This study is the first human study on visualization and quantification of early retention of CD34+ cells delivered transcoronary with a non-occlusive technique. Our principle findings are as follows. First, in contrast to OTW coronary-occluding technique, no intolerance or ventricular arrhythmia occur in patients with a recent MI with cell administration through side-holed perfusion catheter dedicated to cell delivery. Secondly, irrespective of the transcoronary delivery method, early myocardial uptake of 99mTc activity is in the order of ≈5% (range from ≈2% to ≈10%), consistent with the idea that only a small fraction of native (non-engineered) CD34+ cells spontaneously homes to the injured myocardium. Moreover, in the vast majority of patients early myocardial retention of radioactivity is limited to the (viable) peri-infarct zone, indicating that the infarct core zone is not directly accessible to the transcoronary-administered cells.
Coronary Occlusive Versus Non-Occlusive Cell-Delivery: The Rationale
For transcoronary cell delivery, clinical studies have universally
1-
3,
7,
8 adopted the stop-flow technique that is based on the use of the central lumen of a coronary-occluding OTW balloon catheter positioned in the stent. To deliver the cells, the OTW balloon is inflated leading to a flow arrest in the epicardial artery, the guidewire is then removed and the cell suspension is injected.
7 An effective flow arrest on balloon inflation
35 precludes physiological flow-mediated endothelial rolling of the cells in the epicardial artery during cells injection. Then, on balloon deflation there is an instantaneous reactive increase in the epicardial and capillary flow velocity (with normal values exceeded by ≥2-fold
35). Flow-related hydrodynamic forces affect selecin bonds that physiologically form between the administered cells and the endothelium.
11 Moreover, the “waterfall” effect
35 of reactive hyperemia can contribute to a rapid, undesired cell wash-out, thereby limiting the proportion of cells that can effectively interact with the activated endothelium situated downstream (i.e., in the injury zone) and
trans-migrate into the injury zone. In the pig model of myocardial infarction, dynamic scintigraphy of [
18F]-fluorodezoxyglucose (FDG)-labeled progenitor cells recently confirmed a rapid loss of ca
. 80% cells on OTW balloon deflation,
21 whereas in humans repeated balloon occlusions of the infarct artery for cell delivery were reported to stimulate adverse regional no-flow in the infracted myocardial tissue.
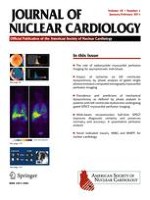
36 In addition, OTW-cell delivery can be associated with the triggering of malignant ventricular arrhythmias
4,
34 (Figure
1). This effect is not unexpected, since the forming myocardial scar is an arrhythmic substrate and ischemia is a well-known arrhythmic trigger. Since prior study protocols included the OTW-balloon IRA occlusions for periods ranging from 3 min
1-
3,
7 to 9 ± 6 min,
8 the pro-arrhythmic effect of IRA occlusions for cell delivery is likely to have been under-reported.
We hypothesized that a non-occlusive, endothelium-targeting cells’ delivery through a side-holed PC might enhance physiological homing (1) by enabling undisturbed physiological rolling in contact with the endothelium of a greater proportion of infused CD34+ cells (increase in an effective contact probability), and (2) by avoiding the accelerated cell wash-out on balloon deflation.
The Magnitude of Early Myocardial Radioactivity Uptake (99mTc-Labeled Cells)
Early myocardial uptake of CD34
+ cells radioactivity was not different in PC- and OTW-delivery (≈5%; Table
2, Figure
3). A similar uptake (5.5%) was determined by PET for
18F-FDG-labeled peripheral CD34
+ cells 1 hour after conventional OTW-delivery.
37 These results do not confirm earlier pilot findings that suggested a higher myocardial uptake of selected CD34
+ cells than the uptake of unselected mononuclear bone marrow cells (14%-39% vs. 1.3%-2.6%, for each group n = 3, PET imaging of
18F-FDG labeled cells).
29 Indeed, the ineffectiveness of ex vivo selection of CD34
+ from the pool of mononuclear cells on the proportion of myocardial engraftment might underlie the lack of outcome difference for the use of selected CD34
+ versus unselected mononuclear cells in a recent large randomized study.
3
Why would a “physiological” delivery not translate into an increased early engraftment of CD34
+ cells? First, the side-holed PC may have failed in the intended delivery of a significant proportion of the cells to the epicardial artery endothelium. Individual cell rolling in contact with the endothelium has been recently visualized (demonstrated qualitatively),
38 but no current imaging technique can quantify this effect (i.e., determine the proportion of cells rolling in contact with endothelium after the PC vs OTW delivery to the epicardial artery). Thus, a later step in the “fate” of the cells was quantified, i.e., the magnitude of early engraftment inferred from percent activity uptake in the myocardium (Figure
3). Secondly, the absence of flow-mediated endothelial rolling during balloon occlusion (IRA flow ceased) and the enhanced rapid cell washout on balloon deflation could both be compensated by a temporary ischemia-induced stimulation of cell-adhesion mechanisms in the injury (border-) zone that may occur with repeated IRA balloon inflations. It has been shown, for instance, that the endothelial expression of P-selectin on cardiac microvessels can be rapidly up-regulated (via transport to the endothelial cell surface) by brief episodes of ischemia.
