Abstract
Objectives:
The study aimed to evaluate the long-term compliance and reasons for discontinuation of intravesical botulinum toxin A (BoNT-A) treatment in women with idiopathic overactive bladder syndrome (iOAB).Methods:
All patients who had been treated with BoNT-A between 2004 and 2010 were invited to join a written survey in June, 2015. Only women with idiopathic OAB symptoms were included. The survey was designed first to assess current urinary symptoms in patients who failed BoNT-A treatment, and second to evaluate their experiences with BoNT-A and subsequent treatments.Results:
In total, 74 patients who discontinued BTX-A treatment were identified. The response rate was 62%. The most common reasons for discontinuation of treatment were insufficient effect (37%), the need for clean intermittent self-catheterization (CISC, 13%), and urinary tract infections (UTI, 9%). 17% of the patients reported that they did not want a new treatment despite good effects. After an average follow-up of 92 months, more than three quarters (76%) of the patients suffered from urgency incontinence. In most patients, incontinence had a great influence on daily life (average score of 7 on a scale of 10). During the time of this survey, 25% of the patients used drugs for their OAB symptoms, 15% were referred for sacral neuromodulation, and 1 patient underwent urinary deviation.Conclusions:
Our study provides important information on the follow-up and the reasons for discontinuation in patients with idiopathic OAB. This information can be used in counselling of patients and further improvement of BoNT-A treatment.Keywords
Overactive Bladder Botulinum Toxin Incontinence Urgency Idiopathic
1. Background
Overactive bladder syndrome is a prevalent disorder which can have a significant impact on quality of life (1). Pharmacologic treatment (antimuscarinics or mirabegron) and conservative management options (lifestyle modifications, bladder training) are the first-line treatment for idiopathic overactive bladder (iOAB) (2). Patients who have symptoms that are refractory to conservative management can be treated with various second-line treatment options including botulinum toxin type A (BoNT-A), percutaneous tibial nerve stimulation (PTNS), or sacral neuromodulation (SNM). The treatment of choice mainly depends on physician experience and patient preference. The advantages of BoNT-A treatment include: reversibility, easily adaptable technique, minimally-invasiveness, and office-based setting. Possible disadvantages are: risk of urinary retention requiring CISC, urinary tract infections, and the need for repeated injections after usually 6 - 12 months. The approval of BoNT-A for idiopathic OAB was based on the 2 phase 3 randomized controlled trials, which demonstrated that BoNT-A 100U significantly improved OAB symptoms and QOL vs. placebo (3, 4). Recently, Nitti et al. showed (in a prospective extension of a phase 3 trial) that the efficacy and safety of BoNT-A are durable after a follow-up of 3.5 years (5). Improvements were seen in overactive bladder symptoms and quality of life, and a high proportion of patients rated their condition as improved/greatly improved.
However, little is known about the patients who discontinue BoNT-A for various reasons. According to a real-life observational study of patients with iOAB who underwent BoNT-A, nearly 70% of patients discontinue treatment after long-term follow-up (6). A better understanding of why most patients discontinue treatment (e.g. insufficient effect, adverse events, and switch to other treatments) is therefore crucial. Moreover, we do not know what happens to patients who fail BoNT-A. Do most patients find a satisfactory treatment for their complaints?
2. Objectives
In this study, we evaluated the long-term data of all patients who discontinued BoNT-A.
3. Methods
In order to evaluate the results of BoNT-A in a homogeneous population, we only included women with idiopathic overactive bladder symptoms in this study. All patients were refractory or intolerant to anticholinergics, pelvic floor rehabilitation, and/or bladder training. Patients with neurogenic bladder dysfunction, interstitial cystitis, or bladder pain syndrome were excluded. All the patients received 200 U of onabotulinum (Allergan, Irvine, CA, USA) in 20 intra-detrusor injections. All patients who had been treated with BoNT-A between 2004 and 2010 were invited to join a written survey in June, 2015. We decided to include only the patients with at least 5 years follow-up in order to evaluate the course of symptoms in long-term. Only women with idiopathic, non-neurogenic OAB wet symptoms were included. The survey was designed for two purposes: first to assess the current urinary symptoms in patients who failed BoNT-A treatment, and second to evaluate their experiences with BoNT-A and subsequent treatments.
