Long Term Efficacy and Assessment of Tumor Response of Transarterial Chemoembolization in Neuroendocrine Liver Metastases: A 15-Year Monocentric Experience
Abstract
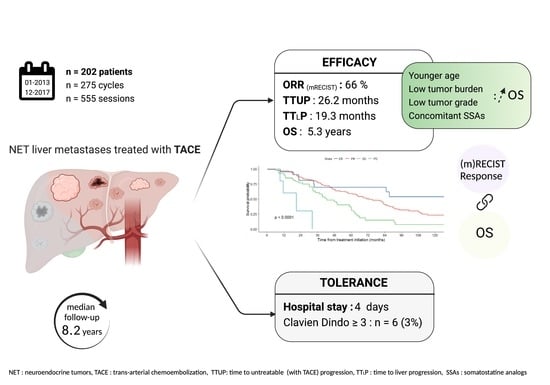
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. TACE
2.3. Imaging Assessments
2.4. Evaluation Criteria
2.5. Adverse Events
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. TACE Procedures
3.3. Survival
3.4. Response to Treatment
3.5. Prognostic Factors
3.6. Adverse Events
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| c-TACE | Conventional TACE (using Lipiodol®) |
| DEB-TACE | Drug-eluting bead TACE |
| RECIST | Response evaluation criteria in solid tumors |
| NET | Neuroendocrine tumor |
| OS | Overall survival |
| mRECIST | Modified response evaluation criteria in solid tumors |
| SSAs | Somatostatin analogs |
| TACE | Transarterial chemoembolization |
| TTLP | Time to liver progression |
| TTUP | Time to untreatable progression |
| WHO | World Health Organization |
References
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.-N.; Rashid, A.; et al. One Hundred Years after “Carcinoid”: Epidemiology of and Prognostic Factors for Neuroendocrine Tumors in 35,825 Cases in the United States. JCO 2008, 26, 3063–3072. [Google Scholar] [CrossRef] [Green Version]
- Riihimäki, M.; Hemminki, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. The Epidemiology of Metastases in Neuroendocrine Tumors: Epidemiology of Metastases. Int. J. Cancer 2016, 139, 2679–2686. [Google Scholar] [CrossRef]
- Niederle, M.B.; Hackl, M.; Kaserer, K.; Niederle, B. Gastroenteropancreatic Neuroendocrine Tumours: The Current Incidence and Staging Based on the WHO and European Neuroendocrine Tumour Society Classification: An Analysis Based on Prospectively Collected Parameters. Endocr.-Relat. Cancer 2010, 17, 909–918. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Bloomston, M.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Clark Gamblin, T.; Celinski, S.A.; Kooby, D.A.; Staley, C.A.; et al. Surgery Versus Intra-Arterial Therapy for Neuroendocrine Liver Metastasis: A Multicenter International Analysis. Ann. Surg. Oncol. 2011, 18, 3657–3665. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.; Öberg, K.; Falconi, M.; Krenning, E.P.; Sundin, A.; Perren, A.; Berruti, A. Gastroenteropancreatic Neuroendocrine Neoplasms: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 844–860. [Google Scholar] [CrossRef]
- Pavel, M.; O’’Toole, D.; Costa, F.; Capdevila, J.; Gross, D.; Kianmanesh, R.; Krenning, E.; Knigge, U.; Salazar, R.; Pape, U.-F.; et al. ENETS Consensus Guidelines Update for the Management of Distant Metastatic Disease of Intestinal, Pancreatic, Bronchial Neuroendocrine Neoplasms (NEN) and NEN of Unknown Primary Site. Neuroendocrinology 2016, 103, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Strosberg, J.R.; Tang, L.; Bellizzi, A.M.; Bergsland, E.K.; O’Dorisio, T.M.; Halperin, D.M.; Fishbein, L.; Eads, J.; Hope, T.A.; et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Pancreatic Neuroendocrine Tumors. Pancreas 2020, 49, 863–881. [Google Scholar] [CrossRef] [PubMed]
- Liapi, E.; Geschwind, J.-F.; Vossen, J.A.; Buijs, M.; Georgiades, C.S.; Bluemke, D.A.; Kamel, I.R. Functional MRI Evaluation of Tumor Response in Patients with Neuroendocrine Hepatic Metastasis Treated with Transcatheter Arterial Chemoembolization. Am. J. Roentgenol. 2008, 190, 67–73. [Google Scholar] [CrossRef]
- Sahu, S.; Schernthaner, R.; Ardon, R.; Chapiro, J.; Zhao, Y.; Sohn, J.H.; Fleckenstein, F.; Lin, M.; Geschwind, J.-F.; Duran, R. Imaging Biomarkers of Tumor Response in Neuroendocrine Liver Metastases Treated with Transarterial Chemoembolization: Can Enhancing Tumor Burden of the Whole Liver Help Predict Patient Survival? Radiology 2016, 283, 883–894. [Google Scholar] [CrossRef]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; The WHO Classification of Tumours Editorial Board. The 2019 WHO Classification of Tumours of the Digestive System. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [Green Version]
- Rindi, G.; Klimstra, D.S.; Abedi-Ardekani, B.; Asa, S.L.; Bosman, F.T.; Brambilla, E.; Busam, K.J.; de Krijger, R.R.; Dietel, M.; El-Naggar, A.K.; et al. A Common Classification Framework for Neuroendocrine Neoplasms: An International Agency for Research on Cancer (IARC) and World Health Organization (WHO) Expert Consensus Proposal. Mod. Pathol. 2018, 31, 1770–1786. [Google Scholar] [CrossRef] [PubMed]
- Guiu, B.; Deschamps, F.; Aho, S.; Munck, F.; Dromain, C.; Boige, V.; Malka, D.; Leboulleux, S.; Ducreux, M.; Schlumberger, M.; et al. Liver/Biliary Injuries Following Chemoembolisation of Endocrine Tumours and Hepatocellular Carcinoma: Lipiodol vs. Drug-Eluting Beads. J. Hepatol. 2012, 56, 609–617. [Google Scholar] [CrossRef]
- de Baere, T.; Arai, Y.; Lencioni, R.; Geschwind, J.-F.; Rilling, W.; Salem, R.; Matsui, O.; Soulen, M.C. Treatment of Liver Tumors with Lipiodol TACE: Technical Recommendations from Experts Opinion. Cardiovasc. Interv. Radiol. 2016, 39, 334–343. [Google Scholar] [CrossRef]
- Lencioni, R.; de Baere, T.; Burrel, M.; Caridi, J.G.; Lammer, J.; Malagari, K.; Martin, R.C.G.; O’Grady, E.; Real, M.I.; Vogl, T.J.; et al. Transcatheter Treatment of Hepatocellular Carcinoma with Doxorubicin-Loaded DC Bead (DEBDOX): Technical Recommendations. Cardiovasc. Interv. Radiol. 2012, 35, 980–985. [Google Scholar] [CrossRef] [Green Version]
- Therasse, P.; Arbuck, S.G.; Eisenhauer, E.A.; Wanders, J.; Kaplan, R.S.; Rubinstein, L.; Verweij, J.; Van Glabbeke, M.; van Oosterom, A.T.; Christian, M.C.; et al. New Guidelines to Evaluate the Response to Treatment in Solid Tumors. JNCI J. Natl. Cancer Inst. 2000, 92, 205–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lencioni, R.; Llovet, J.M. Modified RECIST (MRECIST) Assessment for Hepatocellular Carcinoma. Semin. Liver Dis. 2010, 30, 052–060. [Google Scholar] [CrossRef] [Green Version]
- Dindo, D.; Demartines, N.; Clavien, P.-A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dhir, M.; Shrestha, R.; Steel, J.L.; Marsh, J.W.; Tsung, A.; Tublin, M.E.; Amesur, N.B.; Orons, P.D.; Santos, E.; Geller, D.A. Initial Treatment of Unresectable Neuroendocrine Tumor Liver Metastases with Transarterial Chemoembolization Using Streptozotocin: A 20-Year Experience. Ann. Surg. Oncol. 2017, 24, 450–459. [Google Scholar] [CrossRef]
- Chen, J.X.; Rose, S.; White, S.B.; El-Haddad, G.; Fidelman, N.; Yarmohammadi, H.; Hwang, W.; Sze, D.Y.; Kothary, N.; Stashek, K.; et al. Embolotherapy for Neuroendocrine Tumor Liver Metastases: Prognostic Factors for Hepatic Progression-Free Survival and Overall Survival. Cardiovasc Interv. Radiol. 2017, 40, 69–80. [Google Scholar] [CrossRef]
- Hur, S.; Chung, J.W.; Kim, H.-C.; Oh, D.-Y.; Lee, S.-H.; Bang, Y.-J.; Kim, W.H. Survival Outcomes and Prognostic Factors of Transcatheter Arterial Chemoembolization for Hepatic Neuroendocrine Metastases. J. Vasc. Interv. Radiol. 2013, 24, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Berdelou, A.; Boige, V.; Arfi-Rouche, J.; Malka, D.; Ederhy, S.; Izzedine, H.; Leboulleux, S.; Chougnet, C.N.; Burtin, P.; De Baere, T.; et al. Not All Patients with a Pancreatic Neuroendocrine Tumour Will Benefit from All Approved or Recommended Therapeutic Options: A Real-Life Retrospective Study. Neuroendocrinology 2017, 105, 26–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gowdra Halappa, V.; Corona-Villalobos, C.P.; Bonekamp, S.; Li, Z.; Reyes, D.; Cosgrove, D.; Pawlik, T.M.; Diaz, L.A.; Bhagat, N.; Eng, J.; et al. Neuroendocrine Liver Metastasis Treated by Using Intraarterial Therapy: Volumetric Functional Imaging Biomarkers of Early Tumor Response and Survival. Radiology 2013, 266, 502–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varker, K.A.; Martin, E.W.; Klemanski, D.; Palmer, B.; Shah, M.H.; Bloomston, M. Repeat Transarterial Chemoembolization (TACE) for Progressive Hepatic Carcinoid Metastases Provides Results Similar to First TACE. J. Gastrointest. Surg. 2007, 11, 1680–1685. [Google Scholar] [CrossRef] [PubMed]
- Zappa, M.; Abdel-Rehim, M.; Hentic, O.; Vullierme, M.-P.; Ruszniewski, P.; Vilgrain, V. Liver-Directed Therapies in Liver Metastases from Neuroendocrine Tumors of the Gastrointestinal Tract. Target. Oncol. 2012, 7, 107–116. [Google Scholar] [CrossRef]
- Gupta, S.; Johnson, M.M.; Murthy, R.; Ahrar, K.; Wallace, M.J.; Madoff, D.C.; McRae, S.E.; Hicks, M.E.; Rao, S.; Vauthey, J.-N.; et al. Hepatic Arterial Embolization and Chemoembolization for the Treatment of Patients with Metastatic Neuroendocrine Tumors. Cancer 2005, 104, 1590–1602. [Google Scholar] [CrossRef]
- Sofocleous, C.T.; Petre, E.N.; Gonen, M.; Reidy-Lagunes, D.; Ip, I.K.; Alago, W.; Covey, A.M.; Erinjeri, J.P.; Brody, L.A.; Maybody, M.; et al. Factors Affecting Periprocedural Morbidity and Mortality and Long-Term Patient Survival after Arterial Embolization of Hepatic Neuroendocrine Metastases. J. Vasc. Interv. Radiol. 2014, 25, 22–30. [Google Scholar] [CrossRef]
- Kamat, P.P.; Gupta, S.; Ensor, J.E.; Murthy, R.; Ahrar, K.; Madoff, D.C.; Wallace, M.J.; Hicks, M.E. Hepatic Arterial Embolization and Chemoembolization in the Management of Patients with Large-Volume Liver Metastases. Cardiovasc. Interv. Radiol. 2008, 31, 299–307. [Google Scholar] [CrossRef]
- Kitano, M.; Davidson, G.W.; Shirley, L.A.; Schmidt, C.R.; Guy, G.E.; Khabiri, H.; Dowell, J.D.; Shah, M.H.; Bloomston, M. Transarterial Chemoembolization for Metastatic Neuroendocrine Tumors With Massive Hepatic Tumor Burden: Is the Benefit Worth the Risk? Ann. Surg. Oncol. 2016, 23, 4008–4015. [Google Scholar] [CrossRef]
- Gupta, S. Intra-Arterial Liver-Directed Therapies for Neuroendocrine Hepatic Metastases. Semin. Interv. Radiol. 2013, 30, 028–038. [Google Scholar] [CrossRef] [Green Version]
- Roche, A.; Girish, B.V.; de Baere, T.; Ducreux, M.; Elias, D.; Laplanche, A.; Boige, V.; Schlumberger, M.; Ruffle, P.; Baudin, E. Prognostic Factors for Chemoembolization in Liver Metastasis from Endocrine Tumors. Hepatogastroenterology 2004, 51, 1751–1756. [Google Scholar]
- Brown, K.T.; Koh, B.Y.; Brody, L.A.; Getrajdman, G.I.; Susman, J.; Fong, Y.; Blumgart, L.H. Particle Embolization of Hepatic Neuroendocrine Metastases for Control of Pain and Hormonal Symptoms. J. Vasc. Interv. Radiol. 1999, 10, 397–403. [Google Scholar] [CrossRef]
- Bhagat, N.; Reyes, D.K.; Lin, M.; Kamel, I.; Pawlik, T.M.; Frangakis, C.; Geschwind, J.F. Phase II Study of Chemoembolization With Drug-Eluting Beads in Patients With Hepatic Neuroendocrine Metastases: High Incidence of Biliary Injury. Cardiovasc. Interv. Radiol. 2013, 36, 449–459. [Google Scholar] [CrossRef] [Green Version]
- de Mestier, L.; Lepage, C.; Baudin, E.; Coriat, R.; Courbon, F.; Couvelard, A.; Do Cao, C.; Frampas, E.; Gaujoux, S.; Gincul, R.; et al. Digestive Neuroendocrine Neoplasms (NEN): French Intergroup Clinical Practice Guidelines for Diagnosis, Treatment and Follow-up (SNFGE, GTE, RENATEN, TENPATH, FFCD, GERCOR, UNICANCER, SFCD, SFED, SFRO, SFR). Dig. Liver Dis. 2020, 52, 473–492. [Google Scholar] [CrossRef]
- Okuyama, H.; Ikeda, M.; Takahashi, H.; Ohno, I.; Hashimoto, Y.; Mitsunaga, S.; Sakamoto, Y.; Kondo, S.; Morizane, C.; Ueno, H.; et al. Transarterial (Chemo)Embolization for Liver Metastases in Patients with Neuroendocrine Tumors. Oncology 2017, 92, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.G.; Ulahannan, S.V.; Makorova-Rusher, O.; Rahma, O.; Wedemeyer, H.; Pratt, D.; Davis, J.L.; Hughes, M.S.; Heller, T.; ElGindi, M.; et al. Tremelimumab in Combination with Ablation in Patients with Advanced Hepatocellular Carcinoma. J. Hepatol. 2017, 66, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Characteristic | Study Population (n = 202) |
|---|---|
| Patient Characteristics | |
| Male sex | 101 (50%) |
| Age, mean (SD), years | 60 (13) |
| ECOG PS | |
| 0 | 153 (76%) |
| 1 | 46 (23%) |
| 2 | 3 (1%) |
| Hormone-related symptoms | 75 (37%) |
| Tumor Characteristics | |
| Tumor grade 1 2 3 Primary tumor location Pancreas Small Bowel Lung Other Unknown Extrahepatic metastatic disease Tumor liver involvement <25% 25–50% 50–75% >75% Hyperenhanced liver metastases Treatment Prior systemic treatment lines * 0 1 2 ≥3 Primary tumor resection Concomitant treatment SSAs Everolimus Number of TACE cycles 1 2 ≥3 | 55/143 (38%) 86/143 (60%) 2/143 (1%) 52 (26%) 78 (39%) 44 (22%) 18 (9%) 10 (5%) 125 (63%) 87 (43%) 49 (24%) 35 (17%) 31(15%) 130 (64%) 101 (50%) 48 (24%) 25 (12%) 28 (14%) 138 (68%) 132 (65%) 12 (6%) 146 (72%) 42 (20%) 14 (7%) |
| Characteristic | Number of Session (n = 555) |
|---|---|
| Technique c-TACE DEB-TACE Chemotherapy agent Doxorubicin Idarubicin Oxaliplatin Others Selectivity Total Sectorial Lobar Segmental | 389 (70%) 166 (30%) 488 (88%) 41 (7.4%) 21 (3.8%) 6 (0.7%) 62 (11%) 65 (12%) 306 (55%) 119 (11%) |
| Variable | Overall Patients n = 202 | Overall Cycle n = 275 | Cycle 1 n = 202 | Cycle 2 n = 56 | Cycle 3 or More n = 14 |
|---|---|---|---|---|---|
| RECIST | |||||
| CR | 0 | 0 | 0 | 0 | 0 |
| PR | 102 (50%) | 124 (45%) | 96 (48%) | 22 (41%) | 5 (36%) |
| SD | 94 (47%) | 136 (50%) | 99 (49%) | 28 (52%) | 7(50%) |
| PD | 6 (3%) | 13 (5%) | 7 (3%) | 4 (7%) | 2 (14%) |
| OR rate (CR + PR) | 102 (50%) | 124 (45%) | 96 (48%) | 22 (41%) | 5 (36%) |
| mRECIST | |||||
| CR | 21 (10%) | 22 (8%) | 19 (9%) | 2 (4%) | 1 (7%) |
| PR | 120 (59%) | 158 (58%) | 119 (59%) | 30 (56%) | 7 (50%) |
| SD | 55 (27%) | 80 (29%) | 57 (28%) | 18 (33%) | 4 (29%) |
| PD | 6 (3%) | 13 (5%) | 7 (3%) | 4 (7%) | 2 (14%) |
| OR rate (CR + PR) | 141 (69%) | 180 (66%) | 138 (68%) | 32 (60%) | 8 (57%) |
| Variable | OS Univariate Analysis | OS Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | |
| mRECIST best response SD CR PR PD | - 0.28 0.48 4.25 | 0.13–0.59 0.32–0.71 1.47–12.30 | <0.001 | - 0.46 0.51 4.03 | 0.16–1.31 0.30–0.87 1.25–13.00 | <0.001 |
| RECIST best response SD PR PD | - 0.49 5.21 | 0.34–0.70 1.83–14.80 | <0.001 | - 0.42 4.83 | 0.25–0.71 1.54–15.20 | <0.001 |
| Variable | OS | TTLP | TTUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Gender | 0.3 | 0.26 | 0.5 | ||||||
| Female | - | - | - | ||||||
| Male | 0.84 | 0.59–1.20 | 0.86 | 0.65–1.14 | 0.9 | 0.65–1.25 | |||
| Age | 1.03 | 1.01–1.04 | 0.001 | 1.01 | 1–1.03 | 0.12 | 1.01 | 1–1.03 | 0.13 |
| Tumor localization | 0.7 | 0.058 | 0.3 | ||||||
| Small Bowell | - | - | - | ||||||
| Pancreas | 0.73 | 0.34–1.86 | 1.52 | 1.07–2.15 | 1.12 | 0.74–1.71 | |||
| Lung | 1.02 | 0.64–1.61 | 1.41 | 0.97–2.06 | 1.42 | 0.92–2.17 | |||
| Colon/rectum | 0.8 | 0.80–1.86 | 1.59 | 0.89–2.85 | 1.55 | 0.76–3.15 | |||
| Others | 0.95 | 0.47–1.93 | 1.48 | 0.84–2.61 | 1.66 | 0.92–2.99 | |||
| Grade Tumors | 0.2 | <0.001 | <0.001 | ||||||
| 1 | - | - | - | ||||||
| 2 | 1.38 | 0.87–2.18 | 2.11 | 1.46–3.03 | 1.92 | 1.28–2.88 | |||
| 3 | 5.91 | 0.78–44.87 | 2.32 | 0.31–17.4 | 29.9 | 6.38–141 | |||
| Extrahepatic Disease | <0.001 | 0.82 | 0.048 | ||||||
| No | - | - | - | ||||||
| Yes | 1.96 | 1.34–2.87 | 1.01 | 0.76–1.34 | 1.41 | 1–1.98 | |||
| Liver involvement | <0.001 | 0.85 | 0.016 | ||||||
| <25% | - | - | - | ||||||
| 25–50% | 1.82 | 1.16–2.86 | 1.15 | 0.75–1.67 | 1.45 | 0.96–2.20 | |||
| 50–75% | 1.57 | 0.94–2.61 | 1.09 | 0.67–1.65 | 1.66 | 1.07–2.59 | |||
| >75% | 3.18 | 1.92–5.27 | 1.21 | 0.71–1.94 | 2.02 | 1.25–3.25 | |||
| Hyperenhanced lesion | >0.90 | 0.016 | 0.7 | ||||||
| No | - | - | - | ||||||
| Yes | 1.01 | 0.7–1.45 | 0.82 | 0.62–1.08 | 0.94 | 0.67–1.32 | |||
| Previous treatment lines | <0.001 | 0.03 | 0.002 | ||||||
| 0 | - | - | - | ||||||
| 1 | 1.37 | 0.87–2.16 | 0.9 | 0.63–1.29 | 1.23 | 0.82–1.84 | |||
| ≥2 | 2.35 | 1.55–3.56 | 1.45 | 1.04–2.03 | 2.1 | 1.43–3.11 | |||
| Concomitant SSAs | 0.002 | <0.001 | <0.001 | ||||||
| No | - | - | |||||||
| Yes | 0.55 | 0.38–0.79 | 0.48 | 0.44 | 0.32–0.6 | ||||
| Therapy | 0.6 | 0.36–0.64 | 0.3 | 0.9 | |||||
| Lipiodol-TACE | - | - | - | ||||||
| DEB-TACE | 0.91 | 0.63–1.30 | 1.18 | 0.88–1.57 | 1.03 | 0.75–1.43 | |||
| TACE Selectivity | 0.3 | 0.32 | 0.2 | ||||||
| Segmental | - | - | - | ||||||
| Sectorial | 1.72 | 0.77–3.84 | 1.16 | 0.66–2.06 | 1.35 | 0.69–2.63 | |||
| Lobar | 1.07 | 0.58–1.96 | 0.81 | 0.54–1.22 | 0.85 | 0.52–1.4 | |||
| Total | 1.48 | 0.74–2.96 | 1.05 | 0.63–1.75 | 0.66 | 0.35–1.26 | |||
| Variable | OS | TTLP | TTUP | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95%CI | p | HR | 95%CI | p | HR | 95%CI | p | |
| Age | 1.03 | 1.01–1.06 | 0.006 | 1.02 | 1.0–1.04 | 0.015 | 1.03 | 1.01–1.05 | <0.001 |
| Tumor grade | 0.032 | <0.001 | <0.001 | ||||||
| 1 | - | - | - | ||||||
| 2 | 1.9 | 1.13–3.18 | 2.23 | 1.53–3.25 | 2.5 | 1.61–3.88 | |||
| 3 | 17 | 1.99–150 | 1.2 | 0.15–9.3 | 35.2 | 6.98–178 | |||
| Extrahepatic Disease | 0.053 | ||||||||
| No | - | ||||||||
| Yes | 1.67 | 0.99–2.81 | |||||||
| Liver involvement | <0.001 | 0.012 | |||||||
| <25% | - | - | |||||||
| 25–50% | 2.78 | 1.48–5.22 | 2.07 | 1.24–3.43 | |||||
| 50–75% | 1.47 | 0.71–3.04 | 1.63 | 0.93–2.86 | |||||
| >75% | 5.21 | 2.4–11.3 | 3.49 | 1.81–6.73 | |||||
| Previous treatment lines | 0.058 | 0.001 | <0.001 | ||||||
| 0 | - | - | - | ||||||
| 1 | 0.99 | 0.52–1.89 | 0.96 | 0.6–1.53 | 1.48 | 0.87–2.52 | |||
| ≥2 | 1.98 | 1.09–3.57 | 2.19 | 1.42–3.39 | 2.83 | 1.67–4.78 | |||
| Concomitant SSA | 0.001 | <0.001 | <0.001 | ||||||
| No | - | - | - | ||||||
| Yes | 0.44 | 0.27–0.72 | 0.49 | 0.34–0.72 | 0.41 | 0.26–0.66 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Touloupas, C.; Faron, M.; Hadoux, J.; Deschamps, F.; Roux, C.; Ronot, M.; Yevich, S.; Joskin, J.; Gelli, M.; Barbé, R.; et al. Long Term Efficacy and Assessment of Tumor Response of Transarterial Chemoembolization in Neuroendocrine Liver Metastases: A 15-Year Monocentric Experience. Cancers 2021, 13, 5366. https://doi.org/10.3390/cancers13215366
Touloupas C, Faron M, Hadoux J, Deschamps F, Roux C, Ronot M, Yevich S, Joskin J, Gelli M, Barbé R, et al. Long Term Efficacy and Assessment of Tumor Response of Transarterial Chemoembolization in Neuroendocrine Liver Metastases: A 15-Year Monocentric Experience. Cancers. 2021; 13(21):5366. https://doi.org/10.3390/cancers13215366
Chicago/Turabian StyleTouloupas, Caroline, Matthieu Faron, Julien Hadoux, Frédéric Deschamps, Charles Roux, Maxime Ronot, Steven Yevich, Julien Joskin, Maximiliano Gelli, Rémy Barbé, and et al. 2021. "Long Term Efficacy and Assessment of Tumor Response of Transarterial Chemoembolization in Neuroendocrine Liver Metastases: A 15-Year Monocentric Experience" Cancers 13, no. 21: 5366. https://doi.org/10.3390/cancers13215366