Abstract
Alzheimer's disease (AD) has become one of the most worrying health conditions with no effective treatment available with the increase in population aging. A large number of clinical studies and experiments proved that photobiomodulation (PBM) had a positive effect on AD treatment. The irradiation with red and near-infrared light at a low dose can effectively reduce an accumulation of amyloid-β (Aβ) plaques in the central nervous system, relieving the symptoms of AD. This review summarizes the parameters of PBM for AD treatment studied on cells, animals, and in clinical trials, as well as the dose–effect relationship of PBM treatment for AD. The mechanisms of PBM on the cellular level, which include regulation of microglia and astrocytes that may affect Aβ plaque elimination are also discussed.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 license. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Photobiomodulation (PBM), also known as low level light therapy, has been widely employed in the treatment and regulation of brain diseases in recent years. The visible and near-infrared (NIR) light emitted by the light emitting diode (LED) or lasers in the spectral range of ∼600–1000 nm is found to interfere with brain activities. This kind of treatment mainly acts on a cellular level, triggering a series of biological reactions and influencing cell metabolism [1, 2]. Quite a few studies also exist on PBM application to treat Alzheimer's disease (AD), Parkinson's and other neurodegenerative diseases [3].
AD is a chronic neurodegenerative disease, which is characterized by a gradual loss of cognitive ability, memory and language skills during the disease progression [4]. Nitric oxide (No) effective treatment for AD is currently available and its pathogenesis has not been determined as well. Neuroinflammation and oxidative stress are typical pathological features in the central nervous system (CNS) of AD patients [5, 6]. Two kinds of lesions (i.e. amyloid-β (Aβ) plaques formed by amyloid deposits and nerve entanglement caused by tau protein abnormal hyperphosphorylation) were found to appear in the brain 5–10 years before the development of AD symptoms [7, 8]. These two pathologies can cause neuronal synapses to decrease, leading to impaired neuronal activity, cognition decline and memory loss [9]. Accumulation of Aβ plaques and abnormal hyperphosphorylation of tau protein both appear in the brain of AD patients. However, which kind of lesion appears first is unclear. Reliable pieces of evidence also exist which suggests that the blood flow reduction [10] and the blood-brain barrier (BBB) destruction [11] are the pathological features of AD. Moreover, several trials aimed at eliminating Aβ plaques to improve AD symptoms have been recently reported [12–14].
PBM is assumed to treat AD mainly by improving the activity of neurons, rebuilding the damaged neuron network and stimulating the production of more synapses. The cytochrome C oxidase (CCO) in the mitochondria of neurons is involved in the PBM process as a photoacceptor, stimulation of which causes the mitochondria to produce more adenosine triphosphate (ATP) [15]. Moreover, the microglia and astrocytes distributed throughout the CNS are also simultaneously stimulated by PBM. The microglia are involved in the occurrence of a series of neurodegenerative diseases as a kind of immune response cells. Consequently, microglia activation and related neuroinflammation are the main characteristics of neuropathology [16, 17]. Microglia, being activated by light with different light parameters, can react differently to brain inflammation, producing different immune responses that result in pro- or anti-inflammatory effects [18]. In addition, astrocytes, which maintain the BBB function of the CNS [19–21], can also be affected by PBM and improve AD incidence by increasing blood circulation in the brain [22].
PBM studies employ light irradiation of different parameters. The differences in irradiation wavelength, light source coherence and mode (i.e. continuous wave (CW) or pulsed wave (PW) light with various frequencies), as well as in irradiation dose, lead to significantly different treatment output. The wavelengths are very important in PBM because light at different wavelengths may trigger different photoactivation mechanisms at the molecular level. For instance, the light in the range of ∼600–900 nm is known to mainly stimulate mitochondria, increasing respiration efficiency of cells, and generate more ATP. Therefore, the cell activity is enhanced. However, mitochondria stimulation at other wavelengths is very weak [23]. Different light irradiation dose is achieved by changing the time of irradiation with the same power density for dosage in PBM application. PW light generally has some advantages in PBM treatment concerning the light source mode. In particular, the use of PW light instead of CW light reduces the photo-induced heat generation (photothermal effect) that may cause damage to cells and tissues [24].
This review discusses the mechanisms of PBM on the cellular level and how it regulates microglia and astrocytes that may have a certain effect on Aβ plaque elimination. The dose–effect relationships for PBM in AD treatment are also summarized. This can provide a reference for choosing the parameters for PBM treatment of AD in clinical practice. With light, the wavelength, dose, and frequency will produce the most positive and beneficial effects in AD treatment.
