Published online Jun 26, 2017. doi: 10.5662/wjm.v7.i2.46
Peer-review started: September 5, 2016
First decision: November 10, 2016
Revised: March 10, 2017
Accepted: March 16, 2017
Article in press: March 17, 2017
Published online: June 26, 2017
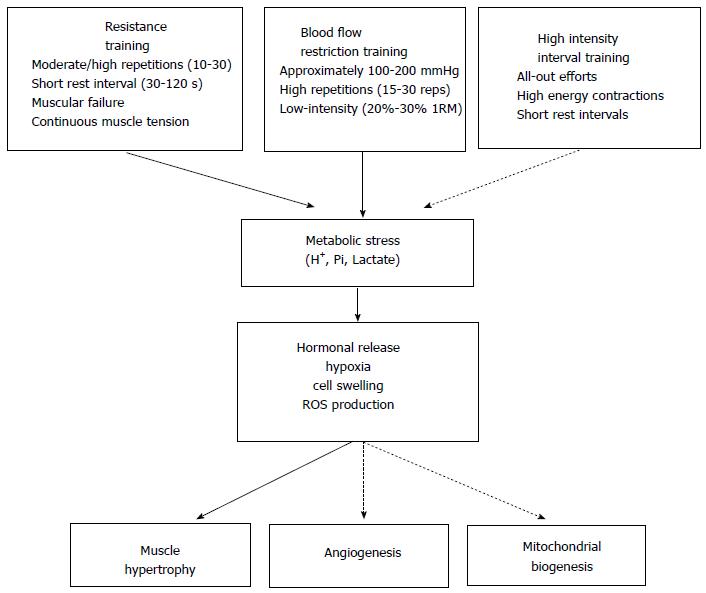
Metabolic stress is a physiological process that occurs during exercise in response to low energy that leads to metabolite accumulation [lactate, phosphate inorganic (Pi) and ions of hydrogen (H+)] in muscle cells. Traditional exercise protocol (i.e., Resistance training) has an important impact on the increase of metabolite accumulation, which influences hormonal release, hypoxia, reactive oxygen species (ROS) production and cell swelling. Changes in acute exercise routines, such as intensity, volume and rest between sets, are determinants for the magnitude of metabolic stress, furthermore, different types of training, such as low-intensity resistance training plus blood flow restriction and high intensity interval training, could be used to maximize metabolic stress during exercise. Thus, the objective of this review is to describe practical applications that induce metabolic stress and the potential effects of metabolic stress to increase systemic hormonal release, hypoxia, ROS production, cell swelling and muscle adaptations.
Core tip: This review aimed to describe practical applications for inducing metabolic stress and the potential effects on the increase of systemic hormonal release, hypoxia, reactive oxygen species production, and cell swelling. These effects are responsible for enhancing muscle adaptations through changes in exercise routines (intensity, volume, rest between sets) and training modes (resistance training, low-intensity resistance training plus blood flow restriction, and high intensity interval training).
- Citation: de Freitas MC, Gerosa-Neto J, Zanchi NE, Lira FS, Rossi FE. Role of metabolic stress for enhancing muscle adaptations: Practical applications. World J Methodol 2017; 7(2): 46-54
- URL: https://www.wjgnet.com/2222-0682/full/v7/i2/46.htm
- DOI: https://dx.doi.org/10.5662/wjm.v7.i2.46
It has been reported that chronic exercise can promote changes in many organs because of cellular adaptations. Skeletal muscle is extremely adjustable in response to contractile activity[1,2], therefore, repeated muscle contractions during exercise can lead to numerous metabolic modifications[3,4]. Overtime, these adaptive responses have shown beneficial effects on health, body composition and performance[5-7].
During acute exercise, the energy used by skeletal muscle contractions are essential in transforming organelles, enzymatic activity, intracellular signaling and transcriptional responses[8-10]. Metabolic stress is a physiological process that occurs during exercise in response to low energy which leads to metabolite accumulation [lactate, phosphate inorganic (Pi) and ions of hydrogen (H+)] in muscle cells[11,12]. Researchers have suggested that metabolic stress has an important impact on hormonal release, hypoxia, cell swelling and production of reactive oxygen species (ROS)[13-15]. All of these components can initiate anabolic signaling for muscle growth and adaptations on energy metabolism[16].
In situations with elevated ATP hydrolysis and glycolytic flux in muscle cells, there is a great accumulation of adenosine monophosphate (AMP) and metabolites[12,17,18]. Furthermore, the reduction of intracellular oxygen levels can also lead to hypoxia[19]. All these metabolic parameters are a powerful stimulus to activate AMP-activated protein kinase (AMPK) and hypoxia-inducible factor (HIF-1α) pathway, the main regulators of mitochondrial biogenesis and angiogenesis[20,21].
Moreover, metabolite accumulation and hypoxia that is produced during exercise may increase ROS production through mitochondrial electron transport chain[22,23]. It is well established that ROS production by endurance exercise has positive effects on mitochondrial adaptations because it stimulates peroxisome proliferator-activated receptor gamma coactivator (PGC-1α) and p38 mitogen-activated protein kinase (p38MAPK) pathways[24]. Scientific evidence shows that suppression of ROS production through the use of the antioxidants impairs some adaptive responses to endurance exercise[25,26], and these results suggest that ROS production has positive effects on mitochondrial adaptations.
Nevertheless, besides stimulating mitochondrial biogenesis and angiogenesis, the metabolic stress also has positive effects on muscle hypertrophy. Resistance training (RT) has great impact on increasing metabolite accumulation, which influences hormonal release, hypoxia, ROS production and cell swelling. All these processes can mediate anabolic signaling that stimulates muscle protein synthesis and activation of satellite cells[13-15].
In this context, changes in acute exercise routines (intensity, volume and rest between sets) are the main factors in determining the magnitude of metabolic stress[27-29]. Furthermore, blood flow restriction training has been considered a tool to maximize metabolic stress[30,31]. Studies have reported great effects of this training method on aerobic adaptations and muscle hypertrophy[32,33].
