Abstract
An equation that accurately estimates the glomerular filtration rate (GFR) in the Japanese population has been proposed; however, the prognostic significance of estimated GFR (eGFR) defined according to this equation has not been reported. In addition, the prognostic significance of eGFR during long-term follow-up after complete coronary revascularization remains unclear. We assessed the prognostic significance of eGFR values, estimated by the new Japanese equation, in a cohort of patients following complete coronary revascularization. We studied consecutive patients with complete revascularization from 1984 to 1992. Patients on dialysis were excluded. A novel Japanese equation was used to estimate the GFR: eGFR=194 × (serum creatinine)−1.094 × (age)−0.287 ( × 0.739 if female). Multivariate Cox proportional hazards regression analyses were performed to determine all-cause and cardiac mortality. We analyzed data of 1809 patients, of whom 571 (31.6%) had an eGFR of ⩾90 ml min−1 per 1.73 m2, 917 (50.7%) had an eGFR of 60–89 ml min−1 per 1.73 m2, 298 (16.5%) had an eGFR of 30–59 ml min−1 per 1.73 m2 and 23 (1.3%) had an eGFR of <30 ml min−1 per 1.73 m2. During follow-up (11.4±2.9 years), there were 397 (22.0%) all-cause and 123 (6.8%) cardiac deaths overall. Patients with an eGFR of 30–59 ml min−1 per 1.73 m2, and <30 ml min−1 per 1.73 m2 revealed significantly greater risk of all-cause mortality than those with eGFR of ⩾90 ml min−1 per 1.73 m2 (hazard ratio (HR) 1.91, P<0.001, HR 3.35, P<0.001, respectively). Furthermore, incidence of cardiac death was higher in patients with an eGFR of 30–59 ml min−1 per 1.73 m2 than those with an eGFR of ⩾90 ml min−1 per 1.73 m2 (HR 2.89, P<0.001). GFR as estimated using the new Japanese equation had a prognostic significance among patients with complete coronary revascularization.
Similar content being viewed by others
Introduction
Individuals with end-stage renal disease, such as those on dialysis, have a significantly high risk for cardiovascular mortality and morbidity.1 Patients with a less-severe impairment of kidney function and who are not on dialysis also carry increased cardiovascular risk when compared with those who have preserved kidney function,2, 3 suggesting that such mild-to-moderate impairments of kidney function have an impact on cardiovascular mortality and morbidity. Therefore, attention has been focused on identifying a less-severe cutoff value for a simple index that accurately reflects the overall kidney function. In this regard, chronic kidney disease has been defined as a glomerular filtration rate (GFR) <60 ml min−1 per 1.73 m2 or the presence of kidney damage, regardless of the cause, for a period of ⩾3 months.4 GFR can be estimated from four variables: age, gender, ethnicity (Black or Caucasian) and serum creatinine (sCr) levels. Commonly, the simplified Modification of Diet in Renal Disease (MDRD) study equation (MDRD equation) is used to estimate the GFR.4, 5, 6 However, this equation is less accurate for Asians and has a greater bias for estimated GFR (eGFR) values <60 ml min−1 per 1.73 m2.7, 8 Accordingly, a modified MDRD equation has been proposed for the Chinese and Japanese populations using a separate correlation coefficient.7, 8, 9 More recently, a novel equation has been reported, one that may more accurately estimate GFR in Japanese populations than the previous modified MDRD equation.10
It has been reported that eGFR values are associated with an increased morbidity and mortality; however, these previous studies were mainly performed on Western populations using the MDRD equation. Therefore, it remains unclear whether GFR estimated by a specific equation for Asians has prognostic significance. Furthermore, the association between GFR estimated by the new Japanese equation and long-term prognosis has not yet been reported.
In addition, the prognostic significance of eGFR values has been studied in several subgroups such as patients with coronary artery disease (CAD), including patients who underwent percutaneous coronary intervention (PCI),11, 12, 13, 14 coronary artery bypass grafting (CABG)15, 16, 17 or survivors of myocardial infarction.18 However, the relationship between eGFR values and the long-term prognosis following complete coronary revascularization has not been established. The aim of this study was to assess the relationship between eGFR by the new Japanese equation, as well as the long-term prognosis in a cohort of patients with CAD following complete revascularization.
Methods
Subjects
Data from consecutive patients who had undergone coronary revascularization, including PCI and CABG, at Juntendo University Hospital (Tokyo, Japan) between January 1984 and December 1992 were analyzed. Patients were enrolled who had achieved complete revascularization, that is, patients in which no un-bypassed major vessels had a stenosis ⩾50%.19, 20 Patients with an untreated neoplasm at baseline and those with associated complex cardiac procedures, such as valve replacement or aneurysm repair at the time of surgical revascularization, as well as non-Japanese patients, were excluded. Patients on dialysis were also excluded to identify the mortality risk associated with eGFR, independent of dialysis. This study was approved by the institute's internal review board, and was performed according to the principles expressed in the Declaration of Helsinki and the ethics policy of the institute.
Data collection
Demographic data, including age and gender, as well as body mass index, coronary risk factors, medication use, revascularization procedure-related factors and comorbidities, were collected using our institutional database.
Equations for eGFR
eGFR was obtained by using the following specific equation for Japanese: GFR=194 × (sCr)−1.094 × (age−0.287 ( × 0.739 if female).10
In this study, patients were stratified as follows: eGFR ⩾90 ml min−1 per 1.73 m2; eGFR=60–89 ml min−1 per 1.73 m2; eGFR=30–59 ml min−1 per 1.73 m2; eGFR <30 ml min−1 per 1.73 m2.21
Definitions of other covariates
Hypertension was defined as a systolic blood pressure ⩾140 mmHg, a diastolic blood pressure ⩾90 mm Hg, or treatment with antihypertensive medications. Diabetes mellitus (DM) was defined as a fasting plasma glucose level ⩾126 mg per 100 ml or treatment with oral hypoglycemic drugs or insulin injections. A current smoker was defined as one who smoked at the time of complete revascularization or who had quit smoking within 1 year before complete revascularization. Patients with isolated PCI were those in whom complete revascularization was achieved by PCI without the need for any bypass grafting.
Outcomes
The follow-up period ended on 30 September 2000. Survival data were collected by serial contact with the patients or their families, and assessed from the medical records of patients who had died and those who continued to be followed up at our hospital. Information about the circumstances and date of death was obtained from the families of patients who died at home, and details of the events or the cause of death was supplied by other hospitals or clinics where the patients had been admitted. The mortality data were categorized according to the causes of death, such as death from all-causes or cardiac deaths using the International Classification of Diseases, Ninth Revision, codes 410–414, 785.51 and 798.
Statistical analysis
Continuous variables are expressed as means±standard deviation, and they were compared using one-way analysis of variance with Dunnett's test. Categorical data are tabulated as frequencies and ratios, and were compared using χ2-test.
Survival was analyzed using the Kaplan–Meier estimate with the log-rank test. The hazard ratio (HR) was calculated using the Cox proportional hazards model. The assumption of proportional hazards was assessed using a log-minus-log survival graph. Univariable analysis was based on the proportional hazard model to determine the association between prognosis and the following variables between the groups: age, gender, body mass index, current smoker, hypertension, DM, total and HDL cholesterol, triglyceride, hemoglobin, atrial fibrillation, previous myocardial infarction, previous stroke, left ventricular ejection fraction, number of diseased vessels, presence or absence of a left main trunk lesion, presence or absence of an arterial bypass graft to the left anterior descending artery, whether complete revascularization was achieved by isolated PCI, and stratified eGFR values. Variables regarded as significant (P⩽0.10) were included in the multivariate analysis. The tests of trends in the HR by the eGFR values were conducted by assigning an ordinal value to each stage in separate models. All multivariable analyses verified the interactions between each variable.
A P-value of <0.05 was considered statistically significant, unless indicated otherwise. All data were analyzed using SPSS version 11.0 for Windows (SPSS Inc., Chicago, IL, USA).
Results
Overall, the data from 1809 eligible patients who underwent complete coronary revascularization during the study period were assessed. Baseline and clinical event data were fully documented during the follow-up period (mean follow-up, 11.4±2.9 years). All patients underwent PCI with simple balloon angioplasty; no patients received stent implantation, as stents were not available at the time when complete revascularization was achieved. All CABG procedures were performed using a conventional cardiopulmonary bypass; arterial grafts were used in 51.1% of cases. None of the patients who underwent complete revascularization during the study period had type-1 DM. During the follow-up period, 397 patients (22.0%) died from any cause and 123 patients (6.8%) died from cardiac causes. Out of 1890 patients, 571 (31.6%) patients had eGFR of ⩾90 ml min−1 per 1.73 m2, 917 (50.7%) patients had eGFR of 60–89 ml min−1 per 1.73 m2, 298 (16.5%) had eGFR of 30–59 ml min−1 per 1.73 m2, 23 (1.3%) patients had eGFR of <30 ml min−1 per 1.73 m2. Mean eGFR values in each group were 119.6±36.9, 75.8±8.2, 52.1±6.8, and 22.2±9.6, respectively. Baseline characteristics of these patients are summarized in Table 1. Patients with a lower eGFR were more likely to be older and male. They also have greater triglyceride level, greater number of diseased vessels and more frequently have atrial fibrillation. As compared with patients with eGFR of ⩾90 ml min−1 per 1.73 m2, patients with eGFR of <30 ml min−1 per 1.73 m2 frequently revealed DM, anemia, impaired left ventricular ejection fraction, history of stroke and greater incidence of angiotensin-converting enzyme inhibitor use.
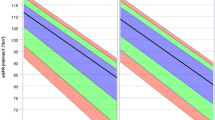
The cumulative survival curves of patients stratified by eGFR values are shown in Figure 1. Patients with a lower eGFR values had lower survival from both all-cause and cardiac-related mortality. Univariable Cox proportional hazard analysis revealed that, as compared with patients with eGFR of ⩾90 ml min−1 per 1.73 m2, the other three groups of patients had significantly greater risk of all-cause and cardiac mortality (Table 2). The other variables that were associated with all-cause mortality according to the univariable analyses were: age (HR 1.07, 95% confidence interval (95% CI) 1.06–1.08, P<0.001), DM (HR 1.35, 95% CI 1.11–1.65, P=0.003), total cholesterol (HR 1.00, 95% CI 0.99–1.01, P=0.060), hemoglobin (HR 0.86, 95% CI 0.81–0.92, P<0.001), atrial fibrillation (HR 2.26, 95% CI 1.78–2.86, P<0.001), previous myocardial infarction (HR 1.72, 1.41–2.11, P<0.001), previous stroke (HR 2.96, 95% CI 2.09–4.20, P<0.001), number of diseased vessels (HR 1.77, 95% CI 1.53–2.04, P<0.001), left main trunk lesion (HR 1.65, 95% CI 1.21–2.24, P=0.002), arterial bypass to left anterior descending (HR 0.82, 95% CI 0.65–1.03, P=0.080), left ventricular ejection fraction (HR 0.98, 95% CI 0.97–0.98, P<0.001) and isolated PCI (HR 0.35, 95% CI 0.26–0.47, P<0.001). The other variables that were associated with cardiac mortality according to the univariable analyses were: age (HR 1.05, 95% CI 1.03–1.08, P<0.001), DM (HR 1.83, 95% CI 1.28–2.61, P=0.001), triglycerides (HR 1.00, 95% CI 1.00–1.01, P=0.016), hemoglobin (HR 0.90, 95% CI 0.80–1.02, P=0.087), atrial fibrillation (HR 2.44, 95% CI 1.60–3.73, P<0.001), previous myocardial infarction (HR 2.12, 1.46–3.09, P<0.001), previous stroke (HR 3.18, 95% CI 1.71–5.92, P<0.001), number of diseased vessels (HR 1.90, 95% CI 1.46–2.49, P<0.001), left main trunk lesion (HR 1.88, 95% CI 1.11–3.19, P=0.018), left ventricular ejection fraction (HR 0.96, 95% CI 0.95–0.97, P<0.001) and isolated PCI (HR 0.40, 95% CI 0.23–0.67, P=0.001).
Cumulative survival curves of patients stratified by estimated glomerular filtration rate (eGFR) values. (a) All-cause death according to the stratified eGFR values. Patients with eGFR of ⩾90 ml min−1 per 1.73 m2 revealed significantly greater cumulative survival than those with eGFR of 60–89 ml min−1 per 1.73 m2 (log-rank test, P=0.018), 30–59 ml min−1 per 1.73 m2 (log-rank test, P<0.001) and <30 ml min−1 per 1.73 m2 (log-rank test, P<0.001). (b) Cardiac death according to the stratified eGFR values. Patients with eGFR of ⩾90 ml min−1 per 1.73 m2 revealed a non-significant tendency toward greater cumulative survival than those with eGFR of 60–89 ml min−1 per 1.73 m2 (log-rank test, P=0.052). They also revealed significantly greater cumulative survival than those with eGFR of 30–59 ml min−1 per 1.73 m (log-rank test, P<0.001), and <30 ml min−1 per 1.73 m2 (log-rank test, P=0.001).
The results of multivariable analyses for all-cause and cardiac mortality are also summarized in Table 2. Patients with eGFR of 30–59 ml min−1 per 1.73 m2 and <30 ml min−1 per 1.73 m2 had significantly greater risk for all-cause mortality, and patients with eGFR of 30–59 ml min−1 per 1.73 m2 had significantly greater risk for cardiac mortality as compared with those with eGFR of ⩾90 ml min−1 per 1.73 m2. Patients with eGFR of <30 ml min−1 per 1.73 m2 revealed a non-significant tendency toward greater risk of cardiac mortality than those with eGFR of ⩾90 ml min−1 per 1.73 m2. Among each stratified eGFR values group, as the eGFR values decreased HR increased in a significant dose-dependent manner for all-cause and cardiac mortality. The HRs of other variables in the multivariable analyses for all cause and cardiac death are shown in Table 3.
We also conducted a sub-group analysis separate from the age, gender, and the presence of hypertension and DM for all cause and cardiac death. As a result, patients with eGFR of <60 ml min−1 per 1.73 m2 showed significantly greater all-cause and cardiac mortality risk than those with eGFR of ⩾90 ml min−1 per 1.73 m2 in all subgroups (Table 4).
Discussion
This study identifies novel findings that provide insights into the relationship between eGFR and cardiovascular diseases. First, we found that when we used new Japanese equation, eGFR values were associated with an increased long-term mortality risk. Second, we also found that there was a strong association between eGFR values and increased long-term mortality in patients with CAD who had achieved complete revascularization. Finally, patients with eGFR of <60 ml min−1 per 1.73 m2 had significantly greater risk of all-cause and cardiac mortality than those with eGFR of ⩾90 ml min−1 per 1.73 m2 regardless of age, gender or hypertensive or diabetic status. Therefore, our findings suggest that baseline GFR values estimated by the new Japanese equation at the time of revascularization were predictive of the long-term all-cause and cardiac mortality among Japanese patients with CAD. In addition, our findings also suggest that eGFR values have a prognostic significance in patients with complete revascularization.
A number of studies have reported the prognostic significance of eGFR in patients with CAD;11, 12, 13, 14, 15, 16, 17, 18 however, the majority of these studies were in Western countries and GFR was estimated by the MDRD equation or by creatinine clearance. Reports from Asian countries are very limited, possibly because of the lack of an accurate equation for eGFR in Asian populations. To the best of our knowledge, there are no studies in which the prognostic significance of eGFR generated by the new Japanese equation has been evaluated. Previous studies have found the MDRD equation to overestimate GFR in a Japanese chronic kidney disease population as compared with GFR measured by using inulin clearance.9, 10 Therefore, a modified MDRD equation for the Japanese population using a separate correlation coefficient has been proposed in a previous study.9 However, in that study, blood samples for sCr were assayed in multiple laboratories and during different periods. Furthermore, data from subjects with GFR >90 ml min−1 per 1.73 m2 were not included in that study. In contrast, this new Japanese equation was derived from a study that used centralized blood assays and enrolled a larger population that included subjects with GFR >90 ml min−1 per 1.73 m2 (Matsuo et al.10) than the study using the MDRD equation with the Japanese coefficient.9 Indeed, the accuracy of the GFR estimation was further improved if the new Japanese equation was used rather than the modified MDRD equation with the Japanese coefficient.10 In these aspects, this study, which clearly showed the prognostic significance of eGFR using an accurate Japanese specific equation, has an important value.
Previous studies in which the prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 (that is, chronic kidney disease stage ⩾3, according to the clinical guideline21) was investigated in patients with CAD, showed that the prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 was 16–46% in Western countries.11, 12, 15, 16, 17, 18 The MDRD equation or creatinine clearance was used in the majority of these studies. On the other hand, the prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 in Japanese patients with CAD was reported as 35 and 39% (Inaguma et al.13 and Furukawa et al.14) using MDRD equation with Japanese coefficient or creatinine clearance. In this study, the prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 was ∼18% using new Japanese equation for eGFR. Considering that patients on dialysis were excluded in this study, a relatively low prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 may be expected in our patient population.
This study is the first report describing the long-term (>10 years) prognostic significance of eGFR values in patients who underwent complete coronary revascularization. There are several reports in which patients with revascularization (PCI or CABG) were assessed;11, 12, 13, 14, 15, 16, 17 however, the follow-up periods in the majority of these studies were ⩽5 years and none of them described whether complete revascularization was achieved. It is important to emphasize the benefit of assessing data only in patients who had achieved complete revascularization, because the importance of completeness of revascularization during long-term follow-up period has been reported.19, 20 In addition, the initial CAD events may be prevented or delayed by complete coronary revascularization, even in patients with severe coronary atherosclerosis. These may minimize the bias of the treatment procedure for the initial CAD events. Therefore, the risk of eGFR for cardiovascular morbidity and mortality among a secondary prevention cohort of patients with CAD could be assessed in this study. Conversely, assessing data only in patients who achieved complete revascularization also introduced potential selection bias in terms of overall mortality rate and prevalence of patients with eGFR≪60 ml min−1 per 1.73 m2, which should be taken into account.
To assess the possible interactions between traditional cardiovascular risk predictors such as age, gender, presence or absence of DM or hypertension, subgroup analyses were also carried out. As shown in Table 4, there were no significant interactions in all subgroups and patients with eGFR <60 ml min−1 per 1.73 m2 had a significant greater risk of both all cause and cardiac mortality than those with eGFR of ⩾90 ml min−1 per 1.73 m2 in each subgroup. These results are consistent with previous studies, investigating the risk of GFR values estimated by the MDRD equation or by creatinine clearance among patients with cardiovascular disease.14, 15, 22
This study has several limitations. First, balloon angioplasty was the sole PCI used in all patients and 51.1% of the CABG procedures involved an arterial graft. It is difficult to determine whether the use of stents and arterial grafts could have improved the results in the recent era of revascularization. It is also difficult to determine the relative importance of improvements in both operator skills and in adjunctive drug therapy. Therefore, further investigation is needed to clarify whether estimating GFR by the new Japanese equation would affect the long-term mortality in the stent and arterial bypass era. Furthermore, the use of some medications, such as angiotensin-converting enzyme inhibitors and statins, which may affect the functional status of the kidney, were different from those in the most recent period. Therefore, the data for the prevalence of patients with eGFR <60 ml min−1 per 1.73 m2 in this study should be interpreted with caution. Finally, eGFR by the new Japanese equation used in this study was not compared with a nuclear renogram, the gold standard for GFR evaluation. As such, the issue is not whether the new Japanese equation is an accurate reflection of GFR but whether it offers prognostic significance.
In conclusion, the eGFR values estimated by the new Japanese equation were associated with an increase in all-cause and cardiac mortality over a 10-year period after the achievement of complete coronary revascularization. This study verifies the prognostic significance of the eGFR by the new Japanese equation among a CAD secondary prevention cohort.
References
Foley RN, Parfrey PS, Sarnak MJ . Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 1998; 32 (5 Suppl 3): S112–S119.
Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY . Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305.
Ruilope LM, Salvetti A, Jamerson K, Hansson L, Warnold I, Wedel H, Zanchetti A . Renal function and intensive lowering of blood pressure in hypertensive participants of the hypertension optimal treatment (HOT) study. J Am Soc Nephrol 2001; 12: 218–225.
National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 (2 Suppl 1): S1–266.
Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D . A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999; 130: 461–470.
Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F . Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 2006; 145: 247–254.
Ma YC, Zuo L, Chen JH, Luo Q, Yu XQ, Li Y, Xu JS, Huang SM, Wang LN, Huang W, Wang M, Xu GB, Wang HY . Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol 2006; 17: 2937–2944.
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Hara S, Ura N, Kiyohara Y, Hirakata H, Watanabe T, Moriyama T, Ando Y, Inaguma D, Narita I, Iso H, Wakai K, Yasuda Y, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S . Estimation of glomerular filtration rate by the MDRD study equation modified for Japanese patients with chronic kidney disease. Clin Exp Nephrol 2007; 11: 41–50.
Imai E, Horio M, Nitta K, Yamagata K, Iseki K, Tsukamoto Y, Ito S, Makino H, Hishida A, Matsuo S . Modification of the Modification of Diet in Renal Disease (MDRD) Study equation for Japan. Am J Kidney Dis 2007; 50: 927–937.
Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A . Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53: 982–992.
Best PJ, Lennon R, Ting HH, Bell MR, Rihal CS, Holmes DR, Berger PB . The impact of renal insufficiency on clinical outcomes in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol 2002; 39: 1113–1119.
Blackman DJ, Pinto R, Ross JR, Seidelin PH, Ing D, Jackevicius C, Mackie K, Chan C, Dzavik V . Impact of renal insufficiency on outcome after contemporary percutaneous coronary intervention. Am Heart J 2006; 151: 146–152.
Inaguma D, Tatematsu M, Shinjo H, Suzuki S, Mishima T, Inaba S, Kurata K, Yuzawa Y, Matsuo S . Relationship between renal function at the time of percutaneous coronary intervention and prognosis in ischemic heart disease patients. Clin Exp Nephrol 2007; 11: 56–60.
Furukawa Y, Ehara N, Taniguchi R, Haruna Y, Ozasa N, Saito N, Doi T, Hoshino K, Tamura T, Shizuta S, Abe M, Toma M, Morimoto T, Teramukai S, Fukushima M, Kita T, Kimura T . Coronary risk factor profile and prognostic factors for young Japanese patients undergoing coronary revascularization. Circ J 2009; 73: 1459–1465.
Cooper WA, O’Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED . Impact of renal dysfunction on outcomes of coronary artery bypass surgery: results from the Society of Thoracic Surgeons National Adult Cardiac Database. Circulation 2006; 113: 1063–1070.
Holzmann MJ, Hammar N, Ahnve S, Nordqvist T, Pehrsson K, Ivert T . Renal insufficiency and long-term mortality and incidence of myocardial infarction in patients undergoing coronary artery bypass grafting. Eur Heart J 2007; 28: 865–871.
Kangasniemi OP, Mahar MA, Rasinaho E, Satomaa A, Tiozzo V, Lepojarvi M, Biancari F . Impact of estimated glomerular filtration rate on the 15-year outcome after coronary artery bypass surgery. Eur J Cardiothorac Surg 2008; 33: 198–202.
Anavekar NS, McMurray JJ, Velazquez EJ, Solomon SD, Kober L, Rouleau JL, White HD, Nordlander R, Maggioni A, Dickstein K, Zelenkofske S, Leimberger JD, Califf RM, Pfeffer MA . Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med 2004; 351: 1285–1295.
Jones EL, Weintraub WS . The importance of completeness of revascularization during long-term follow-up after coronary artery operations. J Thorac Cardiovasc Surg 1996; 112: 227–237.
McLellan CS, Ghali WA, Labinaz M, Davis RB, Galbraith PD, Southern DA, Shrive FM, Knudtson ML . Association between completeness of percutaneous coronary revascularization and postprocedure outcomes. Am Heart J 2005; 150: 800–806.
Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, Hogg RJ, Perrone RD, Lau J, Eknoyan G . National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003; 139: 137–147.
Bax L, Algra A, Mali WP, Edlinger M, Beutler JJ, van der Graaf Y . Renal function as a risk indicator for cardiovascular events in 3216 patients with manifest arterial disease. Atherosclerosis 2008; 200: 184–190.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Kasai, T., Miyauchi, K., Kajimoto, K. et al. Prognostic significance of glomerular filtration rate estimated by the Japanese equation among patients who underwent complete coronary revascularization. Hypertens Res 34, 378–383 (2011). https://doi.org/10.1038/hr.2010.244
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/hr.2010.244
Keywords
This article is cited by
-
Comparison of modification of diet in renal disease and chronic kidney disease epidemiology collaboration formulas in predicting long-term outcomes in patients undergoing stent implantation due to stable coronary artery disease
Clinical Research in Cardiology (2014)
-
Decreased glomerular filtration rate is a significant and independent risk for in-hospital mortality in Japanese patients with acute myocardial infarction: report from the Hokkaido acute myocardial infarction registry
Hypertension Research (2012)
-
Kidney function in patients undergoing coronary revascularization
Hypertension Research (2011)