2. The PBM mechanisms
2.1. The main molecular mechanism of PBM treatment
The photothermal effect caused by laser radiation is well-known and its use in phototherapy is very common [25]. A significant amount of heat is released when a high-power density laser is used to irradiate the tissues, which can even cause the fracture and evaporation of the tissues [26]. However, PBM does not work on cells through heat generation. The power densities of light used in PBM are usually noticeably lower than a few hundreds of milliwatt per square centimeter, and the corresponding temperature rise at this power density is not as significant (mainly under 1 °C) [27]. Schneede et al [28] used a microthermometer to measure the temperature change caused by the 40 mW cm−2 of the laser when irradiating cells to verify whether the heat produced by light with such low power density will damage cells. The results proved that the rise in temperature does not exceed 0.065 °C after monolayer cells were irradiated.
Studies have shown that the light used in PBM is mainly absorbed by CCO in the fourth transport chain of the mitochondrial electron transport chain. CCO changes the redox state of the cells through a series of complex responses [29]. CCO generally has two copper centers and two heme-iron centers. They mainly absorb visible and NIR light between 600 and 1000 nm, which is the range of the optical windows for PBM [30, 31]. The electron excitation energy can be utilized in multiple ways when the photon of the incident light is absorbed by the cell chromophores, particularly increasing the membrane potential of the mitochondria, which results in a rise in cell respiration efficiency [32, 33]. Moreover, oxygen acts as the ultimate acceptor in the electron transport chain whereby 90% of oxygen absorbed by cells is used for cellular respiration to produce ATP, while a small amount of oxygen is converted to reactive oxygen species (ROS) [1, 34]. Furthermore, ROS rapidly regulates gene expression by activating various channels (e.g. NF-kB [35] plays an important role in cell signal transduction, cell cycle process regulation, enzyme activation, nucleic acid and protein synthesis and so on) [36]. Oxygen metabolism is also promoted PBM modulates cells producing more ATP which can result in a brief burst of ROS in cells. In turn, ROS activates transcription factors, leading to the upregulation of various stimulating and protective genes, which are related to cell proliferation and the production of cytokines and growth factors [37]. Even if the irradiation stopped, the expression of the transcription factors will not stop immediately [38]. Excessive ROS may damage the neuronal mitochondrial function [34] and destroy structures of DNA, proteins, and other essential components of cells [39] although mitochondrial respiration is the main source of ROS.
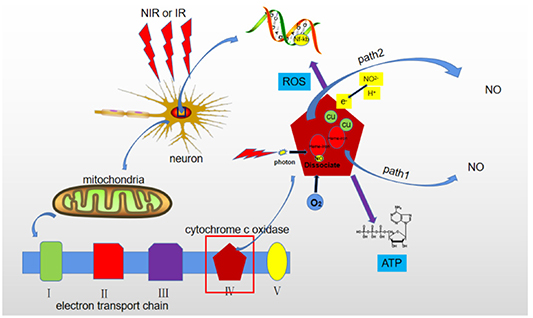
PBM is suggested to affect the neural cell activity through the involvement of a few mechanisms (figure 1). In particular, NO plays an important role in cell activity can be released from CCO through two pathways. The first pathway is via photodissociation when NO is replaced in CCO by molecular oxygen, which leads to increased oxygen consumption by mitochondria followed by a rise in ATP production [40, 41]. CCO acts as a nitrite reductase when the oxygen concentration is too low to replace NO. Photoexcited CCO provides electronic excitation energy to reduce nitrite and release NO [42]. The corresponding chemical equation can be presented as:
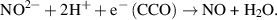
Figure 1. Scheme illustrating PBM mechanisms. When cells are irradiated by red or NIR light, mitochondrial cytochrome C oxidase on the fourth transport chain absorbs photon energy and release ATP, ROS and nitric oxide. ROS can activate the NF-kB channel of cells to release transcription factors. There are two pathways for NO release from CCO: (1) through photodissociation, when the released NO is replaced by molecular oxygen and (2) CCO provides an electron to reduce nitrite to NO.
Download figure:
Standard image High-resolution imageThe released NO at a low concentration triggers a mechanism that is beneficial for the treatment of AD and other neurodegenerative diseases. NO supply improves insufficient blood circulation in the brain (which is common in neurodegenerative diseases [43]), because it can easily penetrate the membrane of the smooth muscle cells of blood and lymphatic vessels, making them diastolic and causing vasodilation [44]. The resulting increase in blood flow helps to repair the brain nerves along with increased ATP production. However, the NO release from CCO immediately stops as the irradiation ends [45]. Therefore, the PBM-induced cell regulation through stimulation of CCO to produce NO does not last long. Both mitochondrial activity and ATP production should be emphasized to be promoted by the light irradiation, resulting in an increased the cell activity and the related cell functions. Figure 1 illustrates the PBM mechanisms on neuron cells.
