused rats through an osmotic minipump also had increased Ang II levels in renal interstitial fluid, which is mediated by the AT1 receptor[81]. Ang II endocytosis with AT1 receptor has been confirmed by the absence of renal Ang II accumulation in AT1a receptor-deficient mice (Agtr1a-/-)[82,83]. Another possible pathway for increasing the intrarenal Ang II level is due to endogenous Ang II production, via markedly augmentation on angiotensinogen[11,84] and renin expression in collecting ducts[85,86], the secretion of renin and prorenin by these cells into the luminal fluid, leading to its increased urinary levels in Ang II-infused hypertensive rats[87]. These results indicating a positive feedback by Ang II in the kidney contradict the well-established view that Ang II has a negative feedback mechanism in the expression and activity in the RAS, where high Ang II levels suppress the release of renin in juxtaglomerular cells and Ang II production in the kidney[88], demonstrating the complexity of the system.
Both Ang II receptors (AT1 and AT2) are expressed in adult kidneys, although AT2 receptor is less expressed than AT1 receptor[79]. This intensely local synthesis of high renal levels of Ang II, and the wide expression of Ang II receptors within the kidney, provides evidence of the pivotal role of Ang II in renal physiology, regulating water and solute reabsorption and renal hemodynamic processes that contribute to Na+ balance and blood pressure regulation. AT1 receptors in the kidney are responsible for the development of hypertension[89-91]. And AT1 receptors within the kidney are necessary for cardiac hypertrophy and hypertension[90,92].
Ang II has many effects on different parts of the kidney. As in the systemic circulation, intrarenal Ang II also is important in renal hemodynamics. Thereby, long-term treatment with Ang II receptor blockers induced unusual proliferative changes in afferent arteriolar smooth muscle cells, narrowing arteriolar lumens and reducing glomerular pressure[93]. Administration of Ang IIthrough an osmotic minipump in hypertensive rats leads to marked suppression of Na+ excretion as well as renal and medullary blood flow[94]. Peritubular capillary Ang II infusion enhanced proximal tubular reabsorption and reduced single nephron glomerular filtration rate in rats[95].
Different targets and signaling pathways regulate Na+ balance within the kidney; rats infused with Ang II showed enhanced ENaC expression[96] and activation of the renal Na+:Cl- cotransporter[97,98]. In vitro studies using isolated basolateral membrane fractions from pig kidney have demonstrated that Ang II stimulates the renal proximal tubule Na+-ATPase activity via PI-PLCβ/PKC pathway[99,100].
It is widely known that intracellular Ca2+ mobilization in proximal tubule cells leads to the activation of many Ca2+-dependent intracellular signaling pathways, including those associated with Na+ reabsorption[101]. Ang II microperfusion techniques in rabbit superficial segment of proximal tubules in vitro regulated Na+ reabsorption via PKC and intracellular Ca2+[102]; low concentrations of Ang II inhibited membrane Ca2+-ATPase via AT1/AT2 receptors heterodimers and PKC in isolated fractions of basolateral membranes of proximal tubule, increasing cytosolic Ca2+ concentration in proximal tubule cells[37,103]. Luminal Ang II stimulates AT1/AT2 receptors heterodimerization that increases sarco/endoplasmic reticulum Ca2+-ATPase activity and promotes Ca2+ mobilization in proximal tubule cells[101].
The intracrine/intracellular system is new paradigm. Cells that express all the necessary components for synthesis can generate Ang II internally[28,29]. Ang II can be secreted and exert autocrine effects, or remain inside the cell and have its effects[6,35]. An alternative way for the intracellular source of Ang II is the internalization of extracellular Ang II after binding to the AT1 surface receptor[82,83]. Not all internalized Ang II-AT1 complex is degraded in lysosomes, thereby increasing its concentration within the cell, and the AT1 receptor may be relocated to other organelles, including the nucleus[101,104-108]. Indeed, subcellular localization of 125I-labeled Ang II in the pig kidney indicates that Ang II generation is predominantly extracellular, followed by AT1 receptor-mediated endocytosis leading to higher intracellular Ang II levels[109]. In accord with this, internalization is seen to be important for AT1a receptor function in polarized proximal tubule epithelial cells, where apical AT1a receptor internalize before interaction with G proteins, which stimulates phospholipase C and cAMP to increase proximal tubule Na+ reabsorption[110,111].
Within the kidney, cells from different segments can generate Ang II or internalize Ang II through the AT1 receptor[109-111]. In vitro and in vivo studies showed that extracellular Ang II accumulates within the kidney via AT1a receptor-mediated endocytosis[82,83,107]. Although many have demonstrated different Ang II intracellular effects, the precise role of intracellular Ang II in nephron segments remains poorly understood. Renal intracellular Ang II increases blood pressure and decreases 24 h urinary Na+ excretion in rats and mices[89,105], suggesting that, like intrarenal Ang II, intracellular Ang II within the kidney also increases Na+ reabsorption and blood pressure.
Endocytosis of Ang II through the AT1 receptor within proximal tubule cells occurs through 2 main pathways: the clathrin-dependent and the microtubule-associated pathway[106]. The canonical clathrin-dependent endocytosis pathway for Ang II occurs in different cell types, such as vascular smooth muscle and human embryonic kidney (HEK-293) cells through the AT1 receptor, c-Src and clathrin Adapter Protein 2[112]. In rabbit proximal tubule cells, the alternative microtubule-associated endocytic pathway rather than the clathrin-dependent pathway participates in the AT1 receptor-mediated uptake of Ang II[113].
Another alternative endocytic pathway for Ang II internalization in proximal tubule cells has been described by Gonzalez-Villalobos et al[114], where anti-megalin antisera interferes with Ang II binding in cell brush-border membrane vesicles extracted from mice, indicating that Ang II internalization is a megalin-dependent process.
Angiotensin receptors are present in the intracellular organelles, including the sarco/endoplasmic reticulum, Golgi apparatus and the nucleus, indicating that Ang II can have many intracellular effects, including modulation of gene expression[33-35]. Proximal tubule cells express angiotensinogen, renin, and ACE mRNAs, suggesting high levels of intracellular Ang II[28,32,73]. Thus, microinjection of Ang II directly in single rabbit proximal tubule cells increased intracellular Ca2+ mobilization through its intracellular AT1 receptors and Ca2+ release from intracellular stores[115]. Ang II induced transcriptional responses of mRNAs for MCP-1, NHE-3 and TGF-β1 stimulating the AT1a receptor in freshly isolated intact rat renal cortical nuclei, indicating that internalized and/or intracellular Ang II acts on nuclear receptors to mediate growth, proinflammatory responses and Na+-retaining effects[108]. Furthermore, in isolated nuclei from kidney cortex from sheep in the absence of cytoplasm, all RAS components (angiotensinogen, ACE and renin) have been identified[116], showing that Ang II can indeed be synthesized within the nucleus.