Urinary symptoms were evaluated with the validated Dutch version of the International Consultation on incontinence questionnaire (ICIQ). Patient experiences with BoNT-A were evaluated using a questionnaire, which addressed the reasons for discontinuation, adverse events, subsequent treatments, and the use of anticholinergic medication. The patients were asked how much burden (pain, stress, anxiety) they experienced with BoNT-A injections on a scale of 0 (not at all) to 10 (very much). They were also asked if they would be willing to undergo BoNT-A treatment again on a scale ranging from 0 (certainly No) to 10 (certainly Yes). The patient charts were reviewed to identify if the patients underwent other treatments after BoNT-A (e.g. sacral neuromodulation, PTNS, urinary deviation, stress incontinence surgery). Since some of the patients received treatment or follow-up in other hospitals, the patients were also asked to indicate if they received any other treatment. The use of medication for urinary symptoms (i.e. anticholinergics and mirabegron) was also recorded.
4. Results
In total, 134 patients were treated with BoNT-A between 2004 and 2010. Of these, 74 patients who discontinued BoNT-A treatment were identified. The results of this long-term observational study have recently been published (7). The mean follow-up was 92 months. Of the 74 questionnaires sent, 46 returned back (response rate of 62%). Of the 28 patients who did not participate in the survey, 16 were deceased, 4 were unreachable, and 8 rejected participation. All the patients (100%) underwent at least one treatment with BoNT-A, 28% had two treatments and 20% had three or more treatments. The baseline patient characteristics are shown in Table 1. In general, the patients rated the burden (e.g. pain, stress, anxiety) of the treatment as low (average score 3.2 out of a scale of 10). Figure 1 shows the main reasons for discontinuation of BoNT-A. The most common reasons for discontinuation of treatment were insufficient effect (37%), the need for clean intermittent self-catheterization (CISC, 13%), and urinary tract infections (UTI, 9%). 17% of the patients reported that they did not want a new treatment despite good effects.
Patient Characteristics at Baseline
| Patient Characteristics at Baseline (N = 46) | |
|---|---|
| Mean age (years) | 67 |
| Urologic history (No. of procedures) | |
| Suburethral sling | 9 |
| Colposuspension (Burch) | 10 |
| Bulking agents | 2 |
| Neuromodulation (PTNS/SNM) | 6 |
| Gynaecologic history (No. of procedures) | |
| Hysterectomy | 34 |
| Prolapse surgery | 11 |
| Comorbidity (No. of patients) | |
| Diabetes | 10 |
| COPD | 4 |
| Cerebrovascular disease | 4 |
Reasons for BoNT-A Discontinuation
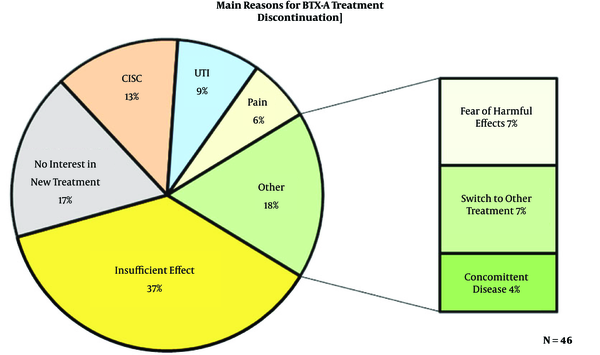
The results of ICIQ are shown in Table 2. patients (80%) suffered from daily incontinence with 63% having moderate to severe leakages. A considerable amount of patients had symptoms (according to the ICIQ) of stress urinary incontinence; 50% had urine loss when coughing or sneezing, and 37% had urine loss during physical exercise. In most patients, incontinence had a great influence on daily life (average score of 7 on a scale of 10). Table 3 shows the subsequent treatments for patients who had BoNT-A failure and current medication use. During the time of this survey, 25% of the patients used drugs for their OAB symptoms, 15% were referred for sacral neuromodulation, and 1 patient underwent urinary deviation. Furthermore, 14 patients (30%) underwent surgery for stress incontinence (mainly trans-obturator tension free tape TOT) during follow-up.
