Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells
Abstract
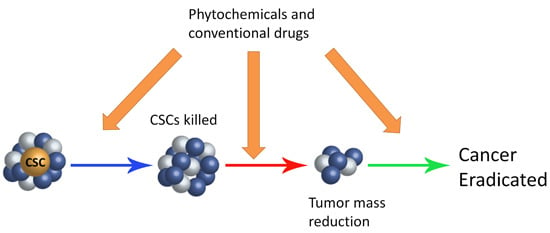
:1. Introduction
| Cellular Capacities | Stem Cells (SCs) | Cancer Stem Cells (CSCs) | Reference |
|---|---|---|---|
| Evade apoptosis | NO | YES | [14] |
| Self-sufficiency in growth signals | NO | YES | [2] |
| Insensitivity to anti-growth signals | NO | YES | [2] |
| Tissue invasion and metastasis | NO | YES | [2] |
| Contact inhibition * | YES | NO | [2] |
| Sustained angiogenesis | NO | YES | [2] |
| Deregulation of cellular energetics | NO | YES | [2] |
| Avoidance of immune destruction | NO | YES | [2] |
| Genome instability and mutations | NO | YES | [2] |
| Tumor-promoting inflammation state | NO | YES | [2] |
| Self-renewal | YES | YES | [14] |
| Quiescence in G0-like phase | YES | YES | [3] |
| Anticancer drug resistance ** | NO | YES | [3] |
2. Isolation of Cancer Stem Cells (CSCs) from Tumors
3. Molecular Mechanisms of Self-Renewal

4. Phytochemicals Able to Kill Cancer Cells
5. Phytochemicals that Selectively Kill Cancer Stem Cells
| Phytochemicals or Extracts | CSCs Type | Molecular Mechanism | Reference |
|---|---|---|---|
| EGCG | Breast cancer | Inhibits Wnt signaling | [88] |
| Piperine | Breast cancer | Inhibits Wnt signaling | [79] |
| Sulforaphane | Breast cancer | Decreases ALDH1 activity | [77] |
| Inhibits Wnt signaling | |||
| Pancreatic cancer | Induces apoptosis, activating caspase 3 | [89] | |
| Downregulates β-catenin | |||
| β-Carotene | Neuroblastoma | Inhibits Wnt signaling | [86] |
| Induces CSC differentiation | |||
| Quercetin | Pancreatic cancer | Inhibits Wnt signaling | [90] |
| Resveratrol | Pancreatic cancer | Induces apoptosis, activating caspase 3 | [91] |
| Colorectal cancer | Inhibits Wnt signaling | [92] | |
| Genistein | Pancreatic cancer | Decreases number of mammospheres | [92] |
| Decrease number of CD44+ cells | |||
| Curcumin | Breast cancer | Decreases number of mammospheres | [79] |
| Decreases ALDH1 activity | |||
| Colon cancer | Decreases ALDH1 activity | [93] | |
| Decreases number of CD44+, CD133+, CD166+ cells | |||
| Induces apoptosis | |||
| Colorectal cancer | Induces G2/M phase arrest | [94] | |
| Downregulates β-catenin | |||
| Prostate cancer | Induces G2/M phase arrest | [95] | |
| Inhibits Wnt signaling | |||
| Sasa quelpaertensis extract | Colon cancer | Induces CSC differentiation | [13] |
| Inhibits Wnt signaling |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Maccalli, C.; de Maria, R. Cancer stem cells: Perspectives for therapeutic targeting. Cancer Immunol. Immunother. 2015, 64, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Molofsky, A.V.; Pardal, R.; Morrison, S.J. Diverse mechanisms regulate stem cell self-renewal. Curr. Opin. Cell Biol. 2004, 16, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer stem cells—Perspectives on current status and future directions: AACR workshop on cancer stem cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [PubMed]
- Boman, B.M.; Wicha, M.; Fields, J.Z.; Runquist, O.A. Symmetric division of cancer stem cells: A key mechanism in tumor growth that should be targeted in future therapeutic approaches. Clin. Pharmacol. Ther. 2007, 81, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Boman, B.M.; Wicha, M.S. Cancer stem cells: A step toward the cure. J. Clin. Oncol. 2008, 26, 2795–2799. [Google Scholar] [CrossRef] [PubMed]
- Ma, I.; Allan, A.L. The role of human aldehyde dehydrogenase in normal and cancer stem cells. Stem Cell Rev. 2011, 7, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Riboli, E.; Norat, T. Epidemiologic evidence of the protective effect of fruit and vegetables on cancer risk. Am. J. Clin. Nutr. 2003, 78, 559S–569S. [Google Scholar] [PubMed]
- Lenzi, M.; Fimognari, C.; Hrelia, P. Sulforaphane as a promising molecule for fighting cancer. Cancer Treat. Res. 2014, 159, 207–223. [Google Scholar] [PubMed]