Another interesting role for intracellular Ang II is encountered in pathological situations. It is thought that intracellular Ang II levels could be altered in different diseases, such as diabetic nephropathy and cardiomyopathy, where hyperglycemia might induce intracellular Ang II production. Indeed, a high glucose concentration induced an increase of ACE mRNA, synthesis and secretion of renin and Ang II in an immortalized murine mesangial cell line[117-119]. Interestingly, an alternative pathway was found for the synthesis of intracellular Ang II in the presence of high glucose in vascular smooth muscle cells. Under normal glucose levels, Ang II is generated by cathepsin D and ACE; however, Ang II is obtained by cathepsin D and chymase action in the presence of high glucose[120,121].
RAS is important in the development of hypertension and cardiovascular diseases[1-4,90]; one of the most common treatments for these diseases is pharmacological inhibition of enzymes and blockade receptors of RAS[122]. Inhibition of renin, the enzyme that initiates RAS, presents a strategy for hypertension therapy (Table 2). Aliskiren is a more selective and potent inhibitor of human renin than other orally active renin inhibitors, remikiren and enalkiren[123]; it can block the generation of active renin in both normotensive and hypertensive human subjects[124]. Aliskiren is as effective as losartan, valsartan and ibesartan (AT1 receptor blockers), atenolol (β blocker) and amlodipine (Ca2+ channel blocker), and has an antihypertensive effect comparable to other major classes of antihypertensive drugs[124,125]. Besides decreasing blood pressure, aliskiren is also renoprotective in diabetic and nondiabetic models of chronic kidney disease, preventing albuminuria in rats[126]. In humans, aliskiren significantly decreases blood pressure, and also the urinary albumin and creatinine ratio in 15 patients with type 2 diabetes mellitus[127].
| Target | Drug | Therapy | Clinical use |
| Renin | Aliskiren | HTN, RF | + |
| Remikiren, enalkiren | HTN | + | |
| ACE | Captopril, lisinopril, trandolapril | HTN, HF, LVD, DN | + |
| Enalapril, enalaprilat, fosinopril, ramipril | HTN, HF | + | |
| Moexipril, quinapril, perindopril, benazepril | HTN | + | |
| AT1 | Losartan, azilsartan, valsartan, ibesartan, candesartan, telmisartan, eprosartan, omesartan | HTN, HF | + |
| Mas | AVE 0991 | HTN | - |
| Ang-(1-7)-CyD | HF | - | |
| ACE2 | Xanthenone | HTN, RF, HF | - |
ACE is another enzyme of the RAS that can be pharmacology inhibited so as to decrease hypertension (Table 2). A total of 17 small orally active ACE inhibitors have recently been synthesized for clinical use, all binding to the active site of the enzyme and interfering with ACE’s ability to bind and cleave its substrates (Ang I and bradykinin, among others)[128,129]. Many ACE inhibitors were approved for hypertension treatment, heart failure and left ventricular dysfunction (e.g., captopril, lisinopril, trandolapril), as also captopril for diabetic nephropathy[129].
Ang II promotes cardiovascular disorders and hypertension via the AT1 receptor, which can be blocked to treat these pathological conditions (Table 2). A total of 8 non-peptide angiotensin-receptor blockers (ARBs) orally active are used clinically for hypertension and cardiovascular diseases (namely losartan, azilsartan, valsartan, ibesartan, candesartan, telmisartan, eprosartan, omesartan), which are all well-tolerated[129,130]. Telmisartan seems more efficacious in decreasing blood pressure than the other ARBs[131,132].
Many patients with hypertension require combination regimens to achieve a significant decrease in blood pressure. In this case, the most commonly used drugs are ARBs and ACE inhibitors, Ca2+ channel blockers (CCB) and diuretics[130]. Long-term treatment triple therapy with olmesartan medoxomil (ARB), amlodipine besylate (CCB) and hydrochlorothiazide (diuretic) in 2112 hypertensive patients with moderate to severe hypertension resulted in 44.5%-79.8% of participants having a decreased the mean blood pressure from 168.6/100.7 mm Hg to 125.0-136.8/77.8-82.5 mmHg, reaching the blood pressure goal[133]. The same triple therapy also proved to be efficient in hypertensive Hispanic/Latin patients[134].
However, even with the successful results obtained by inhibiting the enzymes and receptors of the RAS, many patients do not respond as expected, and cardiovascular disease risks have not decreased to those in normotensive people. Due to the high death rates by heart diseases in the world, which are higher than from many cancers[135], it is important to devise new strategies for the treatment of cardiovascular diseases and hypertension.
Because of the discovery of new components in the RAS that have herein been described, novel Ang II-derived peptides have emerged as excellent target for heart diseases. Since the ACE2/Ang-(1-7)/Mas axis has an opposite and protective effect from the deleterious ACE/Ang II/AT1 axis, it is now the main target for these drugs[14]. Besides inhibiting ACE activity and blocking AT1 receptors responsible for the inhibition of ACE/Ang II/AT1 axis, activation of the ACE2/Ang-(1-7)/Mas axis is a promising alternative means for the treatment of the heart diseases. Nevertheless, this new strategy presents certain problems. First, as a peptide, Ang-(1-7) is proteolytically degraded in the gastrointestinal tract[18]; and second, Ang-(1-7) has a short half-life, complicating its use as an oral pharmacotherapy for hypertension and cardiovascular disease.
The difficulty was overcome after the synthesis of the first nonpeptide compound able to mimic Ang-(1-7) and bind selectively to the Mas receptor[136], the AVE 0991 5-formyl-4-methoxy-2-phenyl-1-{[4-(2-ethyl-aminocarbonylsulfonamido-5-isobutyl-3-thienyl)-phenyl]-methyl}-imidazole (Table 2)[137]. Although this molecule is an antihypertensive candidate because it stimulates NO release in endothelial cells[137], promotes vasorelaxation in mouse and rat aortic rings[138], and attenuates hypertension in SHR[139], clinical trials are needed to see its effects in humans.
Another important achievement has been the inclusion of the heptapeptide in hydroxypropyl-β-cyclodextrin [Ang-(1-7)-CyD], avoiding its proteolytic degradation in the gastrointestinal tract and permitting its oral administration (Table 2)[18]. Cyclodextrins are amphiphilic oligosaccharides that increase drug stability and absorption[140]; after oral administration, they are split up into small saccharides in the colon, leaving Ang-(1-7) to be absorbed[18]. Chronic oral administration of Ang-(1-7)-CyD in isoproterenol-treated rats increases plasma Ang-(1-7) levels, with attenuation of myocardial infarction associated with cardioprotective effects[141].