Results of the ICIQ Questionnaire
| ICIQ Questions | ||
|---|---|---|
| When does urine leak? | ||
| Leaks before you can get to the toilet | 24 | 52% |
| Leaks when you cough or sneeze | 23 | 50% |
| Leaks when you are asleep | 10 | 22% |
| Leaks when you are physically active/exercising | 17 | 37% |
| Leaks when you have finished urinating and are dressed | 9 | 19% |
| Leaks for no obvious reason | 18 | 39% |
| Leaks all the time | 11 | 24% |
| How often do you leak urine? | ||
| Never | 1 | 2% |
| About once a week or less often | 5 | 11% |
| Two or three times a week | 3 | 6% |
| About once a day | 2 | 4% |
| Several times a day | 23 | 50% |
| All the time | 12 | 26% |
| How much do you leak urine? | ||
| None | 1 | 2% |
| A small amount | 16 | 35% |
| A moderate amount | 17 | 37% |
| A large amount | 12 | 26% |
Subsequent Treatments After BoNT-A Failure
| Subsequent Treatments | ||
|---|---|---|
| Sacral Neuromodulation | 7 | 15% |
| Urinary diversion | 1 | 2% |
| Medication | 12 | 26% |
| Mirabegron | 6 | 13% |
| Anticholinergics | 6 | 13% |
5. Discussion
Intravesical injection with BoNT-A is a well-established treatment for overactive bladder syndrome (8, 9). Many studies focus on treatment efficacy and follow-up of patients who were successfully treated. Our study is the first to focus on the failures of BoNT-A treatment. Only a few studies have evaluated the long-term (> 5 years) results in patients with idiopathic OAB. Mohee at al. evaluated a group of 268 patients with OAB over a period of 7 years (6). Over the time, almost two-thirds of patients discontinued treatment, mainly due to tolerability issues. Of all the patients who stopped BoNT-A therapy, 51% went back to a management plan based upon lifestyle changes and anticholinergics. Veeratterapillay et al. reported a discontinuation rate of 25% after 60 months (10). In this study, both patients with idiopathic and patients with neurogenic OAB were included. Most patients discontinued treatment after a single injection.
It is important to know why patients discontinue BoNT-A in order to improve the treatment efficacy and compliance. In our study, the main reasons for discontinuation were insufficient effect, the need for CISC, and urinary tract infections. Most patients stopped therapy after 1 or 2 treatment sessions. Interestingly, 8 patients (17%) in our study discontinued BoNT-A despite satisfactory results and demanded no new treatment. The impact on daily life according to the ICIQ in these patients was considerably lower compared to the rest of the study population (mean score 4.2 vs. 7.6). This might imply that some patients experience a permanent reduction in OAB symptoms after one or two BoNT-A treatments. This reduction can be attributed to the botulinum toxin or other factors that remain uncertain. It is also important to realize that some of the patients discontinued treatment due to other reasons such as fear of harmful effects. Perhaps if these patients are properly informed about BoNT-A, failure can be prevented.
According to the ICIQ results, nearly all (98%) of the patients had a certain degree of urinary incontinence. The majority of them suffered from daily incontinence with a great impact on daily life (average score 7/10). Only 43% received subsequent treatment after failure of BoNT-A (Table 2). Hence, many patients are left untreated with a significant disease burden. We also found a high rate of stress urinary incontinence (according to the ICIQ) in these patients and 30% already underwent surgery for SUI during follow-up. From these results, it is clear that the patients in our study have severe, refractory, mixed urinary incontinence instead of merely OAB symptoms. It is unclear if our patients had all of these symptoms initially or if these symptoms changed over time due to progressive diseases. Since the mean follow-up of our cohort was 92 months, the latter might be well possible. It would be interesting to re-evaluate these patients at our clinic to see if there is a change in bladder function and/or anatomy.
This study had several limitations. The questionnaire used for evaluation of patient experiences was not validated. Also, some of the questions that we used may be prone to recall bias. The response rate in our survey was 62%. Although this response might seem low, only 8 out of 46 patients (17%) declined to participate (the others were deceased or unreachable), which reduces the chance of selection bias. We are also aware of the relatively small sample size and retrospective nature of our study. Nevertheless, current data on long-term follow-up in BoNT-A treatment are still sparse and there are practically no data on patients who fail BoNT-A treatment. Hence, the results of this exploratory study are valuable and can be used to improve future treatment.