39,
40 Recent work in the pig, however, suggested a possible ≈30% reduction in delivery efficiency for cell injection with an inflated vs. non-inflated OTW balloon catheter (
111Indium cell labeling; myocardial retention at one hour of 6.1% ± 2.5% vs 4.1% ± 1%),
41 whereas in this study PC-injections were not less effective than
trans-OTW cells’ administration (Figure
3). Evidence shows that flow-mediated endothelial rolling is mandatory for subsequent cell adhesion and extravasation in the zone of activated endothelium.
5 Even if the side-holed PC method was indeed successful in directing the cells towards the endothelium, it might have no significant impact on the microcirculatory flow (and thus cell uptake) because the “distance” of rolling necessary for optimal down-stream adhesion is undetermined.
5,
38 A third (and not unlikely) possibility is that the pool of native (non-engineered) CD34
+ cells used in clinical studies to-date may contain only a fraction of cells that are “naturally” capable of colonizing the myocardial infarct injury zone (i.e., possess a desirable pattern of receptors and appropriate functional capacity). This concept is exemplified, for instance, by the presence of the CXCR4 receptor in less than 50% of CD34
+ cells.
3 Although CXCR4 is mandatory, it is not sufficient for homing to the injury zone.
42 This indicates that other (yet unidentified) receptors are needed for CD34
+ homing to the injured myocardium, and only a fraction of CD34
+ cells may spontaneously express the “full” (desired) pattern.
Implications of Peri-Infarct Engraftment
It is not unexpected that selective transcoronary approach is ineffective for cell delivery to the non-perfused myocardium because lack of tissue perfusion in the infarcted area would prohibit direct uptake of transcoronary-delivered cells in the infarct central zone (Figure
4I). However, progenitor cells (including bone marrow-derived tissue-committed stem cells)
5,
42,
43 are known to migrate in the gradient of hypoxia-induced chemokines such as SDF-1.
42,
43 Recently, the gradient of SDF-1 has been shown to increase 4-fold from the border to the center of the infarct area,
44 indicating that endogenous mechanisms can stimulate cell migration from the infarct border-zone towards the center of the forming scar. Potential determination of the magnitude of this effect, however, was beyond the scope of this study which was focused on short-term cell tracking (
99mTc half-life is 6.03 h, fraction remaining is 50.2% at 6 h and 6.3% at 24 h). Peri-infarct (rather then infarct central-zone) engraftment might contribute to the apparent ineffectiveness of transcoronary-delivered cells in patients with very large myocardial infarctions,
8 indicating that other delivery strategies
22,
36 need to be considered if cell therapy was aimed at large zones of “irreversible” injury. Due to its relatively low spatial resolution (≈8 mm) SPECT does not allow to evaluate the transmural extent of the infarct.
45 Therefore, the apparent diffuse pattern of early cells’ engraftment (Figure
4II) that was detected in a small proportion of patients (12%) may simply reflect a modest myocardial injury, consistent with the relatively small necrotic enzyme release in this patient group. Maintained myocardial perfusion in the infarct injury zone (prior to cell transfer) in subjects with relatively small MIs (Figure
4II) could explain the “negative” outcome of cell therapy in clinical studies including patients with small MIs (c.f., e.g., the group with LVEF >49% in REPAIR-AMI
7) who are a priori (i.e., without cellular therapy) likely to experience only a mild residual damage.
Strategies to Augment Homing
This study shows that, in humans, altering the transcoronary delivery technique is unlikely to increase the “poor” myocardial retention of native (non-engineered) CD34
+ cells delivered 6-14 days after pPCI (Figure
3). Could the delivery efficiency be increased by changing the timing of cell transplantation? This is unlikely for the reasons as follows. First, the timing of cell injections in our study was consistent with the peak of spontaneous release of CD34
+ cells from the bone marrow after MI,
46,
47 while data in animal models indicate that the mechanisms attracting the cells to the ischemic myocardium do not peak immediately after AMI.
44 Second, clinical studies with cells delivery within the first 24 h of AMI have clearly demonstrated no effect of cells injections
1,
2 whereas functional data from REPAIR-AMI (no treatment effect for cell transfer at ≤4 days, the largest effect at 6-14 days)
7 are consistent with the idea that cell administration should match the peak of their spontaneous release from the bone marrow. Indeed, recent study
11 on the relationship between the time of progenitor cells administration and their recruitment to the infracted human myocardium (peak engraftment at 5-14 days; followed by a reduction in engraftment proportional to the time after AMI) indicate that timing of cell delivery in our study matched the optimal potential for myocardial engraftment.