Another option for the treatment of the deleterious effects of Ang II is activation of ACE2, which, besides increasing Ang II degradation, enhances Ang-(1-7) production; ACE2 activators are an alternative source for controlling hypertension (Table 2). Acute intravenous administration of xanthenone (XNT), which interact with ACE2 in specific sites, promotes conformational changes and increases ACE2 activity. Consequently, it decreases blood pressure, improves cardiac function and decreases renal fibrosis in SHR[142]. It also has antihypertensive effects in rats with pulmonary hypertension[143].
These results together suggest that, besides inhibition of renin and ACE, associated or not with the blocking of AT1 receptor, activation of the ACE2/Ang-(1-7)/Mas axis and its protective effects is emerging as an excellent alternative therapy for the treatment of hypertension and cardiovascular diseases.
The data presented herein show that RAS has passed from being simply an endocrine system to one with paracrine, autocrine and intracrine functions, increasing Ang II concentration in different tissues including the kidney. After years of research, the RAS - previously seen as a simple system with only 2 receptors (AT1 and AT2), and one active peptide (Ang II), turns out to be a complex system, with many new members continuing to be described. In addition to (ACE)/Ang II/AT1 and AT2 axis, other signaling pathways in the RAS, such as ACE2/angiotensin-(1-7)/Mas and Ang IV/AT4, and other active peptide of the RAS, with physiological relevance as Ang III, Ang-(3-4), Ang A and alamandine, are now widely recognized. These newly discovered fragments derived from Ang II can act on the same classic Ang II receptors, AT1 and AT2, or on specific receptors (Mas and AT4) having the same or the opposite effects of Ang II depending on the triggered signaling pathway, in the kidney and other tissues, with many roles seen in physiological and physiopathological conditions. The discovery of renin and prorenin as agonists of PRR receptor, stimulating intracellular pathways and having effects on different cells types in an Ang II-independent manner, raised another axis for this system, namely the prorenin/PRR/MAPK ERK1/2 axis.
Finally, activation of the new ACE2/Ang-(1-7)/Mas axis with opposite and protective effects, compared with ACE/Ang II/AT1 axis, with different drugs such as AVE 0991, the nonpeptide compound mimicking Ang-(1-7) effects, the Ang-(1-7)-CyD, and the XNT, the activator of ACE2 activity, now leading to improved and greater fall in blood pressure creates new possibilities for patients who do not respond as expected to conventional antihypertensive drugs.
A thorough understanding of RAS and all the new possibilities described on this review will certainly contribute to the development of pharmacological approaches, discovery of new drugs and alternative treatments for hypertension, cardiovascular and kidney diseases.
We express our appreciation for the support given by our laboratory colleagues and we especially acknowledge BioMedES for the English revision of the manuscript.
P- Reviewer: Wong KL S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Tigerstedt R, Bergman PG. Niere und Kreislauf. Scand Arch Physiol. 1898;8:223–271. [DOI] [Cited in This Article: ] [Cited by in Crossref: 678] [Cited by in F6Publishing: 678] [Article Influence: 56.5] [Reference Citation Analysis (0)] |
| 2. | Goldblatt H, Lynch J, Hanzal RF, Summerville WW. Studies on experimental hypertension : i. the production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347-379. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1506] [Cited by in F6Publishing: 1263] [Article Influence: 90.2] [Reference Citation Analysis (0)] |
| 3. | Braun-Menendez E, Fasciolo JC, Leloir LF, Muñoz JM. The substance causing renal hypertension. J Physiol. 1940;98:283-298. [PubMed] [Cited in This Article: ] |
| 4. | Page IH, Helmer OM. A crystalline pressor substance (angiotonin) resulting from the reaction between renin and renin-activator. J Exp Med. 1940;71:29-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 294] [Cited by in F6Publishing: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Dzau VJ, Baxter JA, Cantin M, de Bold A, Ganten D, Gross K, Husain A, Inagami T, Menard J, Poole S. Report of the Joint Nomenclature and Standardization Committee of the International Society of Hypertension, American Heart Association and the World Health Organization. J Hypertens. 1987;5:507-511. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Zhuo JL, Ferrao FM, Zheng Y, Li XC. New frontiers in the intrarenal Renin-Angiotensin system: a critical review of classical and new paradigms. Front Endocrinol (Lausanne). 2013;4:166. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 7. | de Kloet AD, Krause EG, Scott KA, Foster MT, Herman JP, Sakai RR, Seeley RJ, Woods SC. Central angiotensin II has catabolic action at white and brown adipose tissue. Am J Physiol Endocrinol Metab. 2011;301:E1081-E1091. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Garg M, Angus PW, Burrell LM, Herath C, Gibson PR, Lubel JS. Review article: the pathophysiological roles of the renin-angiotensin system in the gastrointestinal tract. Aliment Pharmacol Ther. 2012;35:414-428. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 94] [Cited by in F6Publishing: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 9. | Hilzendeger AM, Morgan DA, Brooks L, Dellsperger D, Liu X, Grobe JL, Rahmouni K, Sigmund CD, Mark AL. A brain leptin-renin angiotensin system interaction in the regulation of sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;303:H197-H206. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Probstfield JL, O’Brien KD. Progression of cardiovascular damage: the role of renin-angiotensin system blockade. Am J Cardiol. 2010;105:10A-20A. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Navar LG, Prieto MC, Satou R, Kobori H. Intrarenal angiotensin II and its contribution to the genesis of chronic hypertension. Curr Opin Pharmacol. 2011;11:180-186. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 121] [Cited by in F6Publishing: 117] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 12. | McDonough AA. Mechanisms of proximal tubule sodium transport regulation that link extracellular fluid volume and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2010;298:R851-R861. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 130] [Cited by in F6Publishing: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 13. | Rossier BC, Pradervand S, Schild L, Hummler E. Epithelial sodium channel and the control of sodium balance: interaction between genetic and environmental factors. Annu Rev Physiol. 2002;64:877-897. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 297] [Cited by in F6Publishing: 275] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 14. | Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, Heringer-Walther S, Pinheiro SV, Lopes MT, Bader M. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci USA. 2003;100:8258-8263. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1319] [Cited by in F6Publishing: 1315] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 15. | Harding JW, Cook VI, Miller-Wing AV, Hanesworth JM, Sardinia MF, Hall KL, Stobb JW, Swanson GN, Coleman JK, Wright JW. Identification of an AII(3-8) [AIV] binding site in guinea pig hippocampus. Brain Res. 1992;583:340-343. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 117] [Cited by in F6Publishing: 118] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Fraga-Silva RA, Ferreira AJ, Dos Santos RA. Opportunities for targeting the angiotensin-converting enzyme 2/angiotensin-(1-7)/mas receptor pathway in hypertension. Curr Hypertens Rep. 2013;15:31-38. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 17. | Botelho-Santos GA, Bader M, Alenina N, Santos RA. Altered regional blood flow distribution in Mas-deficient mice. Ther Adv Cardiovasc Dis. 2012;6:201-211. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 18. | Fraga-Silva RA, Costa-Fraga FP, De Sousa FB, Alenina N, Bader M, Sinisterra RD, Santos RA. An orally active formulation of angiotensin-(1-7) produces an antithrombotic effect. Clinics (Sao Paulo). 2011;66:837-841. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 19. | Axelband F, Dias J, Miranda F, Ferrão FM, Reis RI, Costa-Neto CM, Lara LS, Vieyra A. Angiotensin-(3-4) counteracts the Angiotensin II inhibitory action on renal Ca2+-ATPase through a cAMP/PKA pathway. Regul Pept. 2012;177:27-34. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Axelband F, Dias J, Miranda F, Ferrão FM, Barros NM, Carmona AK, Lara LS, Vieyra A. A scrutiny of the biochemical pathways from Ang II to Ang-(3-4) in renal basolateral membranes. Regul Pept. 2009;158:47-56. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Coutinho DC, Foureaux G, Rodrigues KD, Salles RL, Moraes PL, Murça TM, De Maria ML, Gomes ER, Santos RA, Guatimosim S. Cardiovascular effects of angiotensin A: A novel peptide of the renin-angiotensin system. J Renin Angiotensin Aldosterone Syst. 2013;Feb 5; Epub ahead of print. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Lautner RQ, Villela DC, Fraga-Silva RA, Silva N, Verano-Braga T, Costa-Fraga F, Jankowski J, Jankowski V, Sousa F, Alzamora A. Discovery and characterization of alamandine: a novel component of the renin-angiotensin system. Circ Res. 2013;112:1104-1111. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 252] [Cited by in F6Publishing: 265] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 23. | Sampaio WO, Henrique de Castro C, Santos RA, Schiffrin EL, Touyz RM. Angiotensin-(1-7) counterregulates angiotensin II signaling in human endothelial cells. Hypertension. 2007;50:1093-1098. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 198] [Cited by in F6Publishing: 195] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 24. | Liu CP, Chou CT, Chi CC, Lin KL, Cheng HH, Lu YC, Cheng JS, Kuo CC, Liang WZ, Huang IF. Mechanism of [Ca2+]i rise induced by angiotensin 1-7 in MDCK renal tubular cells. J Recept Signal Transduct Res. 2012;32:335-341. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Kohlstedt K, Brandes RP, Müller-Esterl W, Busse R, Fleming I. Angiotensin-converting enzyme is involved in outside-in signaling in endothelial cells. Circ Res. 2004;94:60-67. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 26. | Kohlstedt K, Gershome C, Friedrich M, Müller-Esterl W, Alhenc-Gelas F, Busse R, Fleming I. Angiotensin-converting enzyme (ACE) dimerization is the initial step in the ACE inhibitor-induced ACE signaling cascade in endothelial cells. Mol Pharmacol. 2006;69:1725-1732. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 27. | Nguyen G, Delarue F, Burcklé C, Bouzhir L, Giller T, Sraer JD. Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest. 2002;109:1417-1427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 959] [Cited by in F6Publishing: 886] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 28. | Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol. 2012;302:R494-R509. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Bruneval P, Hinglais N, Alhenc-Gelas F, Tricottet V, Corvol P, Menard J, Camilleri JP, Bariety J. Angiotensin I converting enzyme in human intestine and kidney. Ultrastructural immunohistochemical localization. Histochemistry. 1986;85:73-80. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 131] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Gomez RA, Lynch KR, Chevalier RL, Wilfong N, Everett A, Carey RM, Peach MJ. Renin and angiotensinogen gene expression in maturing rat kidney. Am J Physiol. 1988;254:F582-F587. [PubMed] [Cited in This Article: ] |
| 31. | Ingelfinger JR, Zuo WM, Fon EA, Ellison KE, Dzau VJ. In situ hybridization evidence for angiotensinogen messenger RNA in the rat proximal tubule. An hypothesis for the intrarenal renin angiotensin system. J Clin Invest. 1990;85:417-423. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 267] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 32. | Navar LG, Lewis L, Hymel A, Braam B, Mitchell KD. Tubular fluid concentrations and kidney contents of angiotensins I and II in anesthetized rats. J Am Soc Nephrol. 1994;5:1153-1158. [PubMed] [Cited in This Article: ] |
| 33. | Cook JL, Mills SJ, Naquin RT, Alam J, Re RN. Cleavage of the angiotensin II type 1 receptor and nuclear accumulation of the cytoplasmic carboxy-terminal fragment. Am J Physiol Cell Physiol. 2007;292:C1313-C1322. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 34. | Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol. 2006;40:696-707. [PubMed] [Cited in This Article: ] |
| 35. | Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol. 2004;36:75-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 45] [Cited by in F6Publishing: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Guang C, Phillips RD, Jiang B, Milani F. Three key proteases--angiotensin-I-converting enzyme (ACE), ACE2 and renin--within and beyond the renin-angiotensin system. Arch Cardiovasc Dis. 2012;105:373-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 37. | Axelband F, Assunção-Miranda I, de Paula IR, Ferrão FM, Dias J, Miranda A, Miranda F, Lara LS, Vieyra A. Ang-(3-4) suppresses inhibition of renal plasma membrane calcium pump by Ang II. Regul Pept. 2009;155:81-90. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Shaltout HA, Westwood BM, Averill DB, Ferrario CM, Figueroa JP, Diz DI, Rose JC, Chappell MC. Angiotensin metabolism in renal proximal tubules, urine, and serum of sheep: evidence for ACE2-dependent processing of angiotensin II. Am J Physiol Renal Physiol. 2007;292:F82-F91. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 105] [Cited by in F6Publishing: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 39. | Allred AJ, Diz DI, Ferrario CM, Chappell MC. Pathways for angiotensin-(1---7) metabolism in pulmonary and renal tissues. Am J Physiol Renal Physiol. 2000;279:F841-F850. [PubMed] [Cited in This Article: ] |
| 40. | Gafford JT, Skidgel RA, Erdös EG, Hersh LB. Human kidney “enkephalinase”, a neutral metalloendopeptidase that cleaves active peptides. Biochemistry. 1983;22:3265-3271. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 223] [Cited by in F6Publishing: 238] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987;241:237-247. [PubMed] [Cited in This Article: ] |
| 42. | Young D, Waitches G, Birchmeier C, Fasano O, Wigler M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell. 1986;45:711-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 341] [Cited by in F6Publishing: 348] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 43. | Zohn IE, Symons M, Chrzanowska-Wodnicka M, Westwick JK, Der CJ. Mas oncogene signaling and transformation require the small GTP-binding protein Rac. Mol Cell Biol. 1998;18:1225-1235. [PubMed] [Cited in This Article: ] |