Therefore, the purpose of this paper is to describe practical applications that cause metabolic stress. In addition, we will discuss the potential effects of metabolic stress on the increase of systemic hormonal release, hypoxia, ROS production, and cell swelling for enhancing muscle adaptations.
Skeletal muscle hypertrophy depends on positive muscle protein balance (protein synthesis exceeds breakdown)[34]. Thus, RT is excellent for the stimulation of anabolic signaling and the promotion of muscle hypertrophy[35]. Metabolic stress is one of the primary mechanisms that makes RT increase muscle mass, mainly due to the rise of anabolic hormonal release, hypoxia, ROS production and cell swelling[13]. However, studies have shown that the magnitude of metabolic stress depends on the changes of acute RT program variables[14,15].
Scientific evidence shows that load, number of repetitions, and reset between intervals are important factors to induce metabolite accumulation. Gonzalez et al[29] found that acute RT with moderate repetitions combined with short rest intervals (70% 1RM, 10-12 repetitions and one minute rest interval) shows an increase in blood lactate, serum concentration of lactate dehydrogenase, growth hormone (GH) and cortisol when compared to higher loads, low repetitions combined with longer rest intervals (90% 1RM, 3-5 repetitions and three minute rest intervals). Concerning these findings, duration of rest intervals may reflect directly on the magnitude of metabolic stress. In a review study, researchers demonstrated that short interval sets (less than one minute) are essential in increasing blood lactate and GH production, mainly because of insufficient recovery of phosphocreatine and H+ accumulation[36].
Additionally, Nishimura et al[37] demonstrated higher effects of muscle hypertrophy when RT is performed during hypoxia, possibly because of the strong influence of hormonal release, the recruitment of fast-twitch muscle fibers, ROS production and cell swelling[38]. During RT, muscle contractions compress blood vessels in active muscles, and this occlusion can lead to a reduction of oxygen levels and, consequently, resulting in a hypoxic environment[39]. Intramuscular hypoxia during exercise can increase the necessity of anaerobic latic metabolism by activation of HIF-1α that regulates the expression of glycolytic enzymes[40]. Thus, exercise that produces high levels of lactate can be associated with hypoxia. One study showed that performing hypertrophy-type RT (70% 1RM, 10 repetitions and 90 s rest intervals) induces higher production of lactate and reduction in pH than performing a strength-type RT (85% 1RM, 4-6 repetitions with five minute rest intervals)[41]. In this context, it can be hypothesized that RT can generate hypoxia when performed at moderate/high repetitions combined with short rest intervals, possibly due to a high demand on anaerobic metabolism.
Furthermore, another study found that knee extension RT at low intensity (50% 1RM) generates a significant decrease in muscle oxygenation when compared to high-intensity (80% 1RM) exercise performed with one-second rest between repetitions[42]. These findings suggest, keeping continuous tension on muscles without relaxation can be essential to reducing oxygen levels and maximizing the levels of hypoxia in the skeletal muscle.
Research suggests that ROS production also has important implications on muscle hypertrophy[43,44]. In addition, studies have shown that utilization of antioxidants can modify protein signaling after a RT session and impairs muscle mass gains[45,46]. Muscle contractions during exercise produces ROS at low physiological levels and plays an important role in cell signaling to promote beneficial adaptations[47]. Researchers have found that the production of ROS has an influence in stimulating anabolic signaling, because ROS can act with a signaling molecule to activate the mammalian target of rapamycin (mTOR) through IGF-1 and MAPK pathways[48,49].
Although it is becoming clear that ROS has a profound impact on muscle hypertrophy, the limits of these adaptations are not clear. Hornberger et al[50] observed that selenium-deficient transgenic mice (animals with decreased protein expression of antioxidant enzymes containing selenium) exhibited an increased muscle hypertrophy when stimulated by synergist ablation (a muscle overload model), compared to other animals. In this study, rapamycin treatment (a pharmacological inhibitor of mTOR) completely abolished the hypertrophy effect, thus proving that mTOR is necessary for hypertrophy. It is interesting to note that, contrary to this study (where muscle antioxidant defense was decreased and muscle hypertrophy was optimized), other studies evaluating the impact of antioxidants in humans (through vitamin E and C supplementation) were shown to impair muscle hypertrophy response and cell signaling leading to muscle hypertrophy[45,46]. Several studies have observed that RT increases hypoxia, metabolite accumulation and ROS production, which seems to be strictly related[22,23,51,52]. In this context, we can hypothesize that RT with moderate/high repetitions and short rest intervals can be a stimulus to produce ROS.
Another potent anabolic signaling event produced by RT is cell swelling. Studies have demonstrated that cell swelling mediated by hydration can lead to an increase in protein synthesis and a decrease in proteolysis mainly through the activation of MAPK pathway[53-55]. During intense muscle contractions, veins are obstructed but the arterial system keeps the delivery of blood active[13]. This process can increase intracellular swelling, which leads to an increased pressure against the cytoskeleton. Thus, the cell perceives a threat and initiates an anabolic signaling response to promote reinforcement of its ultrastructure[56]. Studies indicate that cell swelling occurs during metabolite accumulation (lactate, H+ and Pi) which leads to additional intracellular fluid[57,58]. Therefore, it seems reasonable to conclude that RT during hypertrophy causes high metabolite accumulation and can promote more cell swelling than strength RT.
Finally, another aspect that we should consider, especially among well-trained subjects, is RT with moderate/high repetitions until failure. Recent studies show that, when RT is executed with low load (30%-50% 1RM and 25-35 repetitions) until failure, hypertrophy is similar when compared to high load (70%-90% 1RM and 8-12 repetitions)[59-61]. Although no studies have confirmed this hypothesis, we believe that muscular failure can exert additional metabolic stress and then induce anabolic signaling. These findings suggest that the greater time under tension with moderate/high repetitions without relaxation combined with short rest interval and muscular failure can generate a strong hypertrophic response similar to RT with high loads. However, caution should be taken, because restricting rest periods would cause a reduction in the volume performed during a RT session, thus affecting hypertrophy process negatively[62].
