Abstract
Background:
Food allergy is an abnormal immunologic reaction to food proteins. During infancy, allergic colitis presents with bloody stool of a healthy child. calprotectin is released into the intestinal lumen by macrophages and neutrophils and is a reliable and non-invasive biomarker for evaluating inflammation of the digestive system.Objectives:
This study evaluated the changes of fecal calprotectin after modification of mother’s diet, on breastfed infants with food allergy.Methods:
This study was conducted on 29 infants less than one year old with allergic colitis, referred to the Besat hospital of Sanandaj (Iran) from 2013 to 2014. All infants were breast-fed. The fecal calprotectin levels were measured on admission; two and six weeks after starting hypo-allergenic diet for mothers and its levels were correlated with clinical findings.Results:
With the onset of maternal hypoallergenic diet, clinical symptoms showed a statistically significant reduction (P < 0.05). The fecal calprotectin levels decreased during the study. Despite the declining trend of the fecal calprotectin levels, there was no statistical correlation between clinical and laboratory findings (P = 0.741 and P = 0.284).Conclusions:
This study showed that changes on fecal calprotectin levels are not a good indicator for assessment of clinical improvement in food allergy. There was no statistically significant difference between the fecal calprotectin levels on admission, two weeks and six weeks after the intervention.Keywords
1. Background
The incidence of food allergies, like other atopic disorders, has dramatically increased during the past three decades, particularly in developed countries (1). Nowadays, about 3.9% of the American population suffers from the disease (2). About 6% of children experience allergic reactions to food in the first year of their lives. These children are allergic to cow’s milk, eggs and peanuts about 2.5, 1.5 and 1 percent respectively (3). The immaturity of the immune system during infancy allows increased intestinal permeability to food antigens, thus the majority of food hypersensitivity reactions occur at this age (4). Beside those allergens more than 90% of food allergies in children are due also to soy, nuts, wheat, fish and shellfish (3, 5). Typically, food allergies during infancy present with irritability, vomiting, diarrhea and impaired weight gain. These symptoms often begin with feeding of cow’s milk or soy (6). Allergic proctocolitis is the most common allergic reaction. It often occurs as a result of hyper sensibility to cow’s milk protein and can be seen even in exclusively breastfed infants (3, 7). In early life, allergic proctocolitis presents with blood in the stools of a healthy child (8). About 60% of cases occur in breast fed infants, the remaining cases occur in cow’s milk fed infants or soy protein-based formula fed infant (3). The standard method for diagnosis of food allergy is the challenge test: evaluating the symptoms by starting and removal of the suspected food (9). The treatment of food allergy is empiric just by removing some substances from the mother’s diet (3, 4, 6). The gold standard method for detection of improvement is evaluation of patient’s clinical symptoms after starting the diet changes (3). As a result, investigation and follow-up of these patients are always based on clinical response and personal criteria (3). The calprotectin is a sensitive but nonspecific marker that demonstrates inflammation in the gastrointestinal tract (10, 11). This protein is released into the intestinal lumen from macrophages, neutrophils, degradation of cells and apoptosis. This marker is used to indicate disease activity and treatment response in cases of inflammation, such as inflammatory bowel disease (IBD) (10, 12, 13). However, it’s one of the specific markers for other bowel inflammations such bacterial infections (13-15).
2. Objectives
The purpose of this study was to evaluate the diagnostic value of fecal calprotectin as a reliable laboratory marker for therapeutic response in cow’s milk allergy colitis. The investigation was performed due to the high prevalence of cow’s milk allergy colitis in infants, family concern and lack of publications on this issue in Iran.
3. Materials and Methods
This is an interventional prospective cohort study. We enrolled all the breast-fed infants less than one year old with bloody and mucoid stool referred to the gastroenterology clinic of Besat hospital of Sanandaj in Iran from 2013 to 2014. These infants were healthy in every respect; they had a normal weight gain and normal physical examination (except atopic dermatitis). The children were examined by a pediatric gastroenterologist, and if they had inclusion criteria, participated in the study. Therefore the inclusion and exclusion criteria were based on medical history and clinical examination.
The inclusion criteria were: Breast fed Infants less than one year old; Healthy infants with bloody stools; Negative stool culture.