| 44. | Ferrario CM, Martell N, Yunis C, Flack JM, Chappell MC, Brosnihan KB, Dean RH, Fernandez A, Novikov SV, Pinillas C. Characterization of angiotensin-(1-7) in the urine of normal and essential hypertensive subjects. Am J Hypertens. 1998;11:137-146. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 81] [Cited by in F6Publishing: 85] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 45. | Joyner J, Neves LA, Granger JP, Alexander BT, Merrill DC, Chappell MC, Ferrario CM, Davis WP, Brosnihan KB. Temporal-spatial expression of ANG-(1-7) and angiotensin-converting enzyme 2 in the kidney of normal and hypertensive pregnant rats. Am J Physiol Regul Integr Comp Physiol. 2007;293:R169-R177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 46. | De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, Abi-Abib R, Fernandes MS, Santos DP, Caruso-Neves C. Angiotensin II and angiotensin-(1-7) inhibit the inner cortex Na+ -ATPase activity through AT2 receptor. Regul Pept. 2004;120:167-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 59] [Cited by in F6Publishing: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 47. | Lara LS, De Carvalho T, Leão-Ferreira LR, Lopes AG, Caruso-Neves C. Modulation of the (Na(+)+K+)ATPase activity by Angiotensin-(1-7) in MDCK cells. Regul Pept. 2005;129:221-226. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 48. | Lara Lda S, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by Ang-(1-7). Biochem J. 2006;395:183-190. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 60] [Cited by in F6Publishing: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 49. | Castelo-Branco RC, Leite-Delova DC, de Mello-Aires M. Dose-dependent effects of angiotensin-(1-7) on the NHE3 exchanger and [Ca(2+)](i) in in vivo proximal tubules. Am J Physiol Renal Physiol. 2013;304:F1258-F1265. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 50. | Ferrario CM, Varagic J. The ANG-(1-7)/ACE2/mas axis in the regulation of nephron function. Am J Physiol Renal Physiol. 2010;298:F1297-F1305. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 106] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 51. | Kemp BA, Bell JF, Rottkamp DM, Howell NL, Shao W, Navar LG, Padia SH, Carey RM. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60:387-395. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 52. | Padia SH, Kemp BA, Howell NL, Siragy HM, Fournie-Zaluski MC, Roques BP, Carey RM. Intrarenal aminopeptidase N inhibition augments natriuretic responses to angiotensin III in angiotensin type 1 receptor-blocked rats. Hypertension. 2007;49:625-630. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 53. | Padia SH, Howell NL, Siragy HM, Carey RM. Renal angiotensin type 2 receptors mediate natriuresis via angiotensin III in the angiotensin II type 1 receptor-blocked rat. Hypertension. 2006;47:537-544. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 114] [Cited by in F6Publishing: 116] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 54. | Albiston AL, McDowall SG, Matsacos D, Sim P, Clune E, Mustafa T, Lee J, Mendelsohn FA, Simpson RJ, Connolly LM. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J Biol Chem. 2001;276:48623-48626. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 353] [Cited by in F6Publishing: 336] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 55. | Chai SY, Fernando R, Peck G, Ye SY, Mendelsohn FA, Jenkins TA, Albiston AL. The angiotensin IV/AT4 receptor. Cell Mol Life Sci. 2004;61:2728-2737. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 168] [Cited by in F6Publishing: 162] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 56. | Pullman TN, Oparil S, Carone FA. Fate of labeled angiotensin II microinfused into individual nephrons in the rat. Am J Physiol. 1975;228:747-751. [PubMed] [Cited in This Article: ] |
| 57. | Ardaillou R, Chansel D. Synthesis and effects of active fragments of angiotensin II. Kidney Int. 1997;52:1458-1468. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 108] [Cited by in F6Publishing: 102] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 58. | Handa RK. Characterization and signaling of the AT(4) receptor in human proximal tubule epithelial (HK-2) cells. J Am Soc Nephrol. 2001;12:440-449. [PubMed] [Cited in This Article: ] |
| 59. | Yang R, Walther T, Gembardt F, Smolders I, Vanderheyden P, Albiston AL, Chai SY, Dupont AG. Renal vasoconstrictor and pressor responses to angiotensin IV in mice are AT1a-receptor mediated. J Hypertens. 2010;28:487-494. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 24] [Cited by in F6Publishing: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 60. | Matsufuji H, Matsui T, Ohshige S, Kawasaki T, Osajima K, Osajima Y. Antihypertensive effects of angiotensin fragments in SHR. Biosci Biotechnol Biochem. 1995;59:1398-1401. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 55] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 61. | Pentzien AK, Meisel H. Transepithelial transport and stability in blood serum of angiotensin-I-converting enzyme inhibitory dipeptides. Z Naturforsch C. 2008;63:451-459. [PubMed] [Cited in This Article: ] |
| 62. | Dias J, Ferrão FM, Axelband F, Carmona AK, Lara LS, Vieyra A. ANG-(3-4) inhibits renal Na+-ATPase in hypertensive rats through a mechanism that involves dissociation of ANG II receptors, heterodimers, and PKA. Am J Physiol Renal Physiol. 2014;306:F855-F863. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Wan Y, Wallinder C, Plouffe B, Beaudry H, Mahalingam AK, Wu X, Johansson B, Holm M, Botoros M, Karlén A. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem. 2004;47:5995-6008. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 271] [Cited by in F6Publishing: 268] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 64. | Yang R, Smolders I, Vanderheyden P, Demaegdt H, Van Eeckhaut A, Vauquelin G, Lukaszuk A, Tourwé D, Chai SY, Albiston AL. Pressor and renal hemodynamic effects of the novel angiotensin A peptide are angiotensin II type 1A receptor dependent. Hypertension. 2011;57:956-964. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Uddin MN, Nabi AH, Nakagawa T, Ichihara A, Inagami T, Suzuki F. Non-proteolytic activation of prorenin: activation by (pro)renin receptor and its inhibition by a prorenin prosegment, “decoy peptide”. Front Biosci. 2008;13:745-753. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 9] [Cited by in F6Publishing: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 66. | Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K, Connelly KA, Yuen D, Trogadis J, Herzenberg AM. The (Pro)renin receptor: site-specific and functional linkage to the vacuolar H+-ATPase in the kidney. Hypertension. 2009;54:261-269. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 198] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 67. | Gonzalez AA, Luffman C, Bourgeois CR, Vio CP, Prieto MC. Angiotensin II-independent upregulation of cyclooxygenase-2 by activation of the (Pro)renin receptor in rat renal inner medullary cells. Hypertension. 2013;61:443-449. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 51] [Cited by in F6Publishing: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 68. | Kaneshiro Y, Ichihara A, Sakoda M, Takemitsu T, Nabi AH, Uddin MN, Nakagawa T, Nishiyama A, Suzuki F, Inagami T. Slowly progressive, angiotensin II-independent glomerulosclerosis in human (pro)renin receptor-transgenic rats. J Am Soc Nephrol. 2007;18:1789-1795. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 185] [Cited by in F6Publishing: 196] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 69. | Ichihara A, Suzuki F, Nakagawa T, Kaneshiro Y, Takemitsu T, Sakoda M, Nabi AH, Nishiyama A, Sugaya T, Hayashi M. Prorenin receptor blockade inhibits development of glomerulosclerosis in diabetic angiotensin II type 1a receptor-deficient mice. J Am Soc Nephrol. 