Additionally, PBM has been reported to promote neurogenesis and synaptic regeneration. Synapses are important elements in information exchange between neurons and the destruction of synaptic connections between neurons is a known pathological AD feature [46, 47]. Thus, restoring these connections is essential for AD treatment. Furthermore, synaptic and neurons regeneration is stimulated by the brain derived neurotrophic factor (BDNF), a CNS protein that plays a pivotal role in maintaining the activity of existing neurons [48, 49]. Aβ plaque-mediated neurotoxicity and dendritic atrophy have been reported to lead to BDNF reduction in the CNS of transgenic mice [50]. PBM was notably shown to regulate BDNF and promote the restoration of synaptic connections between neurons. Furthermore, Meng et al [51] irradiated SH-SY5Y cells by He–Ne laser (wavelength, 632.8 nm, power density, 12.74 mW cm−2). Cells exposed to PBM had a survival rate of nearly 100%, compared with 60% (the control group was compared) without light and it can rescue the dendritic atrophy by upregulating the BDNF, all the above definitively demonstrated in co-culture model of neuroblastoma cell and Aβ.
The PBM mechanism in anti- inflammation, cerebral blood flow increase, and neural electrical signal modulation has been reported in several clinical trials [52], Moreover, Wang et al demonstrated that PBM increased the concentration of oxidized CCO and electroencephalogram power of the anterior and posterior regions in the human brain. The former explains the main mechanism of the PBM action on CCO in vivo (wavelength, 1064 nm, dose, 13.75 J cm−2) [53]. The concentration of oxidized CCO in the brain will increase when CCO absorbs photons, thus promoting the release of the proton pump, NO, and ATP synthesis which leads to increase cerebral blood flow, cerebral blood volume and cerebral oxygenation. The latter reported that PBM increases the strength of electrophysiological oscillation at alpha (8–13 Hz) and beta (13–30 Hz) bands [54]. These clinical studies have also contributed to our understanding of the mechanisms of PBM in the human brain.
2.2. PBM treatment of AD by regulating microglia
Microglia mediate the endogenous immune response of damage in CNS, playing an important role in several neurodegenerative diseases, including AD. Microglia activation and corresponding neuroinflammation result in neuroprotection or neurotoxicity and are the main features of neuropathology [55]. The proinflammatory response in microglia is mainly mediated by toll-like receptor (TLR) [18]. The Aβ plaques in the CNS of AD patients are a vital factor that activates microglia, promoting the production of IL-1β [56, 57], inducible nitric-oxide synthase [58] and TLR expression [59, 60]. Aβ plaques cannot activate microglia to trigger proinflammatory responses once the TLR is knocked out from microglia [18]. After the activation, microglia can release some cytotoxic agents (e.g. NO, ROS, proteases, and viscous molecules). These overproduced neurotoxic substances promote an inflammatory response in CNS [61]. Moreover, the microglia inflammatory response has two different phenotypes [62]. Microglia cells are in a static state in the absence of pathogens. Microglia cells are activated when pathological markers of AD are detected, releasing inflammatory factors and clearing Aβ plaque by phagocytosis. Microglia partially or completely transform into pro-inflammatory (M1) or anti-inflammatory (M2) states depending on the degree of inflammation [63]. M1 microglia continuously releases ROS, NO, and some other inflammatory factors. Acting as neurotoxic cells, they excessively prune synapses and shed myelin sheath, which eventually leads to information transmission being blocked or even neuron death [64]. These behaviors aggravate CNS damage in AD patients. In contrast, M2 microglia cells are anti-inflammatory, can swallow some Aβ plaques [65], provide nutritional factors and promote the neuronal networks reconstruction [66], and repair brain damage. Leden et al [63] reported the use of CW light source (808 nm laser diode with a power density of 50 mW cm−2) to irradiate primary microglia and BV2 microglia with different light doses. Microglia mainly transited to M1 phenotype at a relatively high dose of 4–30 J cm−2. Conversely, microglia are mainly transited to M2 phenotype at a relatively low dose of 0.2–10 J cm−2. The biphasic response for the dose of light is not applicable for microglia regulation. The PBM effect on microglia is not simply reflected in the inhibition of the activities and functions of cells by high doses and promotion of those low doses, but in the regulation of the microglial phenotype [63]. The different phenotypes of the response of microglia to PBM regulation suggest different ways of microglia activation to treat AD. On the one hand, PBM can inhibit the proinflammatory properties of microglia to limit the harm to CNS. Song et al [60] reported inhibition of the proinflammatory response of microglia via activating the Scr-modulation signal channel. They used He–Ne laser (wavelength, 632.8 nm, power density, 64.6 mW cm−2) to regulate microglia. The results showed that the irradiation significantly reduced the TLR-mediated proinflammatory response in microglia at a dose of 20 J cm−2. On the other hand, light enhances the anti-inflammatory effect by changing the microglia phenotype [63, 66].
