Abstract
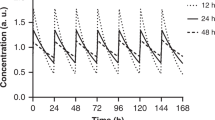
The suboptimal nature of the absorption profiles of human insulin formulations following subcutaneous administration has prompted the development of insulin analogues better suited for therapeutic use in diabetes mellitus. A particular challenge has been to engineer long-acting agents that do not produce unduly variable responses from one injection to another. One recent approach that has met with success has been to acylate, the insulin molecule with a fatty acid, thereby enabling reversible albumin binding. The first clinically available agent of this type is insulin detemir. Pharmacological studies have established that this principle is effective in prolonging action, primarily by retarding absorption. The solubility of insulin detemir in the vial and after injection and an important buffering mechanism effected by plasma albumin binding explain a significant decrease in within-subject variability of pharmacodynamic response observed in repeat isoglycaemic clamp studies where insulin detemir was compared to other basal insulin products. Owing to the extremely high ratio of albumin-binding sites to insulin detemir molecules at therapeutic concentrations, no safety considerations have been identified pertaining to albumin binding. The insulin detemir molecule retains the molecular pharmacological properties of native human insulin, including a physiological balance between metabolic and mitogenic potencies. Thus, insulin detemir offers the promise of an improved tolerability:efficacy ratio in the clinical setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Davies M . The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges. Int J Obes 2004; 28 (Suppl 2): S14–S22.
Home P . The challenge of poorly controlled diabetes mellitus. Diabetes Metab 2003; 29: 101–109.
Saudek CD . Novel forms of insulin delivery. Endocrinol Metab Clin N Am 1997; 26: 599–610.
Emdin SO, Dodson GG, Cutfield JM, Cutfield SM . Role of zinc in insulin biosynthesis. Some possible zinc–insulin interactions in the pancreatic B-cell. Diabetologia 1980; 19: 174–182.
Kruszynska YT, Home PD, Hanning I, Alberti KGMM . Basal and 24-h C-peptide and insulin secretion in normal man. Diabetologia 1987; 30: 16–21.
Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Van Cauter E . Abnormal patterns of insulin secretion in non-insulin-dependent diabetes mellitus. N Engl J Med 1988; 318: 1231–1239.
Kang S, Brange J, Burch A, Volund A, Owens DR . Subcutaneous insulin absorption explained by insulin's physicochemical properties. Evidence from absorption studies of soluble human insulin and insulin analogues in humans. Diabetes Care 1991; 14: 942–948.
Hildebrandt P, Sejrsen P, Nielsen SL, Birch K, Sestoft L . Diffusion and polymerisation determine the insulin absorption from subcutaneous tissue in diabetic patients. Scand J Clin Lab Invest 1985; 45: 685–690.
Brange J, Ribel U, Hansen JF, Dodson G, Hansen MT, Havelund S, Melberg SG, Norris F, Norris K, Snel L . Monomeric insulins obtained by protein engineering and their medical implications. Nature 1988; 333: 679–682.
Lauritzen T, Pramming S, Gale EA, Deckert T, Binder C . Absorption of isophane (NPH) insulin and its clinical implications. BMJ 1982; 285: 159–162.
Hildebrandt P . Subcutaneous absorption of insulin in insulin-dependent diabetic patients. Influence of species, physico-chemical properties of insulin and physiological factors. Dan Med Bull 1991; 38: 337–346.
Heinemann L . Variability of insulin absorption and insulin action. Diabetes Technol Ther 2002; 4: 673–682.
Chen JW, Christiansen JS, Lauritzen T . Limitations to subcutaneous insulin administration in type 1 diabetes. Diabetes Obes Metab 2003; 5: 223–233.
De Meyts P . The structural basis of insulin and insulin-like growth factor-I receptor binding and negative co-operativity, and its relevance to mitogenic vs metabolic signalling. Diabetologia 1994; 37 (Suppl 2): S135–S148.
Brange J, Owens DR, Kang S, Volund A . Monomeric insulins and their experimental and clinical implications. Diabetes Care 1990; 13: 923–954.
Brange J, Volund A . Insulin analogs with improved pharmacokinetic profiles. Adv Drug Deliv Rev 1999; 35: 307–335.
Bolli GB . Physiological insulin replacement in type 1 diabetes mellitus. Exp Clin Endocrinol Diabetes 2001; 109 (Suppl 2): S317–S332.
Gualandi-Signorini AM, Giorgi G . Insulin formulations—a review. Eur Rev Med Pharmacol Sci 2001; 5: 73–83.
Jørgensen LN, Dideriksen LH, Drejer K . Carcinogenic effect of human insulin analogue B10Asp in female rats. Diabetologia 1992; 35: A3.
Hansen BF, Danielsen GM, Drejer K, Sorensen AR, Wiberg FC, Klein HH, Lundemose AG . Sustained signalling from the insulin receptor after stimulation with insulin analogues exhibiting increased mitogenic potency. Biochem J 1996; 315 (Part 1): 271–279.
Kurtzhals P, Schaffer L, Sorensen A, Kristensen C, Jonassen I, Schmid C, Trub T . Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000; 49: 999–1005.
Jehle PM, Micheler C, Jehle DR, Breitig D, Boehm BO . Inadequate suspension of neutral protamine Hagedorn (NPH) insulin in pens. Lancet 1999; 354: 1604–1607.
Owens D, Zinman B, Bolli G . Insulins today and beyond. Lancet 2001; 358: 739–746.
McAulay V, Frier BM . Insulin analogues and other developments in insulin therapy for diabetes. Expert Opin Pharmacother 2003; 4: 1141–1156.
Owens DR, Coates PA, Luzio SD, Tinbergen JP, Kurzhals R . Pharmacokinetics of 125I-labeled insulin glargine (HOE 901) in healthy men: comparison with NPH insulin and the influence of different subcutaneous injection sites. Diabetes Care 2000; 23: 813–819.
Lindholm A . New insulins in the treatment of diabetes mellitus. Best Pract Res Clin Gastroenterol 2002; 16: 475–492.
Markussen J, Hougaard P, Ribel U, Sørensen AR, Sørensen E . Soluble, prolonged-acting insulin derivatives. I: Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B23, B27 and B30. Protein Eng 1987; 1: 205–213.
Markussen J, Diers I, Engesgaard A, Hansen MT, Hougaard P, Langkjaer L, Norris K, Ribel U, Sorensen AR, Sorensen E . Soluble, prolonged-acting insuln derivatives II. Degree of protraction and crystallizability of insulins substituted in positions A17, B8, B13, B27 and B30. Protein Eng 1987; 1: 215–223.
Heinemann L, Linkeschova R, Rave K, Hompesch B, Sedlak M, Heise T . Time–action profile of the long-acting insulin analogue insulin glargine (HOE901) in comparison with those of NPH insulin and placebo. Diabetes Care 2000; 23: 644–649.
Kurtzhals P . Ribel U. Action profile of cobalt(III)–insulin: a novel principle of protraction of potential use for basal insulin delivery. Diabetes 1995; 44: 1381–1385.
Kurtzhals P, Kiehr B, Sørensen A . The Co3+–insulin hexamer is a prolonged-acting insulin prodrug. J Pharm Sci 1995; 84: 1164–1168.
Kurtzhals P, Havelund S, Jonassen I, Kiehr B, Larsen UD, Ribel U, Markussen J . Albumin binding of insulins acylated with fatty acids: characterization of the ligand–protein interaction and correlation between binding affinity and timing of the insulin effect in vivo. Biochem J 1995; 312: 725–731.
Markussen J, Havelund S, Kurtzhals P, Andersen AS, Halstrom J, Hasselager E, Larsen UD, Ribel U, Schaffer L, Vad K, Jonassen I . Soluble fatty acid insulins bind to albumin and show protracted action in pigs. Diabetologia 1996; 39: 281–288.
Havelund S, Plum A, Ribel U, Jonassen I, Vølund A, Colding-Jørgensen M, Markussen J, Kurtzhals P . The mechanism of protraction of insulin detemir, a long-acting, acylated analogue of human insulin. Pharmaceut Res 2004, (in press).
Whittingham JL, Havelund S, Jonassen I . Crystal structure of a prolonged-acting insulin with albumin-binding properties. Biochemistry 1997; 36: 2826–2831.
Kurtzhals P, Colding-Jorgensen M . Albumin binding of insulin detemir reduces the risk for hypoglycaemic events. Diabetes 2004; 53 (Suppl 2): A477.
Hamilton-Wessler M, Ader M, Dea M, Moore D, Jorgensen PN, Markussen J, Bergman RN . Mechanism of protracted metabolic effects of fatty acid acylated insulin, NN304, in dogs: retention of NN304 by albumin. Diabetologia 1999; 42: 1254–1263.
Hamilton-Wessler M, Ader M, Dea MK, Moore D, Loftager M, Markussen J, Bergman RN . Mode of transcapillary transport of insulin and insulin analog NN304 in dog hindlimb: evidence for passive diffusion. Diabetes 2002; 51: 574–582.
Dea MK, Hamilton-Wessler M, Ader M, Moore D, Schaffer L, Loftager M, Volund A, Bergman RN . Albumin binding of acylated insulin (NN304) does not deter action to stimulate glucose uptake. Diabetes 2002; 51: 762–769.
Heise T, Nosek L, Ronn BB, Endahl L, Heinemann L, Kapitza C, Draeger E . Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1. Diabetes 2004; 53: 1614–1620.
Russell-Jones D . Insulin detemir: improving the predictability of glycaemic control. Int J Obes 2004; 28 (Suppl 2): S29–S34.
Kurtzhals P, Havelund S, Jonassen I, Markussen J . Effect of fatty acids and selected drugs on the albumin binding of a long-acting, acylated insulin analogue. J Pharm Sci 1997; 86: 1365–1368.
Sorensen AR, Tornqvist H . Effects of insulin detemir on glucose uptake and utilisation in human and rat adipocytes. Diabetes 2004; 53 (Suppl 2): A558.
Brunner GA, Sendhofer G, Wutte A, Ellmerer M, Sogaard B, Siebenhofer A, Hirschberger S, Krejs GJ, Pieber TR . Pharmacokinetic and pharmacodynamic properties of long-acting insulin analogue NN304 in comparison to NPH insulin in humans. Exp Clin Endocrinol Diabetes 2000; 108: 100–105.
Pieber TR, Plank J, Goerzer E, Sommer R, Wutte A, Sinner F, Bodenlenz M, Endahl L, Draeger E, Zdravkovic M . Duration of action, pharmacodynamic profile and between-subject variability of insulin detemir in subjects with type 1 diabetes. Diabetes 2002; 51 (Suppl 2): A53.
Bodenlenz M, Schaller HC, Wutte A, Jacobsen LV, Druml T, Meinitzer A, Plank J, Balent V, Brandmair H, Brunner GA, Schaupp L, Wach P, Pieber T . Measurement of insulin detemir and human insulin in adipose and muscle tissue of diabetic patients using open-flow microperfusion (Abstract). Diabetologia 2002; 45 (Suppl 2): A797.
Danne T, Lupke K, Walte K, Von Schuetz W, Gall M-A . Insulin detemir is characterised by a consistent pharmacokinetic profile across age groups in children, adolescents, and adults with type 1 diabetes. Diabetes Care 2003; 26: 2359–2364.
Jacobsen LV, Popescu G, Plum A . Pharmacokinetics of insulin detemir in subjects with renal or hepatic impairment. Diabetes 2002; 51 (Suppl 2): A102.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kurtzhals, P. Engineering predictability and protraction in a basal insulin analogue: the pharmacology of insulin detemir. Int J Obes 28 (Suppl 2), S23–S28 (2004). https://doi.org/10.1038/sj.ijo.0802746
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijo.0802746
Keywords
This article is cited by
-
A Review of Insulin Degludec/Insulin Aspart: Pharmacokinetic and Pharmacodynamic Properties and Their Implications in Clinical Use
Clinical Pharmacokinetics (2017)
-
Tageszeitlich flexible Gabe von Insulin degludec bei Patienten mit Typ-1-Diabetes oder Typ-2-Diabetes
MMW - Fortschritte der Medizin (2014)
-
Biosynthetic engineered B28K–B29P human insulin monomer structure in water and in water/acetonitrile solutions
Journal of Biomolecular NMR (2013)
-
Standard and Novel Treatment Options for Metabolic Syndrome and Diabetes Mellitus
Current Treatment Options in Cardiovascular Medicine (2013)
-
Recombinant A22G–B31R-human insulin. A22 addition introduces conformational mobility in B chain C-terminus
Journal of Biomolecular NMR (2012)