2006;17:1950-1961. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 212] [Cited by in F6Publishing: 209] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 70. | Ichihara A, Sakoda M, Kurauchi-Mito A, Nishiyama A, Itoh H. Involvement of receptor-bound prorenin in development of nephropathy in diabetic db/db mice. J Am Soc Hypertens. 2008;2:332-340. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 71. | Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol Renal Physiol. 2011;300:F1310-F1318. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 32] [Cited by in F6Publishing: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Seikaly MG, Arant BS, Seney FD. Endogenous angiotensin concentrations in specific intrarenal fluid compartments of the rat. J Clin Invest. 1990;86:1352-1357. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 320] [Cited by in F6Publishing: 336] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 73. | Navar LG, Nishiyama A. Why are angiotensin concentrations so high in the kidney? Curr Opin Nephrol Hypertens. 2004;13:107-115. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 88] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol. 1997;17:412-422. [PubMed] [Cited in This Article: ] |
| 75. | Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II stimulates renin in inner medullary collecting duct cells via protein kinase C and independent of epithelial sodium channel and mineralocorticoid receptor activity. Hypertension. 2011;57:594-599. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 76. | Lara LS, McCormack M, Semprum-Prieto LC, Shenouda S, Majid DS, Kobori H, Navar LG, Prieto MC. AT1 receptor-mediated augmentation of angiotensinogen, oxidative stress, and inflammation in ANG II-salt hypertension. Am J Physiol Renal Physiol. 2012;302:F85-F94. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 61] [Cited by in F6Publishing: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 77. | Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT(1) receptor. Hypertension. 2002;39:116-121. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 122] [Cited by in F6Publishing: 135] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Zou LX, Imig JD, Hymel A, Navar LG. Renal uptake of circulating angiotensin II in Val5-angiotensin II infused rats is mediated by AT1 receptor. Am J Hypertens. 1998;11:570-578. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 82] [Cited by in F6Publishing: 86] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 79. | Miyata N, Park F, Li XF, Cowley AW. Distribution of angiotensin AT1 and AT2 receptor subtypes in the rat kidney. Am J Physiol. 1999;277:F437-F446. [PubMed] [Cited in This Article: ] |
| 80. | Sharma M, Sharma R, Greene AS, McCarthy ET, Savin VJ. Documentation of angiotensin II receptors in glomerular epithelial cells. Am J Physiol. 1998;274:F623-F627. [PubMed] [Cited in This Article: ] |
| 81. | Nishiyama A, Seth DM, Navar LG. Angiotensin II type 1 receptor-mediated augmentation of renal interstitial fluid angiotensin II in angiotensin II-induced hypertension. J Hypertens. 2003;21:1897-1903. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Li XC, Navar LG, Shao Y, Zhuo JL. Genetic deletion of AT1a receptors attenuates intracellular accumulation of ANG II in the kidney of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2007;293:F586-F593. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]Val5-ANG II in the kidneys and adrenals of AT1a receptor-deficient mice. Am J Physiol Renal Physiol. 2008;294:F293-F302. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 84. | Kobori H, Harrison-Bernard LM, Navar LG. Expression of angiotensinogen mRNA and protein in angiotensin II-dependent hypertension. J Am Soc Nephrol. 2001;12:431-439. [PubMed] [Cited in This Article: ] |
| 85. | Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL, Navar LG. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44:223-229. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 183] [Cited by in F6Publishing: 185] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 86. | Prieto MC, Williams DE, Liu L, Kavanagh KL, Mullins JJ, Mitchell KD. Enhancement of renin and prorenin receptor in collecting duct of Cyp1a1-Ren2 rats may contribute to development and progression of malignant hypertension. Am J Physiol Renal Physiol. 2011;300:F581-F588. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 34] [Cited by in F6Publishing: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 87. | Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG, Prieto MC. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol Renal Physiol. 2011;301:F1195-F1201. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 46] [Cited by in F6Publishing: 49] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 88. | Kurtz A, Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci USA. 1989;86:3423-3427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 90] [Cited by in F6Publishing: 104] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 89. | Li XC, Zhuo JL. Proximal tubule-dominant transfer of AT(1a) receptors induces blood pressure responses to intracellular angiotensin II in AT(1a) receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2013;304:R588-R598. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 90. | Crowley SD, Zhang J, Herrera M, Griffiths R, Ruiz P, Coffman TM. Role of AT₁ receptor-mediated salt retention in angiotensin II-dependent hypertension. Am J Physiol Renal Physiol. 2011;301:F1124-F1130. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab. 2011;13:469-475. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 192] [Cited by in F6Publishing: 196] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 92. | Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest. 2005;115:1092-1099. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 86] [Cited by in F6Publishing: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 93. | Nakanishi K, Nagai Y, Honglan Piao T, Kato H, Yanakieva-Georgieva N, Ishikawa Y, Yoshihara K, Ito K, Yamanaka N, Oite T. Changes in renal vessels following the long-term administration of an angiotensin II receptor blocker in Zucker fatty rats. J Renin Angiotensin Aldosterone Syst. 2011;12:65-74. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Wang CT, Chin SY, Navar LG. Impairment of pressure-natriuresis and renal autoregulation in ANG II-infused hypertensive rats. Am J Physiol Renal Physiol. 2000;279:F319-F325. [PubMed] [Cited in This Article: ] |
| 95. | Mitchell KD, Navar LG. Superficial nephron responses to peritubular capillary infusions of angiotensins I and II. Am J Physiol. 1987;252:F818-F824. [PubMed] [Cited in This Article: ] |