- D’Incalci, M.; Galmarini, C.M. A review of trabectedin (ET-743): A unique mechanism of action. Mol. Cancer Ther. 2010, 9, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Min, S.J.; Lim, J.Y.; Kim, H.R.; Kim, S.J.; Kim, Y. Sasa quelpaertensis leaf extract inhibits colon cancer by regulating cancer cell stemness in vitro and in vivo. Int. J. Mol. Sci. 2015, 16, 9976–9997. [Google Scholar] [CrossRef] [PubMed]
- Signore, M.; Ricci-Vitiani, L.; de Maria, R. Targeting apoptosis pathways in cancer stem cells. Cancer Lett. 2013, 332, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef] [PubMed]
- Croker, A.K.; Goodale, D.; Chu, J.; Postenka, C.; Hedley, B.D.; Hess, D.A.; Allan, A.L. High aldehyde dehydrogenase and expression of cancer stem cell markers selects for breast cancer cells with enhanced malignant and metastatic ability. J. Cell. Mol. Med. 2008, 13, 2236–2252. [Google Scholar] [CrossRef] [PubMed]
- Ginestier, C.; Hur, M.H.; Charafe-Jauffret, E.; Monville, F.; Dutcher, J.; Brown, M.; Jacquemier, J.; Viens, P.; Kleer, C.G.; Liu, S.; et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 2007, 1, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Storms, R.W.; Trujillo, A.P.; Springer, J.B.; Shah, L.; Colvin, O.M.; Ludeman, S.M.; Smith, C. Isolation of primitive human hematopoietic progenitors on the basis of aldehyde dehydrogenase activity. Proc. Natl. Acad. Sci. USA 1999, 96, 9118–9123. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Dontu, G.; Wicha, M.S. Mammary stem cells, self-renewal pathways, and carcinogenesis. Breast Cancer Res. 2005, 7, 86–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brittan, M.; Wright, N. Gastrointestinal stem cells. J. Pathol. 2002, 197, 492–509. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Farabegoli, R.; Iori, R.; Orlandi, M; de Nicola, G.R.; Bagatta, M.; Angelino, D.; Gennari, L.; Ninfali, P. Vitexin-2-O-xyloside, raphasatin and (−)-epigallocatechin-3-gallate synergistically affect cell growth and apoptosis of colon cancer cells. Food Chem. 2013, 138, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, I.A.; Malik, A.; Adhami, V.M.; Asim, M.; Hafeez, B.B.; Sarfaraz, S.; Mukhtar, H. Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 2008, 27, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Wei, F.; Wang, Y.; Wu, B.; Fang, Y.; Xiong, B. EGCG synergizes the therapeutic effect of cisplatin and oxaliplatin through autophagic pathway in human colorectal cancer cells. J. Pharmacol. Sci. 2015, 128, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.C.; Arbab, A.S.; Jardim-Perassi, B.V.; Borin, T.F.; Varma, N.R.; Iskader, A.; Shankar, A.; Ali, M.M.; de Campos, D.A. Effect of curcumin on pro-angiogenic factors in the xenograft model of breast cancer. Anticancer Agents Med. Chem. 2015, in press. [Google Scholar] [CrossRef]
- Rao, C.V; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995, 55, 259–266. [Google Scholar] [PubMed]
- Verma, S.P.; Salamone, E.; Goldin, B. Curcumin and genistein, plant natural products, show synergistic inhibitory effects on the growth of human breast cancer MCF-7 cells induced by estrogenic pesticides. Biochem. Biophys. Res. Commun. 1997, 233, 692–696. [Google Scholar] [CrossRef] [PubMed]
- Khafif, A.; Schantz, S.P.; Chou, T.C.; Edelstein, D.; Sacks, P.G. Quantitation of chemopreventive synergism between (−)-epigallocatechin-3-gallate and curcumin in not normal, premalignant and malignant human oral epithelial cells. Carcinogenesis 1998, 19, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Sreepriya, M.; Bali, G. Effects of administration of embelin and curcumin on lipid peroxidation, hepatic glutathione antioxidant defense and hematopoietic system during N-nitrosodiethylamine/phenobarbital-induced hepatocarcinogenesis in Wistar rats. Mol. Cell. Biochem. 2006, 284, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Koo, J.Y.; Kim, H.J.; Jung, K.O.; Park, K.Y. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J. Med. Food 2004, 7, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Toden, S.; Okugawa, Y.; Jascur, T.; Wodarz, D.; Komarova, N.L.; Buhrmann, C.; Shakibaei, M.; Boland, C.R.; Goel, A. Curcumin mediates chemosensitization to 5-fluorouracil through miRNA-induced suppression of epithelial-to-mesenchymal transition in chemoresistant colorectal cancer. Carcinogenesis 2015, 36, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Sen, S.; Sharma, H.; Singh, N. Curcumin enhances vinorelbine mediated apoptosis in NSCLC cells by the mitochondrial pathway. Biochem. Biophys. Res. Commun. 2005, 331, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kanai, M. Therapeutic applications of curcumin for patients with pancreatic cancer. World J. Gastroenterol. 2014, 20, 9384–9391. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Guha, S.; Krishnan, S.; Diagaradjane, P.; Gelovani, J.; Aggarwal, B.B. Curcumin potentiates antitumor activity of gemcitabine in an orthotopic model of pancreatic cancer through suppression of proliferation, angiogenesis, and inhibition of nuclear factor κB-regulated gene products. Cancer Res. 2007, 67, 3853–3861. [Google Scholar] [CrossRef] [PubMed]
- Lev-Ari, S.; Starr, A.; Katzburg, S.; Berkovich, L.; Rimmon, A.; Ben-Yosef, R.; Vexler, A.; Ron, I.; Earon, G. Curcumin induces apoptosis and inhibits growth of orthotopic human non-small cell lung cancer xenografts. J. Nutr. Biochem. 2014, 25, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Athar, M.; Back, J.H.; Tang, X.; Kim, K.H.; Kopelovich, L.; Bickers, D.L.; Kim, A.L. Resveratrol: A review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007, 224, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; Lee, J.; Lee, S.H. TCF4 is a molecular target of resveratrol in the prevention of colorectal cancer. Int. J. Mol. Sci. 2015, 16, 10411–10425. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.G.; Hong, T.; Shimada, Y.; Komoto, I.; Kawabe, A.; Ding, Y.; Kaganoi, J.; Hashimoto, Y.; Imamura, M. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by resveratrol. Carcinogenesis 2002, 23, 1531–1536. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Bueso-Ramos, C.; Aggarwal, B.B. Suppression of 7,12-dimethylbenz(a)anthracene-induced mammary carcinogenesis in rats by resveratrol: Role of nuclear factor-κB, cyclooxygenase 2, and matrix metalloprotease 9. Cancer Res. 2002, 62, 4945–4954. [Google Scholar] [PubMed]
- Garvin, S.; Ollinger, K.; Dabrosin, C. Resveratrol induces apoptosis and inhibits angiogenesis in human breast cancer xenografts in vivo. Cancer Lett. 2006, 231, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.; Walsh, S.E.; Morrissey, C.; Fitzpatrick, J.M.; Watson, R.W. Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate 2007, 67, 1641–1653. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, B.A.; Narayanan, N.K.; Re, G.G.; Nixon, D.W. Differential expression of genes induced by resveratrol in LNCaP cells: P53-mediated molecular targets. Int. J. Cancer 2003, 104, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.H.; Nihal, M.; Fu, V.X.; Jarrard, D.F.; Ahmad, N. Resveratrol-caused apoptosis of human prostate carcinoma LNCaP cells is mediated via modulation of phosphatidylinositol 3-kinase/Akt pathway and Bcl-2 family proteins. Mol. Cancer Ther. 2006, 5, 1335–1341. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E. Tomatoes, tomato-based products, lycopene, and cancer: Review of the epidemiologic literature. J. Natl. Cancer Inst. 1999, 91, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Seren, S.; Lieberman, R.; Bayraktar, U.D.; Heath, E.; Sahin, K.; Andic, F.; Kucuk, O. Lycopene in cancer prevention and treatment. Am. J. Ther. 2008, 15, 66–81. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, S.; Bidoli, E.; la Vecchia, C.; Talamini, R.; D’Avanzo, B.; Negri, E. Tomatoes and risk of digestive-tract cancers. Int. J. Cancer 1994, 59, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Giovannucci, E.; Rimm, E.B.; Liu, Y.; Stampfer, M.J.; Willett, W.C. A prospective study of tomato products, lycopene, and prostate cancer risk. J. Natl. Cancer Inst. 2002, 94, 391–398. [Google Scholar] [CrossRef] [PubMed]