Individual patient data in this study (number of infused CD34
+ cells × proportion engraftment) show a rough estimate of ≈40 000 to ≈400 000 CD34
+ cells taken up in the peri-infarct area, while it is known that only 1:100 to 1:1000 CD34
+ cells is a non-hematopoetic progenitor. Even if mechanisms other than trans-differentiation (for instance, paracrine effects) were to drive myocardial regeneration,
48,
49 this level of early engraftment appears low relative to an average loss of ≈1 billon myocytes and ≈2-3 billion other (such as endothelial) cells with the typical MI in man.
48 Only a fraction of early engrafted cells can survive and exhibit long-term engraftment, and evidence accumulates that the number of administered cells is likely to limit the magnitude of the pro-regenerative effect in animals and in man.
1,
2,
5,
49,
50
Direct needle injections (transepicardial or transendocardial)
22,
36,
51,
52 may lead to heightened, at least initially, local cells retention but these are neither physiological (for instance, the highly hypoxic milieu in the center of the forming scar may reduce cell viability
51) nor widely applicable in the clinical setting. Other strategies to improve cell homing to the injured myocardial tissue include pre-treatment of the cells (to activate their incorporation in the damaged tissue) or pre-treatment of the target tissue (to augment chemoattractant factors).
5,
49 Experimental work has identified several mechanisms of progenitor cell homing to the infarct injury zone, involving molecules such as HGF, VEGF, ICAM-1, HMGB-1, and the SDF-1-CXCR4 axis.
5,
42,
43,
53 Ex vivo activation of adhesion molecules on the cell surface or over-expression of chemokine receptors (such as CXCR4), the use of bispheric antibodies recognizing myosin light chain, or intramyocardial SDF-1 delivery have all been found promising in animal models.
5,
6,
49 However, in mammals, healing after acute ischemic events is naturally biased towards scar formation and not regeneration of functional tissue
49; a constraint that remains to be overcome by cell-based therapeutic strategies.
Study Limitations
In contrast to some other studies,
1-
3,
7,
8,
36 this study was neither aimed nor powered to evaluate the potential effect of cell transfer on functional recovery of infracted myocardium. Much larger studies (e.g., REGENT with 200 patients randomized)
3 have been unable to resolve this issue conclusively, since differentiation between treatment-induced and spontaneous improvement is extremely difficult with study end-points such as LVEF.
54 We deliberately focused on an “earlier” investigative step, i.e., on a systematic evaluation of CD34
+ cells’ myocardial uptake as a
prerequisite for any potential functional effect(s). Although this study population of 34 subjects may appear modest, recruitment of additional patients would be unlikely to impact the findings since there was clearly no signal of any potential difference between engraftment efficiency with the two techniques tested in this study. This study is not only larger than a number of prior randomized studies focused on stem cell therapy approaches
22 but it is also the largest study to-date with labeled progenitor cells in man (prior ones included a maximum of 5 to 8 subjects with recent MI).
11,
28,
37
Direct labeling with radionuclides provides high-sensitivity cell imaging and is the method of choice for clinical studies that address homing and biodistribution after cell injection.
19,
20,
32 One concern with radioactive labeling is the potential radiation damage to the cell (the likelihood of which increases with the label half-time)
20 and the other is an efflux of the label out of the cells, leading to label loss from viable cells and to “false” indication of cell homing by the extracellular tracer.
20 While these concerns are unlikely to apply in any major part to our study (
99mTc-extametazime spontaneous wash-out from the labeled cells is <10% at 60 min), our use of a radiotracer with a short half-life (
T
1/2 of 6.03 h for
99mTc) reduces the time frame during which cells can be imaged.
19,
32 For this reason, the early retention of radioactivity that we observed might partly reflect the cells uptake in the ischemia-activated microcirculation rather than an effective migration to the injured tissue. Nevertheless, recent double-labeling experiments in the murine heart indicated that γ-counter quantification of
99mTc-labeled progenitor cells homing to ischemic muscle highly correlates with counting of immunofluorescent-stained engrafted cells,
53 providing an additional validation of our technique. Although, due to its longer half-life,
111In-oxime (
T
1/2 of 2.8 days) could potentially allow in vivo cell imaging at later time-points, this compound was consistently shown to importantly reduce viability, migration and proliferation of CD34
+ progenitor cells.
25,
26 Moreover, active elimination of
111In-oxime from the cells (>70% eliminated at 72 hours)
55 would introduce significant errors to the detection of cells at later time-points.