| 96. | Beutler KT, Masilamani S, Turban S, Nielsen J, Brooks HL, Ageloff S, Fenton RA, Packer RK, Knepper MA. Long-term regulation of ENaC expression in kidney by angiotensin II. Hypertension. 2003;41:1143-1150. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 131] [Cited by in F6Publishing: 141] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 97. | Castañeda-Bueno M, Cervantes-Pérez LG, Vázquez N, Uribe N, Kantesaria S, Morla L, Bobadilla NA, Doucet A, Alessi DR, Gamba G. Activation of the renal Na+: Cl- cotransporter by angiotensin II is a WNK4-dependent process. Proc Natl Acad Sci USA. 2012;109:7929-7934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 194] [Cited by in F6Publishing: 210] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 98. | Zhao D, Seth DM, Navar LG. Enhanced distal nephron sodium reabsorption in chronic angiotensin II-infused mice. Hypertension. 2009;54:120-126. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 66] [Cited by in F6Publishing: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 99. | Rangel LB, Caruso-Neves C, Lara LS, Lopes AG. Angiotensin II stimulates renal proximal tubule Na(+)-ATPase activity through the activation of protein kinase C. Biochim Biophys Acta. 2002;1564:310-316. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 100. | Rangel LB, Lopes AG, Lara LS, Carvalho TL, Silva IV, Oliveira MM, Einicker-Lamas M, Vieyra A, Nogaroli L, Caruso-Neves C. PI-PLCbeta is involved in the modulation of the proximal tubule Na+-ATPase by angiotensin II. Regul Pept. 2005;127:177-182. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 101. | Ferrão FM, Lara LS, Axelband F, Dias J, Carmona AK, Reis RI, Costa-Neto CM, Vieyra A, Lowe J. Exposure of luminal membranes of LLC-PK1 cells to ANG II induces dimerization of AT1/AT2 receptors to activate SERCA and to promote Ca2+ mobilization. Am J Physiol Renal Physiol. 2012;302:F875-F883. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 18] [Cited by in F6Publishing: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 102. | Du Z, Ferguson W, Wang T. Role of PKC and calcium in modulation of effects of angiotensin II on sodium transport in proximal tubule. Am J Physiol Renal Physiol. 2003;284:F688-F692. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 38] [Cited by in F6Publishing: 41] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 103. | Assunção-Miranda I, Guilherme AL, Reis-Silva C, Costa-Sarmento G, Oliveira MM, Vieyra A. Protein kinase C-mediated inhibition of renal Ca2+ ATPase by physiological concentrations of angiotensin II is reversed by AT1- and AT2-receptor antagonists. Regul Pept. 2005;127:151-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 17] [Cited by in F6Publishing: 18] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 104. | Zhuo JL, Carretero OA, Li XC. Effects of AT1 receptor-mediated endocytosis of extracellular Ang II on activation of nuclear factor-kappa B in proximal tubule cells. Ann N Y Acad Sci. 2006;1091:336-345. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 20] [Cited by in F6Publishing: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 105. | Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol. 2011;300:F1076-F1088. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 41] [Cited by in F6Publishing: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 106. | Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through a microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol. 2009;297:F1342-F1352. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 49] [Cited by in F6Publishing: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 107. | Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-ANG II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol. 2007;293:C367-C378. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 108. | Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-beta1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol. 2008;294:C1034-C1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 109. | van Kats JP, de Lannoy LM, Jan Danser AH, van Meegen JR, Verdouw PD, Schalekamp MA. Angiotensin II type 1 (AT1) receptor-mediated accumulation of angiotensin II in tissues and its intracellular half-life in vivo. Hypertension. 1997;30:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 126] [Cited by in F6Publishing: 131] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 110. | Schelling JR, Hanson AS, Marzec R, Linas SL. Cytoskeleton-dependent endocytosis is required for apical type 1 angiotensin II receptor-mediated phospholipase C activation in cultured rat proximal tubule cells. J Clin Invest. 1992;90:2472-2480. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 68] [Cited by in F6Publishing: 72] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 111. | Schelling JR, Singh H, Marzec R, Linas SL. Angiotensin II-dependent proximal tubule sodium transport is mediated by cAMP modulation of phospholipase C. Am J Physiol. 1994;267:C1239-C1245. [PubMed] [Cited in This Article: ] |
| 112. | Fessart D, Simaan M, Laporte SA. c-Src regulates clathrin adapter protein 2 interaction with beta-arrestin and the angiotensin II type 1 receptor during clathrin- mediated internalization. Mol Endocrinol. 2005;19:491-503. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 64] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 113. | Li XC, Carretero OA, Navar LG, Zhuo JL. AT1 receptor-mediated accumulation of extracellular angiotensin II in proximal tubule cells: role of cytoskeleton microtubules and tyrosine phosphatases. Am J Physiol Renal Physiol. 2006;291:F375-F383. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 42] [Cited by in F6Publishing: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 114. | Gonzalez-Villalobos R, Klassen RB, Allen PL, Navar LG, Hammond TG. Megalin binds and internalizes angiotensin II. Am J Physiol Renal Physiol. 2005;288:F420-F427. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 50] [Cited by in F6Publishing: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 115. | Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular ANG II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol. 2006;290:F1382-F1390. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 77] [Cited by in F6Publishing: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 116. | Gwathmey TM, Shaltout HA, Pendergrass KD, Pirro NT, Figueroa JP, Rose JC, Diz DI, Chappell MC. Nuclear angiotensin II type 2 (AT2) receptors are functionally linked to nitric oxide production. Am J Physiol Renal Physiol. 2009;296:F1484-F1493. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 67] [Cited by in F6Publishing: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Leite CA, Cristovam PC, Leitão AA, Miranda A, Andrade MC, Di Marco G, Casarini DE, Boim MA. Renin similar to the submaxillary gland form is expressed in mouse mesangial cells: subcellular localization and all generation under control and glucose-stimulated conditions. Cell Physiol Biochem. 2003;13:357-366. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 8] [Cited by in F6Publishing: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 118. | Vidotti DB, Casarini DE, Cristovam PC, Leite CA, Schor N, Boim MA. High glucose concentration stimulates intracellular renin activity and angiotensin II generation in rat mesangial cells. Am J Physiol Renal Physiol. 2004;286:F1039-F1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 161] [Cited by in F6Publishing: 161] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 119. | Singh R, Leehey DJ. Effect of ACE inhibitors on angiotensin II in rat mesangial cells cultured in high glucose. Biochem Biophys Res Commun. 2007;357:1040-1045. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Kumar R, Boim MA. Diversity of pathways for intracellular angiotensin II synthesis. Curr Opin Nephrol Hypertens. 2009;18:33-39. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 37] [Cited by in F6Publishing: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 121. | Lavrentyev EN, Estes AM, Malik KU. Mechanism of high glucose induced angiotensin II production in rat vascular smooth muscle cells. Circ Res. 2007;101:455-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 99] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 122. | Bader M. Tissue renin-angiotensin-aldosterone systems: Targets for pharmacological therapy. Annu Rev Pharmacol Toxicol. 