This effect can be caused by high metabolic stress, leading to anabolic signaling through hypoxia, hormonal release, ROS production and cell swelling (Figure 1).
During the last decade, blood flow restriction training (BFRT), also known as KAATSU or occlusion[63], combined with low-intensity strength training (20%-30% 1RM), has been shown to increase strength and muscle size among trained/untrained athletes[64-66] injured[67] and the elderly[68]. This training model requires the use of cuffs that are placed at the proximal ends of the upper arms or thighs reducing blood flow from the muscle (approximately 100-200 mmHg). Thus, the external pressure applied maintains arterial inflow while blocking venous outflow of blood[69], resulting in an ischemic/hypoxic environment that enhances the training effect[70].
Several studies have compared low-intensity strength training with BFRT and high-intensity without BFRT and demonstrated a significant increase in muscle cross-section area in both exercise protocols[64,69,71,72]. However, RT performed with moderate/high intensities seems to lead to similar degrees of muscle hypertrophy when combined with BFRT. It is not clear if the maximal degree of muscle hypertrophy can be optimized by increasing external loads or if the ceiling for maximal hypertrophy is achieved with low-moderate loads[73].
Cumming et al[74] performed a study with nine healthy volunteers performing five sets of unilateral knee extension at 30% of 1RM until failure combined with BFRT and the same workout without BFRT. Analysis of muscle biopsies revealed a rapid translocation of heat-shock proteins (HSP27 and aB-crystallin) from cytosol to cytoskeletal structures, both of which have been identified as important HSPs for repair and stabilization of stressed and damaged proteins[75]. This indicates that cytoskeletal proteins are stressed during BFRT even without myofibrillar disruptions. Thus, muscle hypertrophy induced by BFRT seems to be mediated by metabolic stress and mechanical tension, and sarcolemmal-bound mechanosensors (i.e., integrins) stimulate intracellular anabolic and catabolic pathways, which convert mechanical energy into chemical signals, promoting protein synthesis instead of degradation[76].
Suga et al[77] investigated metabolic stress (intramuscular phosphocreatine (PCr), Pi, Diprotonated phosphate-H2PO4 and Intramuscular pH) in subjects that performed four unilateral plantar flexion (two min of 30 repetitions/min) using three different intensities (20%, 30% and 40% 1RM) with two resistance exercises (20% 1 RM and 65% 1RM) without BFRT. They concluded that 30% of 1RM induced a similar intramuscular metabolites and pH response than high-intensity RT without BFRT. In addition, Suga et al[31] also showed that multiple low-intensity BFRT sets increase fast-twitch fiber recruitment that could assist the slow twitch fiber to keep the strength during training, however, the authors did not observe statistical significance between multiple sets of high intensity exercise without BFRT. Therefore, these results suggest that multiple-set exercise are more effective than single-set RT.
Previous studies have shown that metabolic stress induced by low-intensity plus BFRT increases GH secretion and muscle hypertrophy[64,65,78], furthermore, this could stimulate metabolic stress markers, such as IL-6[79,80]. The recovery process is initiated by IL-6 by modulating muscle regulatory genes (i.e., MyoD)[81-83] and activating muscle satellite cells[80], and therefore may play a role in regulating muscle growth/hypertrophy[80].
An acute increase in anabolic hormones (e.g., testosterone, GH) has been found during short rest periods (30 to 60 s)[84], however, regarding cytokine production, a recent study compared 30 s vs 90 s of rest after four sets of squat and four sets of bench press with 70% of 1RM until failure without BFRT in healthy adults and observed higher IL-6 levels when 90 s rest was used[85]. In addition, Phillips et al[86] reported greater post-exercise IL-6 concentrations with 65% of 1RM compared to 85% of 1RM with two minutes of recovery. Thus, short rest period induce an acute increase in anabolic hormones, however, it seems that longer recovery intervals combined with higher loads contribute to an increase in IL-6 concentration during RT.
Therefore, changes in variables, such as recovery intervals, volume, intensity, and repetition speed, could be used to optimize the specific adaptation during low-intensity RT plus BFRT.
Studies have investigated the benefits of metabolic stress on skeletal muscle remodeling, angiogenesis, mitochondrial biogenesis, performance, and high-intensity interval training (HIIT) has shown to be a promising training routine. This exercise/training routine is based on high-intensity exercise sets with passive or low-intensity intervals between them. Endurance training adaptations have been found with HIIT[87,88].
The HIIT configuration allows intervals of effort and pause, and the various forms of stimuli can cause adaptations, such as: (1) mechanical stretching and muscle tension; (2) increase of ROS; (3) increase of intramuscular calcium concentrations and (4) changes of energy “status” in the cell.
Two HIIT routines that are commonly used are: four sets of 30 s at 100%[88] and four sets of four minutes[89] at 90%-95% of the maximum power (Pmax), velocity (Vmax) or maximum heart rate (HR max). Wahl et al[90] compared the acute responses of these two routine with another routine done continuously (two hours at 55% Pmax) in triathlon athletes and found that the most intense stimulus (four sets of 30 s at 100%) generated higher metabolic acidosis (pH) and higher concentrations of anabolic hormones (testosterone and GH) after the session. Supporting these results, Wahl et al[91] compared the use of buffer solution (sodium bicarbonate) and placebo with HIIT (four sets of 30 s at 100%), and showed a significant decrease in pH in the placebo group with increases in GH compared to the buffer group. The elevation of these hormones mean hypertrophic adaptations and also important stimuli expression of oxidative enzymes and erythropoiesis, promoting improvements in aerobic performance. This can be explained by the direct stimulation of bone marrow by testosterone, supporting the synthesis of erythropoietin in kidney cells[92].