20 In contrast to
111In-oxime,
26,
27
99mTc-labeling was found not to affect cells’ functional capacity.
27,
28
Presently, we have not performed cell migration
43 or clonogenicity
4 assays because the study was not aimed at the functional effect of cell therapy and, to maximize cell tracking, we decided that in each patient all the isolated CD34
+ cells would be used for transcoronary transfer. Previously, however, we showed that the
trans-catheter passage had a negligible effect on cell viability and clonogenicity.
4
For detailed evaluation of the cells myocardial uptake zone(s), we used SPECT (and not magnetic resonance imaging, MRI) because of its much greater sensitivity for cell tracking (≈10
3 cells with SPECT vs ≈10
5 with MRI).
5,
20,
32,
34 Moreover, MRI cell imaging with the use of iron oxide tracers is prone to false findings as a result of ferritin iron staining in the forming MI scar
32 and of a rapid loss of signal specificity due to phagocytosis of iron particles released from the cells.
19 The lower spatial resolution of SPECT visualization compared to MRI
19,
32 is the cost for high-sensitivity cell tracking,
20,
32 and it has not hampered our qualitative evaluation of the early myocardial homing pattern (Figure
4). Although it would be of interest to quantify in detail the infarct border zone activity, central zone activity, and the remote myocardial activity with our cell injection techniques, this was not feasible since (1) quantification of the labeled cells myocardial uptake was performed on whole body (planar) images, whereas the qualitative evaluation of the pattern of early engraftment (with image fusion) was performed on tomographic images, and (2) with the resolution of SPECT or PET (≈6-8 mm)
45 it is impossible to reliably sub-divide (and quantify) the proportion of early myocardial retention in the respective zones.
19,
20,
32,
45 Indeed, even the use of improved collimators for in vivo imaging or application of histological techniques allow only qualitative rather than quantitative analysis of labeled cells distribution zone(s).
37,
56
SPECT is unable to discriminate between infarcted (dead) versus stunned tissue. Although (to minimize the effect of stunning), we performed SPECT close (≤36-48 h) to cell delivery on the MI day 6-14, the early uptake of radioactivity on the border of perfusion defect in our study (Figure
4) may still reflect, in part, cells’ retention on the border of stunned (rather than infarcted) tissue. This, however, has no major bearing on the interpretation of our SPECT images that demonstrated lack of cell radioactivity early uptake in the non-perfused infarct zone. Intensive development of hybrid imaging techniques (e.g., reporter gene PET combined with MRI using novel paramagnetic tracers
19) is expected to allow longer-term in vivo monitoring of cell engraftment and survival.
19
While evaluation of distinct transcoronary delivery strategies is clearly beyond the scope of small animal models of MI,
57 it is unlikely that performing our study in a large animal could lead to more insights with regard to the efficiency of the distinct transcoronary cell delivery techniques that we tested. We did initially consider a preliminary study in the pig model of MI but we took the decision against it for the following reasons: (1) the PC technique is not novel to interventional cardiology
15,
16 and a pilot study in man already showed that this technique is safe and feasible for transcoronary cell delivery,
4 (2) for the two delivery methods tested, no current imaging technique could quantify the proportion of cells rolling in contact with endothelium or proportion actually extravasating in the injury zone (thus, with this respect, no animal model would be more informative than the human data), (3) preclinical studies are unlikely to predict the effectiveness of cell delivery techniques in patients.
18 Moreover, the coronary-occlusive (OTW) technique has already been used universally in the clinical setting (over 1,500 patients in clinical studies)
1-
3 without any evidence for being required or effective in stimulating engraftment.
6 This necessitated testing the effectiveness of distinct transcoronary delivery techniques directly in man,
6,
11-
13,
18 as a prerequisite to looking for any potential therapeutic effect.
5 The use of histological techniques or other techniques limited to animal models (such as reported gene assays) to evaluate longer-term engraftment
19,
20,
32 would have had a further role in the context of our study hypothesis if the “basic”
99mTc-labeled cell data SPECT had showed any signal of a difference between the two delivery methods that we tested.
The use of a whole-body single-planar scan with a manual LV delineation could be associated with over-estimation of myocardial engraftment due to inclusion of tracer uptake by, for example, part of the lung. An animal study could offer a direct quantification of the labeled cells activity in explanted organs rather than the indirect evaluation from a whole-body (planar) scan. Such study, however, had already been performed, and heart-harvesting studies of radiolabeled cells homing in the pig
33 have been consistent with whole-body cells’ imaging in the pig
41 and in man.
28,
37 In addition, a recent study on homing of
111In-labeled mesenchymal stem cells in the pig
58 reported an excellent correlation (
r = 0.929) between ex vivo γ-activity of the isolated heart and other organs (lungs, kidneys, spleen, urinary bladder) and the organ activity detected on a whole-body scan.