2010;50:439-465. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 231] [Cited by in F6Publishing: 231] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 123. | Dieterle W, Corynen S, Mann J. Effect of the oral renin inhibitor aliskiren on the pharmacokinetics and pharmacodynamics of a single dose of warfarin in healthy subjects. Br J Clin Pharmacol. 2004;58:433-436. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 124. | Pimenta E, Oparil S. Role of aliskiren in cardio-renal protection and use in hypertensives with multiple risk factors. Ther Clin Risk Manag. 2009;5:459-464. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 3] [Cited by in F6Publishing: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 125. | Dietz R, Dechend R, Yu CM, Bheda M, Ford J, Prescott MF, Keefe DL. Effects of the direct renin inhibitor aliskiren and atenolol alone or in combination in patients with hypertension. J Renin Angiotensin Aldosterone Syst. 2008;9:163-175. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 31] [Cited by in F6Publishing: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 126. | Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W. Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension. 2008;52:130-136. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 224] [Cited by in F6Publishing: 238] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 127. | Persson F, Rossing P, Schjoedt KJ, Juhl T, Tarnow L, Stehouwer CD, Schalkwijk C, Boomsma F, Frandsen E, Parving HH. Time course of the antiproteinuric and antihypertensive effects of direct renin inhibition in type 2 diabetes. Kidney Int. 2008;73:1419-1425. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 128. | Ondetti MA, Rubin B, Cushman DW. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977;196:441-444. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1433] [Cited by in F6Publishing: 1260] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 129. | Zaman MA, Oparil S, Calhoun DA. Drugs targeting the renin-angiotensin-aldosterone system. Nat Rev Drug Discov. 2002;1:621-636. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 328] [Cited by in F6Publishing: 294] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 130. | Mallat SG. What is a preferred angiotensin II receptor blocker-based combination therapy for blood pressure control in hypertensive patients with diabetic and non-diabetic renal impairment? Cardiovasc Diabetol. 2012;11:32. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 19] [Cited by in F6Publishing: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Sasaki T, Noda Y, Yasuoka Y, Irino H, Abe H, Adachi H, Hattori S, Kitada H, Morisawa D, Miyatake K. Comparison of the effects of telmisartan and olmesartan on home blood pressure, glucose, and lipid profiles in patients with hypertension, chronic heart failure, and metabolic syndrome. Hypertens Res. 2008;31:921-929. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 14] [Cited by in F6Publishing: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 132. | Nishimura T, Hashimoto J, Ohkubo T, Kikuya M, Metoki H, Asayama K, Totsune K, Imai Y. Efficacy and duration of action of the four selective angiotensin II subtype 1 receptor blockers, losartan, candesartan, valsartan and telmisartan, in patients with essential hypertension determined by home blood pressure measurements. Clin Exp Hypertens. 2005;27:477-489. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 33] [Cited by in F6Publishing: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 133. | Kereiakes DJ, Chrysant SG, Izzo JL, Littlejohn T, Oparil S, Melino M, Lee J, Fernandez V, Heyrman R. Long-term efficacy and safety of triple-combination therapy with olmesartan medoxomil and amlodipine besylate and hydrochlorothiazide for hypertension. J Clin Hypertens (Greenwich). 2012;14:149-157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 15] [Cited by in F6Publishing: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 134. | Lewin AJ, Kereiakes DJ, Chrysant SG, Izzo JL, Oparil S, Lee J, Fernandez V, Melino M. Triple-combination treatment with olmesartan medoxomil/amlodipine/ hydrochlorothiazide in Hispanic/Latino patients with hypertension: the TRINITY study. Ethn Dis. 2014;24:41-47. [PubMed] [Cited in This Article: ] |
| 135. | Pons F, Lupón J, Urrutia A, González B, Crespo E, Díez C, Cano L, Cabanes R, Altimir S, Coll R. Mortality and cause of death in patients with heart failure: findings at a specialist multidisciplinary heart failure unit. Rev Esp Cardiol (Engl Ed). 2010;63:303-314. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1] [Cited by in F6Publishing: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 136. | Pinheiro SV, Simões e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, Pesquero JB, Walther T, Alenina N, Bader M. Nonpeptide AVE 0991 is an angiotensin-(1-7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44:490-496. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 127] [Cited by in F6Publishing: 125] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 137. | Wiemer G, Dobrucki LW, Louka FR, Malinski T, Heitsch H. AVE 0991, a nonpeptide mimic of the effects of angiotensin-(1-7) on the endothelium. Hypertension. 2002;40:847-852. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 140] [Cited by in F6Publishing: 139] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 138. | Lemos VS, Silva DM, Walther T, Alenina N, Bader M, Santos RA. The endothelium-dependent vasodilator effect of the nonpeptide Ang(1-7) mimic AVE 0991 is abolished in the aorta of mas-knockout mice. J Cardiovasc Pharmacol. 2005;46:274-279. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 95] [Cited by in F6Publishing: 93] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 139. | Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin-(1-7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290:H684-H691. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 142] [Cited by in F6Publishing: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 140. | Uekama K. Design and evaluation of cyclodextrin-based drug formulation. Chem Pharm Bull (Tokyo). 2004;52:900-915. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 282] [Cited by in F6Publishing: 261] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 141. | Marques FD, Ferreira AJ, Sinisterra RD, Jacoby BA, Sousa FB, Caliari MV, Silva GA, Melo MB, Nadu AP, Souza LE. An oral formulation of angiotensin-(1-7) produces cardioprotective effects in infarcted and isoproterenol-treated rats. Hypertension. 2011;57:477-483. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 102] [Cited by in F6Publishing: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 142. | Hernández Prada JA, Ferreira AJ, Katovich MJ, Shenoy V, Qi Y, Santos RA, Castellano RK, Lampkins AJ, Gubala V, Ostrov DA. Structure-based identification of small-molecule angiotensin-converting enzyme 2 activators as novel antihypertensive agents. Hypertension. 2008;51:1312-1317. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 197] [Cited by in F6Publishing: 201] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 143. | Ferreira AJ, Shenoy V, Yamazato Y, Sriramula S, Francis J, Yuan L, Castellano RK, Ostrov DA, Oh SP, Katovich MJ. Evidence for angiotensin-converting enzyme 2 as a therapeutic target for the prevention of pulmonary hypertension. Am J Respir Crit Care Med. 2009;179:1048-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 190] [Cited by in F6Publishing: 197] [Article Influence: 13.1] [Reference Citation Analysis (0)] |