Mitochondrial biogenesis is another adaptation of great importance in this process and one of the most studied. A key molecule for this adaptation is PGC-1α, a coactivator of several transcription factors related to metabolic and mitochondrial adaptations[93]. Burgomaster et al[87] found that six weeks of HIIT (three times per week, four to six sets of 30 at 100%) and continuous training (five times per week, 40 to 60 min at 55% VO2max) showed significant improvements in mitochondrial functions with optimization lipid oxidation, increased activity of oxidative enzymes (citrate synthase and 3-hydroxyacyl CoA dehydrogenase) and contents of PGC-1α. The important finding of this study was the difference in the duration of training sessions, ranging from approximately 1.5 h to 4.5 h per week for HIIT and continuous training, respectively.
Due to the importance of PGC-1α, the expression and activation of proteins that stimulate it has great relevance. Two proteins, which are unquestionably stimulated by metabolic stress, are p38MAPK and AMPK[94-96]. Gibala et al[97] showed a significant increase in phosphorylation of AMPK and p38MAPK after acute sessions of HIIT (four sets of 30 s at 100%), and despite a great increase in mRNA of PGC-1α, its protein content did not change. Additionally, Little et al[98], using the same protocol of exercises, showed significantly higher values of p38MAPK after exercise, as well as an increase of 750% of mRNA PGC-1α and 66% of protein already in the nucleus of muscle cells, confirming the potential of these training routine.
Mitochondrial biogenesis and angiogenesis are essential for aerobic adaptations and improvement of performance. Considering the efficiency of HIIT (short training repetitions and metabolic stress), with BFRT seems to be beneficial to increase vascular adaptations. Consequently, Taylor et al[32] compared acute HIIT (four sets of 30 s at 100%), with HIIT + BFR (cuff in the thigh, two minutes, 130 mmHg). The results of these biopsies (vastus lateralis) showed a significant increase in p38MAPK after HIIT and HIIT+BFRT, with no differences between them. After three hours of exercise, a significant increase in mRNA PGC-1α was observed, vascular endothelial growth factor (VEGF) and its receptor (VEGFR-2), however mRNA of HIF-1α only increased in HIIT + BFRT. These results indicate that HIIT by itself is capable of stimulating angiogenesis, but the fact that only HIIT + BFRT increased HIF-1α cannot be overlooked, because it is a key factor for hypoxia and metabolic stress. Low PO2 increases concentrations, favoring translocation to the nucleus and subsequent activation of VEGF in the human skeletal muscle[99].
Changes in acute exercise routine variables, such as intensity, volume, recovery interval and type of training are determinants that influence the magnitude of metabolic stress. Despite, traditional training protocol, such as RT, increase metabolite accumulation and influence hormonal release, hypoxia, ROS production and cell swelling. In this review, we discussed that low-intensity RT plus BFRT and HIIT are alternative exercise routines that increase metabolic stress and muscle adaptation among different populations. However, the difference between exercise protocols used in literature and different levels of physical fitness should be considered when interpreting the results.
Manuscript source: Invited manuscript
Specialty type: Medical Laboratory Technology
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Dobrian AD, Elner VM, Kimchi A, Li W, Ni Y S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Coffey VG, Shield A, Canny BJ, Carey KA, Cameron-Smith D, Hawley JA. Interaction of contractile activity and training history on mRNA abundance in skeletal muscle from trained athletes. Am J Physiol Endocrinol Metab. 2006;290:E849-E855. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 103] [Cited by in F6Publishing: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Ferraro E, Giammarioli AM, Chiandotto S, Spoletini I, Rosano G. Exercise-induced skeletal muscle remodeling and metabolic adaptation: redox signaling and role of autophagy. Antioxid Redox Signal. 2014;21:154-176. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 124] [Cited by in F6Publishing: 133] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 3. | Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab. 2013;17:162-184. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1197] [Cited by in F6Publishing: 1228] [Article Influence: 111.6] [Reference Citation Analysis (0)] |
| 4. | Hood DA, Irrcher I, Ljubicic V, Joseph AM. Coordination of metabolic plasticity in skeletal muscle. J Exp Biol. 2006;209:2265-2275. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 250] [Cited by in F6Publishing: 253] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | Camera DM, Smiles WJ, Hawley JA. Exercise-induced skeletal muscle signaling pathways and human athletic performance. Free Radic Biol Med. 2016;98:131-143. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 74] [Cited by in F6Publishing: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Carter CS, Hofer T, Seo AY, Leeuwenburgh C. Molecular mechanisms of life- and health-span extension: role of calorie restriction and exercise intervention. Appl Physiol Nutr Metab. 2007;32:954-966. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 7. | Kraemer WJ, Deschenes MR, Fleck SJ. Physiological adaptations to resistance exercise. Implications for athletic conditioning. Sports Med. 1988;6:246-256. [PubMed] [Cited in This Article: ] |
| 8. | Adhihetty PJ, Irrcher I, Joseph AM, Ljubicic V, Hood DA. Plasticity of skeletal muscle mitochondria in response to contractile activity. Exp Physiol. 2003;88:99-107. [PubMed] [Cited in This Article: ] |
| 9. | Flück M, Hoppeler H. Molecular basis of skeletal muscle plasticity--from gene to form and function. Rev Physiol Biochem Pharmacol. 2003;146:159-216. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 296] [Cited by in F6Publishing: 290] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 10. | Coffey VG, Hawley JA. The molecular bases of training adaptation. Sports Med. 2007;37:737-763. [PubMed] [Cited in This Article: ] |
| 11. | Tesch PA, Colliander EB, Kaiser P. Muscle metabolism during intense, heavy-resistance exercise. Eur J Appl Physiol Occup Physiol. 1986;55:362-366. [PubMed] [Cited in This Article: ] |