5.1. Conclusion
Our study provides important information on the follow-up and reasons for discontinuation of BoNT-A in patients with idiopathic OAB. Most patients do not find a satisfactory treatment for their symptoms and are left with a significant disease burden. This information can be used in counselling of patients and further improvement of BoNT-A treatment.
References
-
1.
Irwin DE, Kopp ZS, Agatep B, Milsom I, Abrams P. Worldwide prevalence estimates of lower urinary tract symptoms, overactive bladder, urinary incontinence and bladder outlet obstruction. BJU Int. 2011;108(7):1132-8. [PubMed ID: 21231991]. https://doi.org/10.1111/j.1464-410X.2010.09993.x.
-
2.
Lucas MG, Bosch RJ, Burkhard FC, Cruz F, Madden TB, Nambiar AK, et al. EAU guidelines on surgical treatment of urinary incontinence. Actas Urol Esp. 2013;37(8):459-72. [PubMed ID: 23835037]. https://doi.org/10.1016/j.acuro.2013.02.002.
-
3.
Dmochowski R, Chapple C, Nitti VW, Chancellor M, Everaert K, Thompson C, et al. Efficacy and safety of onabotulinumtoxinA for idiopathic overactive bladder: a double-blind, placebo controlled, randomized, dose ranging trial. J Urol. 2010;184(6):2416-22. [PubMed ID: 20952013]. https://doi.org/10.1016/j.juro.2010.08.021.
-
4.
Sievert KD, Chapple C, Herschorn S, Joshi M, Zhou J, Nardo C, et al. OnabotulinumtoxinA 100U provides significant improvements in overactive bladder symptoms in patients with urinary incontinence regardless of the number of anticholinergic therapies used or reason for inadequate management of overactive bladder. Int J Clin Pract. 2014;68(10):1246-56. [PubMed ID: 24754838]. https://doi.org/10.1111/ijcp.12443.
-
5.
Nitti VW, Ginsberg D, Sievert KD, Sussman D, Radomski S, Sand P, et al. Durable Efficacy and Safety of Long-Term OnabotulinumtoxinA Treatment in Patients with Overactive Bladder Syndrome: Final Results of a 3.5-Year Study. J Urol. 2016;196(3):791-800. [PubMed ID: 27038769]. https://doi.org/10.1016/j.juro.2016.03.146.
-
6.
Mohee A, Khan A, Harris N, Eardley I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (OAB). BJU Int. 2013;111(1):106-13. [PubMed ID: 22672569]. https://doi.org/10.1111/j.1464-410X.2012.11282.x.
-
7.
Marcelissen TA, Rahnama'i MS, Snijkers A, Schurch B, De Vries P. Long-term follow-up of intravesical botulinum toxin-A injections in women with idiopathic overactive bladder symptoms. World J Urol. 2017;35(2):307-11. [PubMed ID: 27272312]. https://doi.org/10.1007/s00345-016-1862-y.
-
8.
Habashy D, Losco G, Tse V, Collins R, Chan L. Botulinum toxin (OnabotulinumtoxinA) in the male non-neurogenic overactive bladder: clinical and quality of life outcomes. BJU Int. 2015;116 Suppl 3:61-5. [PubMed ID: 26176660]. https://doi.org/10.1111/bju.13110.
-
9.
Cui Y, Wang L, Liu L, Zeng F, Niu J, Qi L, et al. Botulinum toxin-A injections for idiopathic overactive bladder: a systematic review and meta-analysis. Urol Int. 2013;91(4):429-38. [PubMed ID: 23970316]. https://doi.org/10.1159/000351037.
-
10.
Veeratterapillay R, Harding C, Teo L, Vasdev N, Abroaf A, Dorkin TJ, et al. Discontinuation rates and inter-injection interval for repeated intravesical botulinum toxin type A injections for detrusor overactivity. Int J Urol. 2014;21(2):175-8. [PubMed ID: 23819724]. https://doi.org/10.1111/iju.12205.