2.3. PBM treatment of AD by regulating astrocytes
Astrocytes are the most widely distributed glial cells in the mammalian brain. The cell body extends into long and separate projections, which are distributed between the cell bodies of the nerve cells. Their main function is to support and separate the nerve cells. Moreover, astrocytes also participate in BBB formation, the destruction of which is considered to be an important link in AD pathogenesis [11, 19, 67]. Astrocytes are also responsible for the protection and defense functions of the CNS [20]. In addition, similarly to the microglia, astrocytes exhibit two phenotypes with completely different functions and properties to respond to CNS inflammation. A1 astrocytes are known to have a damaging effect on neurons, while A2 astrocytes, in contrast, mediate nerve cells protection and repair [68]. A1 astrocytes release NO, ROS, and proinflammatory factors, which are toxic to glial cells and cause them to lose their phagocytic function to eliminate Aβ plaques [69]. A2 astrocytes release ATP, GABA [70], neurotrophic factors and cytokines that promote the proliferation and differentiation of neurons and the repair of synapses [71].
Oxidative stress and neuroinflammation are important processes in AD pathology [5, 6]. Aβ plaque induce the assembly of NADH oxidase in astrocytes, which activates astrocytes to produce superoxide anions (a type of ROS that is toxic to neurons) and proinflammatory factors. NADH oxidase is also important for ROS production by microglia [72]. Yang et al [73] used CW light of He–Ne laser (wavelength, 632.8 nm, dose, 16.2 J cm−2) to regulate the astrocytes co-cultured with Aβ. ROS detection and IL-1β were used to evaluate the response to oxidative stress and inflammatory effects mediated by Aβ in astrocytes treated with PBM [74]. The superoxide anions and IL-1β was shown to significantly rise compared with the control group when astrocytes were co-cultured with Aβ [72]. The levels of superoxide anions and IL-1β were similar to those in the control group after light exposure, showing that the PBM of astrocytes can result in the reduction of oxidative stress and inflammatory effects induced by Aβ. Bungart et al [75] used locally delivered bioluminescence resonance energy transfer to quantum dots (BRET-QDots) nanoparticles as the internal light sources for PBM in microglia because the light produced by external sources (i.e. ordinary lasers or LEDs) strongly attenuates when penetrates the skull to reach the cortex. Consequently, the oxidative stress and inflammatory response in astrocytes were significantly reduced under PBM treatment. This study suggests a noninvasive method for deeper light penetration in PBM for AD treatment.
3. Dose–effect relationships of PBM for AD
3.1. The biphasic response of PBM
Cells usually do not reveal a noticeable response to light irradiation when the light power density is too low or the irradiation time is too short. Moreover, the cell activity is inhibited by a high dose of irradiation causing significant photothermal or other damages [76, 77]. The dose-dependent response in PBM is often called a biphasic response, also known as the Arndt–Schulz law [78]. Sharma et al reported a PBM dose-response test using primary nerve cells with NIR CW light irradiation (wavelength, 810 nm, power density, 25 mW cm−2). ATP production, Ca2+ influx, and the mitochondrial membrane potential significantly increased under irradiation at a low light dose of 3 J cm−2. However, when the cells were irradiated with a higher dose of 30 J cm−2, higher amounts of ROS and NO were simultaneously generated [76]. Thus, an appropriate dose is very important for PBM to be effective. No absolute values for effective PBM doses have been established as they depend on the experimental conditions. Appropriate irradiation doses in a practical PBM application should be determined according to the specific situation.
Some glial cells (including microglia and astrocytes) often exhibit a triphasic response to PBM although neurons have a biphasic response to PBM. Moreover, microglia and astrocytes differentiate into two phenotypes at different irradiation doses [66, 79]. Under irradiation at relatively low doses, both microglia and astrocytes transform into phenotypes that are beneficial to neurons but they release proinflammatory factors and neurotoxic factors under high irradiation doses as aforementioned [66, 71].
3.2. PBM for AD treatment in cell and animal models and in clinical trials
PBM effects are mostly studied using cells in vitro, while in vivo research is mainly focused on mouse AD models, except for a small number of recent clinical trials. These PBM studies aim to determine the most effective PBM parameters to reduce the content of Aβ plaques and their damage to CNS.