| 12. | Volianitis S, Secher NH, Quistorff B. The intracellular to extracellular proton gradient following maximal whole body exercise and its implication for anaerobic energy production. Eur J Appl Physiol. 2010;109:1171-1177. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Schoenfeld BJ. The mechanisms of muscle hypertrophy and their application to resistance training. J Strength Cond Res. 2010;24:2857-2872. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 485] [Cited by in F6Publishing: 523] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 14. | Schoenfeld BJ. Potential mechanisms for a role of metabolic stress in hypertrophic adaptations to resistance training. Sports Med. 2013;43:179-194. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 239] [Cited by in F6Publishing: 260] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 15. | Pearson SJ, Hussain SR. A review on the mechanisms of blood-flow restriction resistance training-induced muscle hypertrophy. Sports Med. 2015;45:187-200. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 189] [Cited by in F6Publishing: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 16. | Ozaki H, Loenneke JP, Buckner SL, Abe T. Muscle growth across a variety of exercise modalities and intensities: Contributions of mechanical and metabolic stimuli. Med Hypotheses. 2016;88:22-26. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 47] [Cited by in F6Publishing: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Robergs RA, Ghiasvand F, Parker D. Biochemistry of exercise-induced metabolic acidosis. Am J Physiol Regul Integr Comp Physiol. 2004;287:R502-R516. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 651] [Cited by in F6Publishing: 665] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 18. | Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA, Kiens B. Isoform-specific and exercise intensity-dependent activation of 5’-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528 Pt 1:221-226. [PubMed] [Cited in This Article: ] |
| 19. | Casey DP, Joyner MJ. Local control of skeletal muscle blood flow during exercise: influence of available oxygen. J Appl Physiol (1985). 2011;111:1527-1538. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 62] [Cited by in F6Publishing: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Niemi H, Honkonen K, Korpisalo P, Huusko J, Kansanen E, Merentie M, Rissanen TT, André H, Pereira T, Poellinger L. HIF-1α and HIF-2α induce angiogenesis and improve muscle energy recovery. Eur J Clin Invest. 2014;44:989-999. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 21. | Marcinko K, Steinberg GR. The role of AMPK in controlling metabolism and mitochondrial biogenesis during exercise. Exp Physiol. 2014;99:1581-1585. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 56] [Cited by in F6Publishing: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Clanton TL. Hypoxia-induced reactive oxygen species formation in skeletal muscle. J Appl Physiol (1985). 2007;102:2379-2388. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 261] [Cited by in F6Publishing: 256] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 23. | Morales-Alamo D, Calbet JA. Free radicals and sprint exercise in humans. Free Radic Res. 2014;48:30-42. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Ji LL, Kang C, Zhang Y. Exercise-induced hormesis and skeletal muscle health. Free Radic Biol Med. 2016;98:113-122. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 25. | Strobel NA, Peake JM, Matsumoto A, Marsh SA, Coombes JS, Wadley GD. Antioxidant supplementation reduces skeletal muscle mitochondrial biogenesis. Med Sci Sports Exerc. 2011;43:1017-1024. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 137] [Cited by in F6Publishing: 135] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 26. | Merry TL, Ristow M. Do antioxidant supplements interfere with skeletal muscle adaptation to exercise training? J Physiol. 2016;594:5135-5147. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 153] [Cited by in F6Publishing: 177] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 27. | Spiering BA, Kraemer WJ, Anderson JM, Armstrong LE, Nindl BC, Volek JS, Maresh CM. Resistance exercise biology: manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008;38:527-540. [PubMed] [Cited in This Article: ] |
| 28. | Goto K, Ishii N, Kizuka T, Takamatsu K. The impact of metabolic stress on hormonal responses and muscular adaptations. Med Sci Sports Exerc. 2005;37:955-963. [PubMed] [Cited in This Article: ] |
| 29. | Gonzalez AM, Hoffman JR, Townsend JR, Jajtner AR, Boone CH, Beyer KS, Baker KM, Wells AJ, Mangine GT, Robinson EH. Intramuscular anabolic signaling and endocrine response following high volume and high intensity resistance exercise protocols in trained men. Physiol Rep. 2015;3:pii: e12466. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 27] [Cited by in F6Publishing: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Sugaya M, Yasuda T, Suga T, Okita K, Abe T. Change in intramuscular inorganic phosphate during multiple sets of blood flow-restricted low-intensity exercise. Clin Physiol Funct Imaging. 2011;31:411-413. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 36] [Cited by in F6Publishing: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Suga T, Okita K, Takada S, Omokawa M, Kadoguchi T, Yokota T, Hirabayashi K, Takahashi M, Morita N, Horiuchi M. Effect of multiple set on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. Eur J Appl Physiol. 2012;112:3915-3920. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 92] [Cited by in F6Publishing: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 32. | Taylor CW, Ingham SA, Ferguson RA. Acute and chronic effect of sprint interval training combined with postexercise blood-flow restriction in trained individuals. Exp Physiol. 2016;101:143-154. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 44] [Cited by in F6Publishing: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Slysz J, Stultz J, Burr JF. The efficacy of blood flow restricted exercise: A systematic review & amp; meta-analysis. J Sci Med Sport. 2016;19:669-675. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 128] [Cited by in F6Publishing: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 34. | Phillips SM. Physiologic and molecular bases of muscle hypertrophy and atrophy: impact of resistance exercise on human skeletal muscle (protein and exercise dose effects). Appl Physiol Nutr Metab. 2009;34:403-410. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 73] [Cited by in F6Publishing: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 35. | Damas F, Phillips S, Vechin FC, Ugrinowitsch C. A review of resistance training-induced changes in skeletal muscle protein synthesis and their contribution to hypertrophy. Sports Med. 2015;45:801-807. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 125] [Cited by in F6Publishing: 133] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 36. | Henselmans M, Schoenfeld BJ. The effect of inter-set rest intervals on resistance exercise-induced muscle hypertrophy. Sports Med. 2014;44:1635-1643. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 26] [Cited by in F6Publishing: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 37. | Nishimura A, Sugita M, Kato K, Fukuda A, Sudo A, Uchida A. Hypoxia increases muscle hypertrophy induced by resistance training. Int J Sports Physiol Perform. 2010;5:497-508. [PubMed] [Cited in This Article: ] |