The exclusion criteria were: Abnormal weight gain; Fever; Severe and projectile vomiting; Failure to respond to diet therapy after four weeks.
were The samples were selected among infants less than one year old with suspected cow’s milk protein allergy colitis referred to the gastroenterology clinic of Besat hospital. According to the results of a similar study (16), the sample size was calculated 20 patients. Finally, twenty nine patients were enrolled in the study. Initially, a questionnaire depicting demographic characteristics, history (diarrhea, blood or mucus in the stool and time of onset symptoms) and physical examination findings was completed. Every child was examined three times: at admission, two and six week after starting elimination of the cow’s proteins from their mothers. A separate questionnaire was completed at each visit. The amount of improvement and the level of fecal calprotectin were recorded as well. There is no gold standard test for allergy relief. In this study, we used the diet changes and assessment of clinical symptoms as the gold standard to detect recovery. Stool samples were sent to a special laboratory, and level of calprotectin measured by Calprotection Kits (Buhlmann Co, Switzerland) with ELISA method. The normal range of fecal calprotectin varies according to age. Values in the first year of life are less than 350 μg/g, in children less than 275 μg/g and in adults less than 50 μg/g (13) This issue has also been considered in this study. All mothers and the infants older than 6 months received hypoallergenic regime removing all foods containing cow’s proteins. Then clinical and laboratory response was assessed. Clinical response was determined by the absence of blood and mucus in stool during the first days of regime application according to stool analysis and mothers’ observation.
3.1. Ethical Approval
The study protocol was approved by the ethics committee of Kurdistan University of Medical Sciences. Before the study, informed consent was obtained from parents of participating infants.
3.2. Statistical Analysis
Data on each patient was collected after six weeks and statistical analysis was performed by SPSS software (version 22.0). The mean and median for quantitative data and the frequency and percentage for quality data were calculated. Then difference between quantitative variables was noted before and after the intervention. Spearman correlation coefficient was used to evaluate the correlation between level of fecal calprotectin and severity of symptoms.
4. Results
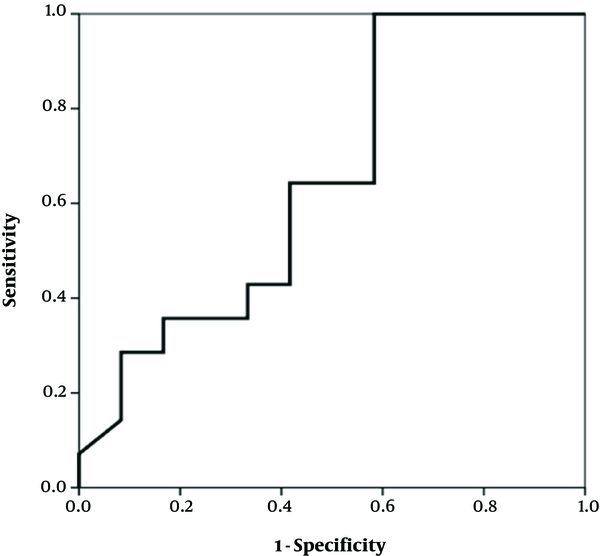
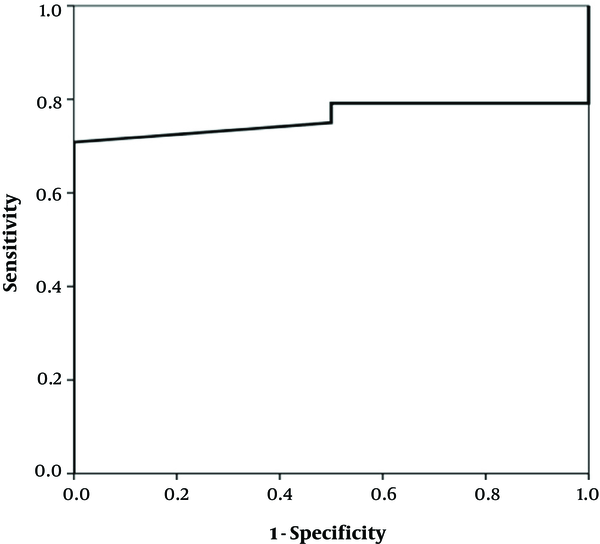
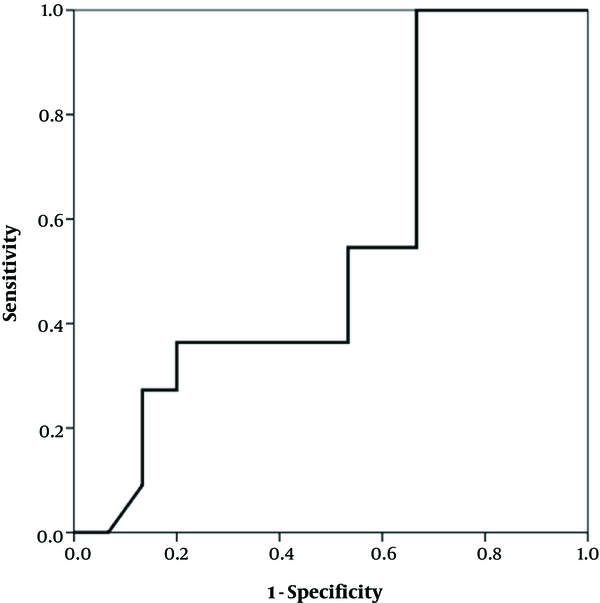