Table 1 summarized the PBM research on AD—cell models, which combined cells related to AD pathogenesis with Aβ. PBM studies were aimed at possibly reducing the toxic effect of Aβ on cells. The light dose range was 0.1–20 J cm−2. He–Ne CW laser emitting at 632.8 nm was used as a light source in most of the studies [51, 60, 73, 80–82]. In particular, Liang et al used the He–Ne laser to irradiate nerve cells with doses of 0.1–2 J cm−2 and found that PBM inhibited neuronal apoptosis mediated by Aβ 25–35 through activating the Akt/GSK3β/β-catenin pathway [80], protein kinase C [81], and promoting Yes-associated protein cytoplasmic translocation [82]. Studies have shown that LED light can also effectively reduce the cell apoptosis in the nerve cells co-cultured with Aβ model [83]. Moreover, Bungart et al [75] applied BRET-QDots nanoparticles as nanosized light sources localized in situ and emitting at 800 nm to reduce oxidative stress and expression of inflammatory factors induced by Aβ in astrocytes [84]. In addition, red (670 nm) and NIR (1,068 nm) light was also shown to reduce the neuronal cell death induced by Aβ [85, 86]. The reported cell studies can be assumed to have shown that PBM can reduce Aβ toxicity to nerve cells both directly and indirectly (by interfering with the functional properties of microglia [60] and astrocytes [73, 75]).
Table 1. Reports on PBM of AD cell models.
| Model | Wavelength and source | Dose | Mode | Coherence | Effect | References |
|---|---|---|---|---|---|---|
| Human neuroblastoma (SH-EP) cells + Aβ42 | Laser (670 nm) | 1.04 J cm−2 | CW | Yes | Irradiated cells had less Aβ aggregates amounts and higher ATP level than the nonirradiated cells | [85] |
| Monolayer cell cultures of PC12 + Aβ 25–35 | GaAlAs LED (640 ± 15 nm) | 32.4 J cm−2 | CW | No | Treatment of cells with LED irradiation diminished Aβ 25–35 induced apoptosis | [83] |
| SH-SY5Y cells or primary hippocampal neurons of rat + Aβ 25–35 | He–Ne laser (632.8 nm) | 0.5, 1, 2, or 4 J cm−2 | CW | Yes | Cell viability was increased and the apoptosis rate was decreased after treatment with PBM | [51] |
| Primary rat astrocytes + Aβ 1–42 | He–Ne laser (632.8 nm) | 16.2 J cm−2 | CW | Yes | PBM suppressed ROS production and inflammatory response induced by Aβ in primary rat astrocytes | [73] |
| CAD neuronal cells + Aβ (1–42) | LED (1068 ± 12.5 nm) | Five sets of 0.9 J cm−2 | PW on 600 Hz | No | PBM can effectively reduce the cell decay rate but cannot promote cell proliferation | [86] |
| Astrocytes + Aβ peptide | 800 nm-emitting BRET-QDots | N/A | CW | No | Attenuating superoxide anion production and inflammatory marker expression induced by Aβ | [75] |
| Microglial and neuronal cells | He‐Ne laser (632.8 nm) | 20 J cm−2 | CW | Yes | PBM could attenuate toll-like receptor (TLR)-mediated proinflammatory responses in microglia | [60] |
| PC12 cells + Aβ 25–35 | He–Ne laser (632.8 nm) | 2 J cm−2 | CW | Yes | PBM attenuated Aβ-induced apoptosis through the Akt/GSK3β/β-catenin pathway | [80] |
| PC12 cells + Aβ 25–35 | He–Ne laser (632.8 nm) | 0.156–1.248 J cm−2 | CW | Yes | PBM inhibited apoptosis induced in PC 12 cell by Aβ 25–35 | [81] |
| PC12 cells + Aβ 25–35 | He–Ne laser (632.8 nm) | 2 J cm−2 | CW | Yes | PBM reduced nerve cell apoptosis through inhibiting YAP translocated from cytoplasm to nucleus | [82] |
Abbreviation: N/A, not available
Table 2 summarizes the in vivo studies of AD treatment with PBM. Transgenic mice or normal mice that have been injected with Aβ peptide are often used as subjects in AD animal studies and a variety of methods can be used to evaluate the therapeutic effect. Measuring the contents of deposition of Aβ plaques in brains [12, 87] and testing the behaviors of mice (e.g. Morris water maze test) [12] are two main methods to evaluate the treatment effect. Light doses used in the in vivo studies are usually higher than in vitro, reaching 80 J cm−2, because the light is strongly attenuated by biological tissues before reaching the cerebral cortex [88, 89].
Table 2. Reports on PBM of AD in vivo models.
| Model | Wavelengths and source | Dose | Mode | Coherence | Effect | Reference |
|---|---|---|---|---|---|---|
| APP/PS1 mice | He‐Ne laser (632.8 nm) | Approximately 2 J cm−2 reaching the interior of the hippocampus | CW | Yes | PBM effectively reduces Aβ plaques levels and amyloid plaque burden in mice. | [12] |
| Male swiss mice injected with Aβ 25–35 | Laser at 850 nm combined with NIR LED (850 nm) and red LED (625 nm) | 8.4 J cm−2 | PW at 10 Hz on head and 1000 Hz on abdomen (50% duty cycle) | Both | PBM inhibited oxidative stress and inflammation in the Aβ 25–35 mice model | [13] |
| Male Sprague Dawley rats injected with Aβ 1–42 | Laser at 808 nm | 15 J cm−2 reaching cerebral cortex tissue, 5 J cm−2 at hippocampus | CW | Yes | PBM treatment reduced Aβ-induced hippocampal neurodegeneration and recognition memory impairments. | [90] |
| K3&APP/PS1 transgenic mouse model | LED (670 nm) | 4 J cm−2 each time, a total of 80 J cm−2 | CW | No | PBM reduced oxidative stress and mitochondrial dysfunction in susceptible regions of the brain. | [89] |
| APP/PS1 transgenic mouse model | GaAlAs diode laser emitting (808 ± 10 nm) | 1.2–12 J cm−2 | CW and PW at 100 Hz | Yes | PBM can effectively reduce the Aβ plaques burden in the brain of the mice. | [91] |
| Mongrel male mice injected with Aβ 1–42 | Laser (1267 nm) | 18 J cm−2, 25 J cm−2, 32 J cm−2 and 39 J cm−2 | CW | Yes | PBM reduced the Aβ plaques accumulation in the brain. | [87] |
Several mechanisms were shown to be involved in PBM-induced reduction of the burden of Aβ plaques on the CNS. For example, Zhang et al [12] used He–Ne laser to irradiate amyloid precursor protein (APP)/PS1 transgenic mice and found an activation of the PKA/SIRT1 signal. This pathway causes a change in the expression of APP to nonamyloid protein. Consequently, levels of Aβ plaques in the hippocampus and cortical areas of the irradiated mice were reduced by half compared with those in the nonirradiated mice. Additionally, Lu et al [90] reported neuroinflammation, oxidative stress and so on in mice that were injected Aβ peptide without light exposure to determine whether PBM can inhibit Aβ-induced neurotoxicity in mice. However, mice treated with PBM (wavelength of 808 nm, dose reach the cerebral cortex of 15 J cm−2) could suppress AB-induced neurodegeneration in the hippocampus and recognition memory impairment. In addition, Blivet et al [13] and Purushothuman et al [89] reported the use of LED light sources (wavelengths of 850, 625 and 670 nm) to irradiate animals, which also resulted in a reduction in plaque size in the cortex and hippocampus and inhibited the Aβ-induced damage including oxidative stress and neuroinflammation to the mouse nervous system. No significant difference was observed between PBM performed with coherent or non-coherent light sources.
A few ways exists to evaluate the condition and treatment effect of AD patients in clinics, including MMSE score, magnetic resonance imaging and computed tomography [92]. Figure 2 shows that the structure of the treatment device in these clinic trials is similar to a helmet, making it stable and fixed on the patient's head. In addition, Saltmarche et al [93] mentioned a combination of transnasal (figure 2(A)) and transcranial (figure 2(B)) light treatments, which may achieve a higher dose of light reaching the cerebral cortex in comparison with just wearing a helmet-type light source on the head. Thus, they set the transcranial light power density (41 mW cm−2) almost twice as large as the intranasal single diode (23 mW cm−2). Moreover, recent studies have reported the depth at which NIR light (808 nm) can penetrate the skull of a human corpse to 40 mm, although with substantial attenuation (∼5 orders of magnitude) [94] . These experiments suggest that PBM may improve the patient's mood, sleep, and blood flow in the brain [92, 93, 95, 96].
Figure 2. The PBM irradiation modes in clinic. (A) Transcranial light. (B) Transnasal light.
Download figure:
Standard image High-resolution imageAs shown in tables 2 and 3, the power range of direct irradiation to the mouse skull in the PBM treatment animals model is below 80 J cm−2 [12, 13, 87, 89–91], while it can reach several hundred joules in clinical trials [92, 93, 95]. The light doses used in clinical studies were significantly higher compared with animal studies. Red or NIR light (630–810 nm) has been reported to have average penetration rates of 60%–70% and 0.2%–10% for C57BL/6 mouse and human skulls [88], respectively. The human skull and scalp are much thicker compared with the mice, which leads to a much stronger light attenuation.
Table 3. Reports on PBM applications for AD in clinic.
| Object | Wavelength and source | Dose | Mode | Coherence | Effect | Reference |
|---|---|---|---|---|---|---|
| Five patients with mild to moderately severe cognitive impairment | LED (810 nm) | The dose is 639 J cm−2 for the weeks 1–2 and 375 J cm−2 per week for the weeks 3–12. | PW at 10 Hz | No | Increased function, better sleep, fewer angry outbursts, less anxiety, and wandering were reported post-PBM | [93] |
| 81 patients aged from 34 to 79 | Visible light | Output power: 20 mW; irradiation time: 20–40 min (400–800 J). | CW or PW or combined modes | Yes | The patients developed less dementia | [92] |
| 81 patients aged from 34 to 79 | Visible light | Output power: 20 mW; irradiation time: 20–40 min (400–800 J). | CW or PW or combined modes | Yes | Improved the brain blood circulation and the metabolism of the nerve centre | [95] |
| 11 patients aged from 40 to 85 | 1100 LEDs set in 15 arrays of 70 LEDs/array (1060–1080 nm) | The patients were treated for 6 min every day for 28 consecutive days, but the exact dose is unknown. | PW at 10 Hz and 50% duty cycle | No | A trend of improvement in executive functioning, clock drawing, immediate recall, praxis memory, visual attention and task switching | [96] |
3.3. Wavelengths of light used in PBM for AD treatment
The light of different wavelengths can stimulate cells through different pathways. For example, while red/NIR light in the ∼600–900 nm range is absorbed by mitochondria, which promotes ATP production and leads to changes in the levels of ROS, NO, cytokines, and proinflammatory factors [97], green light is found to regulate the ion selectivity of the cell membrane [23]. Thus, involving different cell regulation mechanisms by tuning irradiation wavelengths is possible. Moreover, choosing an appropriate wavelength for PBM of AD as its pathogenesis is ultimately important. Furthermore, the CNS environment is very complex and any impact on all cell types involved in the AD pathogenesis should be considered.
Tables 1 and 2 show the use of red and NIR light with wavelengths of 632.8, 670, 800, 808, 810, 850, 1,068 and 1,267 nm is reported for both in vitro and in vivo studies. He–Ne laser with a wavelength of 632.8 nm is inexpensive, commonly available and easy to use, making it a preferable light source in many studies. Overall, the employed wavelengths are mainly within the range of light absorbed by CCO in the mitochondria (i.e. ∼600–1000 nm) [98], which can effectively enhance the activity of nerve cells [99], the phagocytic function and anti-inflammatory properties of glial cells [100]. Cells irradiated by the light of these wavelengths can reduce the adverse effects of Aβ on nerve cells [51, 80–83, 85, 86] and regulate the inflammatory responses and the ability of glial cells to swallow Aβ plaques [60, 73, 75].
For the in vivo studies of PBM, the ability of light to penetrate animal tissues is necessary. If the light is exhausted before reaching the cerebral cortex, the cells cannot respond. Jagdeo et al [101] tested the penetration rate of 633 and 830 nm light through the skull. The results showed that an 830 nm infrared light had better penetration ability than that of 633 nm red light, However, it does not necessarily mean that light with a longer wavelength is more penetrating than light with a shorter wavelength [102]. Wang et al [103] investigated the relationship between the penetrating power of biological tissues using two combined beams of light with different wavelengths. The penetration through brain tissues was be found to be better for the combination of 680 and 810 nm than for the combination of 980 and 1064 nm. A choice of wavelength for PBM in vivo is determined by not only by cellular responses, but also by the ability to penetrate tissues (e.g. scalp and skull) with high efficiency.
3.4. Light doses used for PBM in AD treatment
Table 1 shows that the light doses used in PBM cell studies are mainly in the range of 0.3–20 J cm−2.The light within this dose range can causes various cell responses, while stronger light can inhibit cell activities and functions. Meng et al [51] used the He–Ne laser irradiation of 0.5, 1, 2, and 4 J cm−2 to treat the SH-SY5Y neuroblastoma cells or primary rat hippocampal neurons co-cultured with Aβ peptide. The results showed that the BDNF level was upregulated along with the increase in length, branching, and spine density of dendrites in hippocampal neurons. The light treatment with a dose of 2 J cm−2 was found to be the most effective, inhibiting the neurotoxicity of Aβ to nerve cells. Moreover, only irradiation with a dose of 2 J cm−2 produced a noticeable effect on the BDNF expression and the dendrites growth in nerve cells. Neither too low nor too high dose could effectively eliminate the effects mediated by Aβ. The obtained results are therefore in line with the PBM biphasic dose response [78].
The studies in most cases show that the effective light doses in PBM on animal models are higher compared with PBM on cells. This is understandable because the light is partly attenuated by the hair, scalp and skull in the animal experiments. The light exponentially attenuates in the brain cortex, with a dose becoming negligible at some depth [104]. However, if the irradiation dose is too high, light can cause local temperature rise, resulting in biological tissues damage in most cases [87]. PBM studies conducted in a genetically modified mouse model showed that although the light with a dose of 39 J cm−2 was most effective in reducing Aβ plaque deposition, it leads to an increase in the temperature of the scalp by 2 °C. Moreover, light with doses of 18 and 25 J cm−2 could not affect the Aβ plaques deposition. Hence, the dose of 32 J cm−2 was found to be the most appropriate for PBM [87]. In summary, the therapeutic effect of PBM in animal experiments has to be considered along with the negative effects of intense light and light-generated temperature rise,
3.5. Light modes used for PBM in AD treatment
As shown in tables 1 and 2, most PBM studies utilize CW light sources. No definite conclusion exists on the difference between the PBM mechanism induced by PW and CW light However, studies show that the PW mode is preferable to CW one in terms of lower heat generation and higher cell stimulation response [105]. This is evidently associated with that PW mode has a no light time for cells or animals. Consequently, heat at the irradiated location is not continuously produced and photothermal damage to cells and tissues is reduced. Moreover, the peak power (PP) of PW light is higher compared with CW light, meaning that PW light can penetrate deeper into biological tissues. The calculation of pulse PP involves the output frequency of the light source and the duration of each light on cycle. The calculation of the duty cycle is from the equation [24]:

where DR is the duty ratio (i.e. the ratio of times when the light is on and off), F is the output frequency of the light source, and DT is the duty time when the light source is on in each output cycle. PP can be expressed by the following equation:

where PP is the peak power and AP is the average power.
Typically, the DR of PW is relatively small, so the PP is significantly higher than AP. Thus, PW light penetrates deeper than CW light, assuming that the attenuation of biological tissue remains unaffected [106].
Finally, studies have shown that PW can regulate some biological activities that CW cannot. El Sayed et al found that PW at 20 Hz can increase the amount of degranulation of mast cells [105]. Moreover, PW can give cells time to rest during light, which may enhance the cell metabolism and responses [107].
3.6. Light sources used in PBM for AD treatment
Lasers and LEDs are the main light sources employed in the PBM treatment of AD. Tables 1 and 2 summarize the reports showing that 11 of all 16 reviewed studies used coherent light sources (i.e. lasers), and the rest used LEDs or a combination of LEDs and lasers. The effect of laser and LED light sources on cell biology appears to be very close [88]. However, the laser light can penetrate deeper into biological tissues because of better directionality [108]. Henderson et al found that the LED light of 0.05 W and 810 nm and LED light of 0.2 W and 650/880 nm could not penetrate the human skin within 1.9 mm, while ∼11.5% photons of the laser (15 W, 810 or 980 nm) could penetrate through the skin [106]. However, the spectral width of laser emission is narrower compared with LED. In addition, LEDs are significantly less expensive. Moreover, the area illuminated by the LED device is much larger compared with the laser. A larger area can be irradiated by a single LED source. In conclusion, laser and LED light both have their own advantages. The above characteristics can be considered when choosing the light source in the actual situation.
4. Outlooks
No major breakthroughs are currently demonstrated though significant costs have been invested in developing drugs to treat AD [109]. Moreover, a large number of in vitro and in vivo AD model studies, along with several clinical trials, revealed that PBM is of great potential for AD treatment. It is also noninvasive and has no side effects. The dose–response relationship directly determines the therapeutic effect in the PBM application in the treatment of AD. The determination of reasonable PBM parameters for AD treatment is a key step to improve its efficiency. A large number of PBM reports have been published, including studies of the PBM mechanisms and methods to evaluate the treatment effect, which is beneficial for the future clinical trials. However, PBM still has a long way to go before it would be an effective way to treat AD. The PBM parameters, in particular, the dose–effect relationship for PMB in AD treatment, are still yet to be explored and optimized. PBM provides a new and promising alternative for AD therapy in clinics with the advantages of high safety, low cost, and easy implementation in clinical trials.
Acknowledgments
This work has been partially supported by the National Natural Science Foundation of China (61835009/61620106016), Shenzhen Basic Research Project (JCYJ20170412105003520) and Shenzhen International Cooperation Project (GJHZ20180928161811821, GJHZ20190822095420249).
Data availability statement
All data that support the findings of this study are included within the article (and any supplementary content files).