| 38. | Scott BR, Slattery KM, Sculley DV, Dascombe BJ. Hypoxia and resistance exercise: a comparison of localized and systemic methods. Sports Med. 2014;44:1037-1054. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 39. | Tamaki T, Uchiyama S, Tamura T, Nakano S. Changes in muscle oxygenation during weight-lifting exercise. Eur J Appl Physiol Occup Physiol. 1994;68:465-469. [PubMed] [Cited in This Article: ] |
| 40. | Favier FB, Britto FA, Freyssenet DG, Bigard XA, Benoit H. HIF-1-driven skeletal muscle adaptations to chronic hypoxia: molecular insights into muscle physiology. Cell Mol Life Sci. 2015;72:4681-4696. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 54] [Cited by in F6Publishing: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 41. | Nicholson G, Mcloughlin G, Bissas A, Ispoglou T. Do the acute biochemical and neuromuscular responses justify the classification of strength- and hypertrophy-type resistance exercise? J Strength Cond Res. 2014;28:3188-3199. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 11] [Cited by in F6Publishing: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 42. | Tanimoto M, Ishii N. Effects of low-intensity resistance exercise with slow movement and tonic force generation on muscular function in young men. J Appl Physiol (1985). 2006;100:1150-1157. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 169] [Cited by in F6Publishing: 156] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 43. | Wadley GD. A role for reactive oxygen species in the regulation of skeletal muscle hypertrophy. Acta Physiol (Oxf). 2013;208:9-10. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 6] [Cited by in F6Publishing: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 44. | Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S. Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med. 2013;19:101-106. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 205] [Cited by in F6Publishing: 215] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 45. | Bjørnsen T, Salvesen S, Berntsen S, Hetlelid KJ, Stea TH, Lohne-Seiler H, Rohde G, Haraldstad K, Raastad T, Køpp U. Vitamin C and E supplementation blunts increases in total lean body mass in elderly men after strength training. Scand J Med Sci Sports. 2016;26:755-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 63] [Cited by in F6Publishing: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 46. | Paulsen G, Hamarsland H, Cumming KT, Johansen RE, Hulmi JJ, Børsheim E, Wiig H, Garthe I, Raastad T. Vitamin C and E supplementation alters protein signalling after a strength training session, but not muscle growth during 10 weeks of training. J Physiol. 2014;592:5391-5408. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 97] [Cited by in F6Publishing: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 47. | Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1005-L1028. [PubMed] [Cited in This Article: ] |
| 48. | Handayaningsih AE, Iguchi G, Fukuoka H, Nishizawa H, Takahashi M, Yamamoto M, Herningtyas EH, Okimura Y, Kaji H, Chihara K. Reactive oxygen species play an essential role in IGF-I signaling and IGF-I-induced myocyte hypertrophy in C2C12 myocytes. Endocrinology. 2011;152:912-921. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 64] [Cited by in F6Publishing: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 49. | Kefaloyianni E, Gaitanaki C, Beis I. ERK1/2 and p38-MAPK signalling pathways, through MSK1, are involved in NF-kappaB transactivation during oxidative stress in skeletal myoblasts. Cell Signal. 2006;18:2238-2251. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 206] [Cited by in F6Publishing: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 50. | Hornberger TA, McLoughlin TJ, Leszczynski JK, Armstrong DD, Jameson RR, Bowen PE, Hwang ES, Hou H, Moustafa ME, Carlson BA. Selenoprotein-deficient transgenic mice exhibit enhanced exercise-induced muscle growth. J Nutr. 2003;133:3091-3097. [PubMed] [Cited in This Article: ] |
| 51. | Morales-Alamo D, Ponce-González JG, Guadalupe-Grau A, Rodríguez-García L, Santana A, Cusso MR, Guerrero M, Guerra B, Dorado C, Calbet JA. Increased oxidative stress and anaerobic energy release, but blunted Thr172-AMPKα phosphorylation, in response to sprint exercise in severe acute hypoxia in humans. J Appl Physiol (1985). 2012;113:917-928. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 52. | Korthuis RJ, Granger DN, Townsley MI, Taylor AE. The role of oxygen-derived free radicals in ischemia-induced increases in canine skeletal muscle vascular permeability. Circ Res. 1985;57:599-609. [PubMed] [Cited in This Article: ] |
| 53. | Lang F, Busch GL, Ritter M, Völkl H, Waldegger S, Gulbins E, Häussinger D. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247-306. [PubMed] [Cited in This Article: ] |
| 54. | Grant AC, Gow IF, Zammit VA, Shennan DB. Regulation of protein synthesis in lactating rat mammary tissue by cell volume. Biochim Biophys Acta. 2000;1475:39-46. [PubMed] [Cited in This Article: ] |
| 55. | Zhou X, Naguro I, Ichijo H, Watanabe K. Mitogen-activated protein kinases as key players in osmotic stress signaling. Biochim Biophys Acta. 2016;1860:2037-2052. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 56. | Lang F. Mechanisms and significance of cell volume regulation. J Am Coll Nutr. 2007;26:613S-623S. [PubMed] [Cited in This Article: ] |
| 57. | Sjøgaard G, Adams RP, Saltin B. Water and ion shifts in skeletal muscle of humans with intense dynamic knee extension. Am J Physiol. 1985;248:R190-R196. [PubMed] [Cited in This Article: ] |
| 58. | Sjøgaard G. Water and electrolyte fluxes during exercise and their relation to muscle fatigue. Acta Physiol Scand Suppl. 1986;556:129-136. [PubMed] [Cited in This Article: ] |