- García-Closas, R.; Castellsague´, X.; Bosch, X.; Gonzàlez, C.A. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int. J. Cancer 2005, 117, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, V.; Fleshner, N.E.; Sugar, L.M.; Klotz, L.H. Antioxidants block prostate cancer in lady transgenic mice. Cancer Res. 2004, 64, 5891–5896. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.; Bosin, E.; Feldman, B.; Giat, Y.; Miinster, A.; Danilenko, M.; Sharoni, Y. Lycopene is a more potent inhibitor of human cancer cell proliferation than either alpha-carotene or betacarotene. Nutr. Cancer 1995, 24, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Shimoi, K.; Saka, N.; Kaji, K.; Nozawa, R.; Kinae, N. Metabolic fate of luteolin and its functional activity at focal site. Biofactors 2000, 12, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.F.; Yang, W.E.; Chang, H.R.; Chu, S.C.; Hsieh, Y.S. Luteolin induces apoptosis in oral squamous cancer cells. J. Dent. Res. 2008, 87, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, X.H.; Wang, Z.J. Flavones and flavonols exert cytotoxic effects on a human oesophageal adenocarcinoma cell line (OE33) by causing G2/M arrest and inducing apoptosis. Food Chem. Toxicol. 2008, 46, 2042–2053. [Google Scholar] [CrossRef] [PubMed]
- Ju, W.; Wang, X.; Shi, H.; Chen, W.; Belinsky, S.A.; Lin, Y. A critical role of luteolin-induced reactive oxygen species in blockage of tumor necrosis factor-activated nuclear factor κB pathway and sensitization of apoptosis in lung cancer cells. Mol. Pharmacol. 2007, 71, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Lim do, Y.; Jeong, Y.; Tyner, A.L.; Park, J.H. Induction of cell cycle arrest and apoptosis in HT-29 human colon cancer cells by the dietary compound luteolin. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G66–G75. [Google Scholar] [CrossRef] [PubMed]
- Selvendiran, K.; Koga, H.; Ueno, T.; Yoshida, T.; Maeyama, M.; Torimura, T.; Yano, H.; Kojiro, M.; Sata, M. Luteolin promotes degradation in signal transducer and activator of transcription 3 in human hepatoma cells: An implication for the antitumor potential of flavonoids. Cancer Res. 2006, 66, 4826–4834. [Google Scholar] [CrossRef] [PubMed]
- Chiu, F.L.; Lin, J.K. Downregulation of androgen receptor expression by luteolin causes inhibition of cell proliferation and induction of apoptosis in human prostate cancer cells and xenografts. Prostate 2008, 68, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Zhang, Q.; Shen, W.; Zhu, J. Antiproliferative and chemosensitizing effects of luteolin on human gastric cancer AGS cell line. Mol. Cell. Biochem. 2008, 313, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.L.; Dia, V.P.; Wallig, M.; Gonzalez de Mejia, E. Luteolin and gemcitabine protect against pancreatic cancer in an orthotopic mouse model. Pancreas 2015, 44, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Hurley, T.G.; Olendzki, B.C.; Teas, J.; Ma, Y.; Hampl, J.S. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J. Natl. Cancer Inst. 1998, 90, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Adlercreutz, H.; Honjo, H.; Higashi, A.; Fotsis, T.; Hamalainen, E.; Hasegawa, T.; Okada, H. Urinary excretion of lignans and isoflavonoid phytoestrogens in Japanese men and women consuming a traditional Japanese diet. Am. J. Clin. Nutr. 1991, 54, 1093–1100. [Google Scholar] [PubMed]
- Ziegler, R.G.; Hoover, R.N.; Pike, M.C.; Hildesheim, A.; Nomura, A.M.; West, D.W.; Wu-Williams, A.H.; Kolonel, L.N.; Horn-Ross, P.L.; Rosenthal, J.F.; Hyer, M.B. Migration patterns and breast cancer risk in Asian-American women. J. Natl. Cancer Inst. 1993, 85, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Goodman, M.T.; Wilkens, L.R.; Hankin, J.H.; Lyu, L.C.; Wu, A.H.; Kolonel, L.N. Association of soy and fiber consumption with the risk of endometrial cancer. Am. J. Epidemiol. 1997, 146, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, R.M.; Banerjee, S.; Li, Y.; Aboukameel, A.; Kucuk, O.; Sarkar, F.H. Cisplatin-induced antitumor activity is potentiated by the soy isoflavone genistein in BxPC-3 pancreatic tumor xenografts. Cancer 2006, 106, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Qi, J.; Li, X.T.; Zhou, K.; Xn, J.H.; Zhou, Y.; Zhang, C.Q.; Xu, J.P.; Zhou, R.J. ATRA and Genistein synergistically inhibit the metastatic potential of human lung adenocarcinoma cells. Int. J. Clin. Exp. Med. 2015, 8, 4220–4227. [Google Scholar] [PubMed]