The study enrolled 29 infants under one year, seventeen (58.6%) of whom were female and twelve (41.4%) were male. Their mean age was about 4 months (117.2 days, SD: 65.7). The growth indexes (weight, height and head circumference) were within the normal range at the beginning and after two and six weeks (Table 1). There was a significant difference in the clinical findings (bloody and mucoid diarrhea) before and after initiation of the diet (Table 2). The change in the fecal calprotectin level and improvement of diarrhea and blood in the stool are shown in Figures 1 - 2. The area under the curve in Figure 1 was determined to 0.652, suggesting that changes in fecal calprotectin level was not a good indicator for improvement of diarrhea (P = 0.19). In Figure 2, the area under the curve is estimated to 0.760 which suggests that fecal calprotectin was not a reliable marker to assess blood in the stool in cases with allergic colitis (P = 0.229). Also the area under the curve in Figure 3 was estimated 0.548 suggesting that the level of fecal calprotectin was not a suitable marker for evaluation of mucus in the stool (P = 0.678). The medium of fecal calprotectin levels at baseline, after 2 and 6 weeks of starting the study was 209.1 (SD: 387.9), 189.5 (SD: 382.4) and 125.2 (SD: 105.4), respectively. There was no statistically significant difference between the fecal calprotectin levels at two and six weeks after admission with the beginning of the study (P = 0.741, P = 0.284).
The Growth Index of Patients (Central Index and Distribution)
| Growth Index | Median | SD | 50th Percentile |
|---|---|---|---|
| Beginning | |||
| Weight, kg | 6.4 | 1.4 | 5.4 |
| Height, cm | 62.1 | 5.2 | 61 |
| Head circumference, cm | 40.5 | 2.3 | 40.5 |
| After 2 weeks | |||
| Weight, kg | 6.8 | 1.3 | 5.9 |
| Height, cm | 63.1 | 5.1 | 62.5 |
| Head circumference, cm | 40.9 | 2.3 | 41 |
| After 6 weeks | |||
| Weight, kg | 7.3 | 1.2 | 6.5 |
| Height, cm | 65.1 | 4.9 | 6.4 |
| Head circumference, cm | 41.8 | 2.1 | 42 |
Comparing Intensity of Diarrhea, Blood and Mucus in Stool
| Medium | Middle | SD | P-Value | |
|---|---|---|---|---|
| Intensity of diarrhea | ||||
| Beginning | 5.8 | 5.5 | 1.2 | |
| After 2 weeks | 3.7 | 3.5 | 2.1 | < 0.001 |
| After 6 weeks | 1.3 | 1 | 1.4 | < 0.001 |
| Blood in the stool | ||||
| Beginning | 1.1 | 0 | 1.7 | |
| After 2 weeks | 0.3 | 0 | 0.7 | 0.003 |
| After 6 weeks | 0.2 | 0 | 0.7 | 0.008 |
| Mucus in the stool | ||||
| Beginning | 3.9 | 4.5 | 2.2 | |
| After 2 weeks | 2.2 | 2.5 | 2.0 | < 0.001 |
| After 6 weeks | 0.8 | 0 | 1.3 | < 0.001 |
Fecal calprotectin levels and improvement of diarrhea
Fecal calprotectin levels and blood in stool
Fecal calprotectin levels and mucus in stool
5. Discussion
The calprotectin is a protein which is released into the intestinal lumen from stimulated neutrophils, macrophages, cell destruction and apoptosis. The level of fecal calprotectin in the process of bowel inflammation due to invasion of neutrophils to the digestive system is higher than that in the plasma, for this reason, the fecal calprotectin is used as a reliable and non-invasive marker for evaluation of inflammation. Our study showed that there was no statistical correlation between clinical symptoms and laboratory findings despite that the level of fecal calprotectin showed a relative decline during 6 weeks. The fecal calprotectin was not a good predicting factor for clinical improvement in cow’s milk allergy colitis. In contrast to our study, Beser et al. (17) showed that the average level of fecal calprotectin was significantly different after starting the diet (P < 0.001). Baldassarre et al. (18) found that the average level of fecal calprotectin was significantly higher in the patients than in the control group (P < 0.001), and after starting the diet its level dropped to 50% (P < 0.001). The same results were obtained by Pohl et al. (12). These studies reported that fecal calprotectin can be used as a marker for screening therapeutic response to hypoallergenic diet. Ezri and Nydegger (13) showed that fecal calprotectin correlated with age and the cut-off point for children less than one year was 350 μg/g. Fagerberg et al. (19) demonstrated that fecal calprotectin in IBD is much higher than in normal cases. The presence of neutrophils is more prominent in colonic inflammation and infections while in allergy the presence of eosinophils is evident.
We had two limitations in the study. First, the cost of fecal calprotectin test was high and this after treatment may occur later than the clinical response, hindered us in using it in more patients. Second, some patients did not appear for follow up visits, due to the remoteness of their residence. However, despite these limitations, the results of the study suggest that fecal calprotectin is not an appropriate laboratory marker for diagnosis and treatment. In general it can be concluded, that calprotectin level in stool is not a good indicator of disease improvement and the clinical findings are much more reliable than the laboratory tests.
For future studies a greater sample size and longer follow up time is recommended as the fecal calprotectin level decrease after treatment may occur later .than the clinical response, so it is necessary to wait a longer time to obtain a paraclinical response to treatment.
Acknowledgements
References
-
1.
Prescott SL, Pawankar R, Allen KJ, Campbell DE, Sinn JKH, Fiocchi A, et al. A global survey of changing patterns of food allergy burden in children. World Allergy Organ J. 2013;6(1):21. [PubMed ID: 24304599]. [PubMed Central ID: PMC3879010]. https://doi.org/10.1186/1939-4551-6-21.
-
2.
Taylor-Black S, Wang J. The prevalence and characteristics of food allergy in urban minority children. Ann Allergy Asthma Immunol. 2012;109(6):431-7. [PubMed ID: 23176883]. [PubMed Central ID: PMC4280673]. https://doi.org/10.1016/j.anai.2012.09.012.
-
3.
Anna NW, Hugh AS, Scott HS. Food Allergy and Adverse Reactions to Foods. In: Richard EB, Robert MK, Bonita FS, Joseph WSGI, Nina FS, editors. Nelson Textbook of Pediatrics. 20 ed. Philadelphia: Elsevier; 2016. p. 1137-42.
-
4.
Torrente F, Murch S. Food allergy enteropathy. In: Kleinman RE, Sanderson IR, Goulet O, editors. Walker's Pediatric Gastrointestinal Disease. 5 ed. Hamilton: McGraw-Hill Medical Publishing Division; 2008. p. 329-37.
-
5.
Berni Canani R, Di Costanzo M, Troncone R. The optimal diagnostic workup for children with suspected food allergy. Nutrition. 2011;27(10):983-7. [PubMed ID: 21907896]. https://doi.org/10.1016/j.nut.2011.07.006.
-
6.
Markowitz ME, Liacouras CA. Allergic and Eosinophilic Gastrointestinal Disease. In: Wyllie R, Hyams JS, editors. Pediatric Gastrointestinal and Liver Disease. 4 ed. Philadelphia: Elsevier; 2011. p. 395-404000. https://doi.org/10.1016/b978-1-4377-0774-8.10038-7.
-
7.
Troncone R, Discepolo V. Colon in food allergy. J Pediatr Gastroenterol Nutr. 2009;48 Suppl 2:S89-91. [PubMed ID: 19300136]. https://doi.org/10.1097/MPG.0b013e3181a15d1a.
-
8.
Academy of Breastfeeding Medicine. ABM Clinical Protocol #24: Allergic Proctocolitis in the Exclusively Breastfed Infant. Breastfeed Med. 2011;6(6):435-40. [PubMed ID: 22050274]. https://doi.org/10.1089/bfm.2011.9977.
-
9.
Vandenplas Y, Koletzko S, Isolauri E, Hill D, Oranje AP, Brueton M, et al. Guidelines for the diagnosis and management of cow's milk protein allergy in infants. Arch Dis Child. 2007;92(10):902-8. [PubMed ID: 17895338]. [PubMed Central ID: PMC2083222]. https://doi.org/10.1136/adc.2006.110999.
-
10.
Berni Canani R, Rapacciuolo L, Romano MT, Tanturri de Horatio L, Terrin G, Manguso F, et al. Diagnostic value of faecal calprotectin in paediatric gastroenterology clinical practice. Dig Liver Dis. 2004;36(7):467-70. [PubMed ID: 15285526]. https://doi.org/10.1016/j.dld.2004.02.009.
-
11.
Bjarnason I, Sherwood R. Fecal calprotectin: a significant step in the noninvasive assessment of intestinal inflammation. J Pediatr Gastroenterol Nutr. 2001;33(1):11-3. [PubMed ID: 11479401].
-
12.
Pohl J, Azuma L, Watts M, Easley D. Fecal Calprotectin and Cow??S Milk Protein Allergy in Infants. J Pediatr Gastroenterol Nutr. 2005;41(4):515. https://doi.org/10.1097/01.mpg.0000181929.86800.78.
-
13.
Ezri J, Nydegger A. [Pediatrics. Fecal calprotectin in children: use and interpretation]. Rev Med Suisse. 2011;7(277):69-70. French. [PubMed ID: 21309180].
-
14.
Paduchova Z, Durackova Z. Fecal calprotectin as a promising marker of inflammatory diseases. Bratisl Lek Listy. 2009;110(10):598-602. [PubMed ID: 20017448].
-
15.
Abraham BP, Kane S. Fecal markers: calprotectin and lactoferrin. Gastroenterol Clin North Am. 2012;41(2):483-95. [PubMed ID: 22500530]. https://doi.org/10.1016/j.gtc.2012.01.007.
-
16.
Walkiewicz D, Werlin SL, Fish D, Scanlon M, Hanaway P, Kugathasan S. Fecal calprotectin is useful in predicting disease relapse in pediatric inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(5):669-73. https://doi.org/10.1002/ibd.20376.
-
17.
Beser OF, Sancak S, Erkan T, Kutlu T, Cokugras H, Cokugras FC. Can Fecal Calprotectin Level Be Used as a Markers of Inflammation in the Diagnosis and Follow-Up of Cow's Milk Protein Allergy? Allergy Asthma Immunol Res. 2014;6(1):33-8. [PubMed ID: 24404391]. [PubMed Central ID: PMC3881398]. https://doi.org/10.4168/aair.2014.6.1.33.
-
18.
Baldassarre ME, Laforgia N, Fanelli M, Laneve A, Grosso R, Lifschitz C. Lactobacillus GG improves recovery in infants with blood in the stools and presumptive allergic colitis compared with extensively hydrolyzed formula alone. J Pediatr. 2010;156(3):397-401. [PubMed ID: 19880141]. https://doi.org/10.1016/j.jpeds.2009.09.012.
-
19.
Fagerberg UL, Loof L, Myrdal U, Hansson LO, Finkel Y. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J Pediatr Gastroenterol Nutr. 2005;40(4):450-5. [PubMed ID: 15795593].