| 59. | Mitchell CJ, Churchward-Venne TA, West DW, Burd NA, Breen L, Baker SK, Phillips SM. Resistance exercise load does not determine training-mediated hypertrophic gains in young men. J Appl Physiol (1985). 2012;113:71-77. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 402] [Cited by in F6Publishing: 428] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 60. | Morton RW, Oikawa SY, Wavell CG, Mazara N, McGlory C, Quadrilatero J, Baechler BL, Baker SK, Phillips SM. Neither load nor systemic hormones determine resistance training-mediated hypertrophy or strength gains in resistance-trained young men. J Appl Physiol (1985). 2016;121:129-138. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 251] [Cited by in F6Publishing: 243] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 61. | Schoenfeld BJ, Peterson MD, Ogborn D, Contreras B, Sonmez GT. Effects of Low- vs. High-Load Resistance Training on Muscle Strength and Hypertrophy in Well-Trained Men. J Strength Cond Res. 2015;29:2954-2963. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 199] [Cited by in F6Publishing: 233] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 62. | Helms ER, Cronin J, Storey A, Zourdos MC. Application of the Repetitions in Reserve-Based Rating of Perceived Exertion Scale for Resistance Training. Strength Cond J. 2016;38:42-49. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 89] [Cited by in F6Publishing: 110] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 63. | Abe T, Beekley MD, Hinata S, Koizumi K, Sato Y. Day-today change in muscle strength and MRI-measured skeletal muscle size during 7 days KAATSU resistance training: A case study. Int J KAATSU Train Res. 2005;1:71-76. [Cited in This Article: ] |
| 64. | Takarada Y, Sato Y, Ishii N. Effects of resistance exercise combined with vascular occlusion on muscle function in athletes. Eur J Appl Physiol. 2002;86:308-314. [PubMed] [Cited in This Article: ] |
| 65. | Wilson JM, Lowery RP, Joy JM, Loenneke JP, Naimo MA. Practical blood flow restriction training increases acute determinants of hypertrophy without increasing indices of muscle damage. J Strength Cond Res. 2013;27:3068-3075. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 80] [Cited by in F6Publishing: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 66. | Clark BC, Manini TM, Hoffman RL, Williams PS, Guiler MK, Knutson MJ, McGlynn ML, Kushnick MR. Relative safety of 4 weeks of blood flow-restricted resistance exercise in young, healthy adults. Scand J Med Sci Sports. 2011;21:653-662. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 111] [Cited by in F6Publishing: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Loenneke JP, Young KC, Wilson JM, Andersen JC. Rehabilitation of an osteochondral fracture using blood flow restricted exercise: a case review. J Bodyw Mov Ther. 2013;17:42-45. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 29] [Cited by in F6Publishing: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 68. | Libardi CA, Chacon-Mikahil MP, Cavaglieri CR, Tricoli V, Roschel H, Vechin FC, Conceição MS, Ugrinowitsch C. Effect of concurrent training with blood flow restriction in the elderly. Int J Sports Med. 2015;36:395-399. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 65] [Cited by in F6Publishing: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 69. | Kaijser L, Sundberg CJ, Eiken O, Nygren A, Esbjörnsson M, Sylvén C, Jansson E. Muscle oxidative capacity and work performance after training under local leg ischemia. J Appl Physiol (1985). 1990;69:785-787. [PubMed] [Cited in This Article: ] |
| 70. | Takada S, Okita K, Suga T, Omokawa M, Kadoguchi T, Sato T, Takahashi M, Yokota T, Hirabayashi K, Morita N. Low-intensity exercise can increase muscle mass and strength proportionally to enhanced metabolic stress under ischemic conditions. J Appl Physiol (1985). 2012;113:199-205. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 75] [Cited by in F6Publishing: 85] [Article Influence: 7.1] [Reference Citation Analysis (1)] |
| 71. | Farup J, de Paoli F, Bjerg K, Riis S, Ringgard S, Vissing K. Blood flow restricted and traditional resistance training performed to fatigue produce equal muscle hypertrophy. Scand J Med Sci Sports. 2015;25:754-763. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 107] [Cited by in F6Publishing: 123] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 72. | Ellefsen S, Hammarström D, Strand TA, Zacharoff E, Whist JE, Rauk I, Nygaard H, Vegge G, Hanestadhaugen M, Wernbom M. Blood flow-restricted strength training displays high functional and biological efficacy in women: a within-subject comparison with high-load strength training. Am J Physiol Regul Integr Comp Physiol. 2015;309:R767-R779. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 87] [Cited by in F6Publishing: 75] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Laurentino G, Ugrinowitsch C, Aihara AY, Fernandes AR, Parcell AC, Ricard M, Tricoli V. Effects of strength training and vascular occlusion. Int J Sports Med. 2008;29:664-667. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 88] [Cited by in F6Publishing: 80] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 74. | Cumming KT, Paulsen G, Wernbom M, Ugelstad I, Raastad T. Acute response and subcellular movement of HSP27, αB-crystallin and HSP70 in human skeletal muscle after blood-flow-restricted low-load resistance exercise. Acta Physiol (Oxf). 2014;211:634-646. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 52] [Cited by in F6Publishing: 52] [Article Influence: 5.2] [Reference Citation Analysis (1)] |
| 75. | Noble EG, Milne KJ, Melling CW. Heat shock proteins and exercise: a primer. Appl Physiol Nutr Metab. 2008;33:1050-1065. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 104] [Cited by in F6Publishing: 100] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 76. | Zou K, Meador BM, Johnson B, Huntsman HD, Mahmassani Z, Valero MC, Huey KA, Boppart MD. The α₇β1-integrin increases muscle hypertrophy following multiple bouts of eccentric exercise. J Appl Physiol (1985). 2011;111:1134-1141. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 48] [Cited by in F6Publishing: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Suga T, Okita K, Morita N, Yokota T, Hirabayashi K, Horiuchi M, Takada S, Omokawa M, Kinugawa S, Tsutsui H. Dose effect on intramuscular metabolic stress during low-intensity resistance exercise with blood flow restriction. J Appl Physiol (1985). 2010;108:1563-1567. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 85] [Cited by in F6Publishing: 91] [Article Influence: 6.5] [Reference Citation Analysis (5)] |