- Zhang, Y.; Vareed, S.K.; Nair, M.G. Human tumor cell growth inhibition by nontoxic anthocyanidins, the pigments in fruits and vegetables. Life Sci. 2005, 76, 1465–1472. [Google Scholar] [CrossRef] [PubMed]
- AbdullRazis, A.F.; Iori, R.; Ioannides, C. The natural chemopreventive phytochemical R-sulforaphane is a far more potent inducer of the carcinogen-detoxifying enzyme systems in rat liver and lung than the S-isomer. Int. J. Cancer 2011, 128, 2775–2782. [Google Scholar] [CrossRef] [PubMed]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Felletti, M.; Blasa, M.; Angelino, D.; Celeghini, C.; Corallini, A.; Ninfali, P. Total extracts of Beta vulgais var. cicla seeds versus its purified phenolic components: Antioxidant activities and antiprolferative effects against colon cancer cells. Phytochem. Anal. 2011, 22, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, R.; de Bartolomeo, A.; Rosignoli, P.; Servili, M.; Selvaggini, R.; Montedoro, G.F.; di Saverio, C.; Morozzi, G. Virgin olive oil phenols inhibit proliferation of human Promyelocytic leukemia cells (HL60) by inducing apoptosis and differentiation. J. Nutr. 2006, 136, 614–619. [Google Scholar] [PubMed]
- Hashim, Y.Z.; Worthington, J.; Allsopp, P.; Ternan, N.G.; Brown, E.M.; McCann, M.J.; Rowland, I.R.; Esposto, S.; Servili, M.; Gill, C.I. Virgin olive oil phenolics extract inhibit invasion of HT115 human colon cancer cells in vitro and in vivo. Food Funct. 2014, 5, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Chu, Y.L.; Ho, C.T.; Chung, J.G.; Lai, C.I.; Su, Y.C.; Kuo, Y.H.; Sheen, L.Y. Antcin K, an active triterpenoid from the fruiting bodies of Basswood-cultivated Antrodia cinnamomea, inhibits metastasis via suppression of integrin-mediated adhesion, migration, and invasion in human hepatoma cells. J. Agric. Food Chem. 2015, 63, 4561–4569. [Google Scholar] [CrossRef] [PubMed]
- Ruhul Amin, A.R.M.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J. Clin. Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef] [PubMed]
- Zanichelli, F.; Capasso, S.; Cipollaro, M.; Pagnotta, E.; Cartenì, M.; Casale, F.; Iori, R.; Galderisi, U. Dose-dependent effects of R-sulforaphane isothiocyanate on the biology of human mesenchymal stem cells, at dietary amounts, it promotes cell proliferation and reduces senescence and apoptosis, while at anti-cancer drug doses, it has a cytotoxic effect. Age 2012, 34, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Rausch, V.; Baumann, B.; Apel, A.; Beckermann, B.M.; Groth, A.; Mattern, J.; Li, Z.; Kolb, A.; Moldenhauer, G.; et al. Sulforaphane targets pancreatic tumour-initiating cells by NF-κB-induced antiapoptotic signalling. Gut 2009, 58, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a dietary component of broccoli/broccoli sprouts, inhibits breast cancer stem cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, L. Discovery and development of sulforaphane as a cancer chemopreventive phytochemical. Acta Pharmacol. Sin. 2007, 28, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Kakarala, M.; Brenner, D.E.; Khorkaya, H.; Cheng, C.; Tazi, K.; Ginestier, C.; Liu, S.; Dontu, G.; Wicha, M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010, 122, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.; Nerlich, A.G.; Iancu, C.M.; Cilli, M.; Schleicher, E.; Vene, R.; Dell’Eva, R.; Jochum, M.; Albini, A.; Pfeffer, U. The chemopreventive polyphenol curcumin prevents hematogenous breast cancer metastases in immunodeficient mice. Cell. Physiol. Biochem. 2007, 19, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Lao, C.D.; Ruffin, M.T.; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 17, 6–10. [Google Scholar]
- Chearwae, W.; Anuchapreeda, S.; Nandigama, K.; Ambudkar, S.V.; Limtrakul, P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem. Pharmacol. 2004, 68, 2043–2052. [Google Scholar] [CrossRef] [PubMed]
- Limtrakul, P.; Chearwae, W.; Shukla, S.; Phisalphong, C.; Ambudkar, S.V. Modulation of function of three ABC drug transporters, P-glycoprotein (ABCB1), mitoxantrone resistance protein (ABCG2) and multidrug resistance protein 1 (ABCC1) by tetrahydrocurcumin, a major metabolite of curcumin. Mol. Cell. Biochem. 2007, 296, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Khar, A. Biological effects of curcumin and its role in cancer chemoprevention and therapy. Anticancer Agents Med. Chem. 2006, 6, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Park, S.; Kim, Y. Effect of β-carotene on cancer cell stemness and differentiation in SK-N-BE(2)C neuroblastoma cells. Oncol. Rep. 2013, 30, 1869–1877. [Google Scholar] [PubMed]
- Kang, S.I.; Shin, H.S.; Kim, H.M.; Hong, Y.S.; Yoon, S.A.; Kang, S.W.; Kim, J.H.; Ko, H.C.; Kim, S.J. Anti-obesity properties of a Sasa quelpaertensis extract in high-fat diet-induced obese mice. Biosci. Biotechnol. Biochem. 2012, 76, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Zhang, X.; Rieger-Christ, K.M.; Summerhayes, I.C.; Wazer, D.E.; Paulson, K.E.; Yee, A.S. Suppression of Wnt signaling by the green tea compound (−)-epigallocatechin 3-gallate (EGCG) in invasive breast cancer cells. Requirement of the transcriptional repressor HBP1. J. Biol. Chem. 2006, 281, 10865–10875. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Tang, S.N.; Zhu, W.; Meeker, D.; Shankar, S. Sulforaphane synergizes with quercetin to inhibit self-renewal capacity of pancreatic cancer stem cells. Front. Biosci. 2011, 3, 515–528. [Google Scholar] [CrossRef]
- Zhou, W.; Kallifatidis, G.; Baumann, B.; Rausch, V.; Mattern, J.; Gladkich, J.; Giese, N.; Moldenhauer, G.; Wirth, T.; Buchler, M.W.; et al. Dietary polyphenol quercetin targets pancreatic cancer stem cells. Int. J. Oncol. 2010, 37, 551–561. [Google Scholar] [PubMed]
- Shankar, S.; Nall, D.; Tang, S.N.; Meeker, D.; Passarini, J.; Sharma, J.; Srivastava, R.K. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial–mesenchymal transition. PLoS ONE 2011, 6, e16530. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, P.; Padhye, S.; Ahmad, A.; Sarkar, F.H. Novel strategies targeting cancer stem cells through phytochemicals and their analogs. Drug Deliv. Transl. Res. 2013, 3, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, J.; Banerjee, S.; Kanwar, S.S.; Yu, Y.; Patel, B.B.; Sarkar, F.H.; Majumdar, A.P. Curcumin enhances dasatinib-induced inhibition of growth and transformation of colon cancer cells. Int. J. Cancer 2011, 128, 951–961. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.S.; Marlow, B.P.; Gupta, N.; Narayan, S. β-catenin-mediated transactivation and cell–cell adhesion pathways are important in curcumin (diferuylmethane)-induced growth arrest and apoptosis in colon cancer cells. Oncogene 2002, 21, 8414–8427. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Gaascht, F.; Cronauer, M.; Henry, E.; Dicato, M.; Diederich, M. Anti-proliferative potential of curcumin in androgen-dependent prostate cancer cells occurs through modulation of the Wingless signaling pathway. Int. J. Oncol. 2011, 38, 603–611. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarpa, E.-S.; Ninfali, P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. Int. J. Mol. Sci. 2015, 16, 15727-15742. https://doi.org/10.3390/ijms160715727
Scarpa E-S, Ninfali P. Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells. International Journal of Molecular Sciences. 2015; 16(7):15727-15742. https://doi.org/10.3390/ijms160715727
Chicago/Turabian StyleScarpa, Emanuele-Salvatore, and Paolino Ninfali. 2015. "Phytochemicals as Innovative Therapeutic Tools against Cancer Stem Cells" International Journal of Molecular Sciences 16, no. 7: 15727-15742. https://doi.org/10.3390/ijms160715727