| 78. | Fujita S, Abe T, Drummond MJ, Cadenas JG, Dreyer HC, Sato Y, Volpi E, Rasmussen BB. Blood flow restriction during low-intensity resistance exercise increases S6K1 phosphorylation and muscle protein synthesis. J Appl Physiol (1985). 2007;103:903-910. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 289] [Cited by in F6Publishing: 294] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 79. | Patterson SD, Leggate M, Nimmo MA, Ferguson RA. Circulating hormone and cytokine response to low-load resistance training with blood flow restriction in older men. Eur J Appl Physiol. 2013;113:713-719. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 40] [Cited by in F6Publishing: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 80. | Serrano AL, Baeza-Raja B, Perdiguero E, Jardí M, Muñoz-Cánoves P. Interleukin-6 is an essential regulator of satellite cell-mediated skeletal muscle hypertrophy. Cell Metab. 2008;7:33-44. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 569] [Cited by in F6Publishing: 568] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 81. | Warren GL, Hulderman T, Jensen N, McKinstry M, Mishra M, Luster MI, Simeonova PP. Physiological role of tumor necrosis factor alpha in traumatic muscle injury. FASEB J. 2002;16:1630-1632. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 182] [Cited by in F6Publishing: 172] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 82. | Gleeson M, Bishop NC. The T cell and NK cell immune response to exercise. Ann Transplant. 2005;10:43-48. [PubMed] [Cited in This Article: ] |
| 83. | Tidball JG. Inflammatory processes in muscle injury and repair. Am J Physiol Regul Integr Comp Physiol. 2005;288:R345-R353. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 829] [Cited by in F6Publishing: 845] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 84. | Kraemer WJ, Ratamess NA. Hormonal responses and adaptations to resistance exercise and training. Sports Med. 2005;35:339-361. [PubMed] [Cited in This Article: ] |
| 85. | Rossi FE, Gerosa-Neto J, Zanchi NE, Cholewa JM, Lira FS. Impact of Short and Moderate Rest Intervals on the Acute Immunometabolic Response to Exhaustive Strength Exercise: Part I. J Strength Cond Res. 2016;30:1563-1569. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 16] [Cited by in F6Publishing: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Phillips MD, Mitchell JB, Currie-Elolf LM, Yellott RC, Hubing KA. Influence of commonly employed resistance exercise protocols on circulating IL-6 and indices of insulin sensitivity. J Strength Cond Res. 2010;24:1091-1101. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 35] [Cited by in F6Publishing: 33] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 87. | Burgomaster KA, Howarth KR, Phillips SM, Rakobowchuk M, Macdonald MJ, McGee SL, Gibala MJ. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J Physiol. 2008;586:151-160. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 719] [Cited by in F6Publishing: 763] [Article Influence: 44.9] [Reference Citation Analysis (1)] |
| 88. | Gibala MJ, Little JP, van Essen M, Wilkin GP, Burgomaster KA, Safdar A, Raha S, Tarnopolsky MA. Short-term sprint interval versus traditional endurance training: similar initial adaptations in human skeletal muscle and exercise performance. J Physiol. 2006;575:901-911. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 674] [Cited by in F6Publishing: 647] [Article Influence: 35.9] [Reference Citation Analysis (1)] |
| 89. | Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Bach R. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665-671. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 701] [Cited by in F6Publishing: 710] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 90. | Wahl P, Mathes S, Köhler K, Achtzehn S, Bloch W, Mester J. Acute metabolic, hormonal, and psychological responses to different endurance training protocols. Horm Metab Res. 2013;45:827-833. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 39] [Cited by in F6Publishing: 39] [Article Influence: 3.5] [Reference Citation Analysis (1)] |
| 91. | Wahl P, Zinner C, Achtzehn S, Bloch W, Mester J. Effect of high- and low-intensity exercise and metabolic acidosis on levels of GH, IGF-I, IGFBP-3 and cortisol. Growth Horm IGF Res. 2010;20:380-385. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 58] [Cited by in F6Publishing: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 92. | Shahidi NT. A review of the chemistry, biological action, and clinical applications of anabolic-androgenic steroids. Clin Ther. 2001;23:1355-1390. [PubMed] [Cited in This Article: ] |
| 93. | Hood DA. Mechanisms of exercise-induced mitochondrial biogenesis in skeletal muscle. Appl Physiol Nutr Metab. 2009;34:465-472. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 149] [Cited by in F6Publishing: 160] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 94. | Jäger S, Handschin C, St-Pierre J, Spiegelman BM. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1alpha. Proc Natl Acad Sci USA. 2007;104:12017-12022. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 1688] [Cited by in F6Publishing: 1806] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 95. | Knutti D, Kressler D, Kralli A. Regulation of the transcriptional coactivator PGC-1 via MAPK-sensitive interaction with a repressor. Proc Natl Acad Sci USA. 2001;98:9713-9718. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 207] [Cited by in F6Publishing: 215] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 96. | Fan M, Rhee J, St-Pierre J, Handschin C, Puigserver P, Lin J, Jäeger S, Erdjument-Bromage H, Tempst P, Spiegelman BM. Suppression of mitochondrial respiration through recruitment of p160 myb binding protein to PGC-1alpha: modulation by p38 MAPK. Genes Dev. 2004;18:278-289. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 240] [Cited by in F6Publishing: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 97. | Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1alpha in human skeletal muscle. J Appl Physiol (1985). 2009;106:929-934. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 257] [Cited by in F6Publishing: 268] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 98. | Little JP, Safdar A, Bishop D, Tarnopolsky MA, Gibala MJ. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1303-R1310. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 209] [Cited by in F6Publishing: 216] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 99. | Ameln H, Gustafsson T, Sundberg CJ, Okamoto K, Jansson E, Poellinger L, Makino Y. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009-1011. [PubMed] [DOI] [Cited in This Article: ] [Cited by in Crossref: 178] [Cited by in F6Publishing: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |