Abstract
Epidemiological studies have shown an inverse association between high-density lipoprotein cholesterol (HDL-C) levels and risk of ischemic heart disease. In addition, a low level of HDL-C has been shown to be a risk factor for other diseases not related to atherosclerosis. However, recent studies have not supported a causal effect of HDL-C in the development of atherosclerosis. Furthermore, new drugs markedly elevating HDL-C levels have been disappointing with respect to clinical endpoints. Earlier, most studies have focused almost exclusively on the total HDL-C without regard to the chemical composition or multiple subclasses of HDL particles. Recently, there have been efforts to dissect the HDL fraction into as many well-defined subfractions and individual molecules of HDL particles as possible. On the other hand, the focus is shifting from the structure and composition to the function of HDL particles. Biomarkers and mechanisms that could potentially explain the beneficial characteristics of HDL particles unrelated to their cholesterol content have been sought with sophisticated methods such as proteomics, lipidomics, metabonomics, and function studies including efflux capacity. These new approaches have been used in order to resolve the complex effects of diseases, conditions, environmental factors, and genes in relation to the protective role of HDL but high-throughput methods are still needed for large-scale epidemiological studies.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
- High-density lipoproteins
- Cholesterol
- Atherosclerosis
- Coronary heart disease
- Apolipoproteins
- Cholesterol efflux
- Diabetes
- Obesity
- Cancer
- Proteomics
- Lipidomics
- Metabonomics
Low levels of high-density lipoprotein (HDL) particles in the plasma of patients with coronary heart disease (CHD) were observed already in the early 1950s (Barr et al. 1951; Nikkilä 1953). At that time the lipoprotein fraction was called alpha lipoprotein as electrophoresis methods were used. Twenty years later the same relationship was confirmed in large epidemiological studies using ultracentrifugation or precipitation methods. The new interest for HDL research was then stimulated by the reverse cholesterol transport hypothesis (Glomset et al. 1966) and a Lancet review written by Miller and Miller (1975). These authors proposed that low plasma HDL concentration accelerates the development of atherosclerosis by impairing the clearance of cholesterol from the arterial wall.
1 Protective Role of HDL: Evidence from Epidemiological Studies
The protective role of HDL has been well established in several epidemiological studies. An increment of 2.5 % corresponding to 1 mg/dl or 0.04 mmol/l is associated with a 2 and 3 % reduction in CHD risk in men and women, respectively (Gordon et al. 1989; Jacobs et al. 1990). So far, it is mainly the total cholesterol concentration in HDL particles that has been determined using standardized methods applicable also to routine clinical work. However, cholesterol represents only a small fraction, approximately 15 %, of the HDL particle mass. Furthermore, the proportion of free cholesterol to esterified cholesterol varies between lipoprotein particles.
The relationship between HDL-C and CHD is complex and HDL-C may not be an appropriate indicator of the impact of this lipoprotein fraction on cardiovascular risk. This has been demonstrated, e.g., by the fact that carotid intima media thickness is not increased in apoA-I(Milano) mutation carriers with very low levels of HDL-C (Sirtori et al. 2001). Moreover, recently, drugs that increase HDL cholesterol (HDL-C) level have failed to reduce cardiovascular risk, and Mendelian randomization studies have failed to show a causal relationship between HDL-C and cardiovascular diseases (van Capelleveen et al. 2013).
Despite this controversy, the hard evidence for low HDL-C level as a risk factor of atherosclerosis will be described in the first part of this presentation. It is noteworthy that the cholesterol concentration in HDL fraction is not necessarily associated with the antiatherogenic properties of HDL. Therefore, later in this chapter, other potential HDL-related biomarkers for the prevention of atherosclerosis by HDL will be presented. These include not only the other major lipid and apolipoprotein components of HDL but also minor bioactive lipid molecules residing in the HDL particles. Furthermore, the physicochemical characteristics of various subfractions of HDL as well as the large number of molecules circulating more or less firmly bound to HDL particles may contribute to the antiatherogenic potential of HDL via antioxidative and anti-inflammatory effects or cholesterol transport capacity.
2 HDL Cholesterol as a Risk Factor for Atherosclerosis and Its Complications
Until recently, the clinical evaluation of HDL as a risk factor has focused almost exclusively on the total HDL-C without regard to the chemical composition or multiple subclasses of HDL particles. A large body of epidemiological research has shown a solid inverse and independent relationship between HDL-C and the risk of cardiovascular disease (Toth et al. 2013).
Gofman et al. first reported an inverse association between HDL-C levels and the risk of ischemic heart disease (Gofman et al. 1966). This was shown in case–control studies from Framingham and Livermore cohorts with 10–12 years of follow-up. Later, the inverse relationship has been observed also in several larger studies in the USA (the Honolulu Heart Program, Rhoads et al. 1976, and the Framingham Heart Study, Gordon et al. 1977), in Norway (the Tromsø Heart Study, Miller et al. 1977), in Germany (the Prospective Cardiovascular Münster Study, Assmann et al. 1996), and in Israel (the Israeli Ischemic Heart Disease Study, Goldbourt et al. 1997). Recent meta-analyses have corroborated the relationship between HDL-C and atherosclerosis and its complications (Chirovsky et al. 2009; Boekholdt et al. 2013; Touboul et al. 2014). The association is independent of triglyceride levels and other risk factors (Goldbourt et al. 1997). Many CHD risk algorithms have included HDL-C as a factor to improve the prediction of CHD events (Cooper et al. 2005; Halcox et al. 2013; Hippisley-Cox et al. 2013; Tehrani et al. 2013).
Recently, the picture has become less clear. Mendelian randomization studies have not supported a causal effect of HDL-C in the atherosclerotic disease process (Voight et al. 2012; Holmes et al. 2014). Moreover, statin trials have shown that HDL-C is predictive among patients treated with statin even at low LDL levels (Barter et al. 2007), whereas it is not predictive among patients taking placebo (Ridker et al. 2010; Mora et al. 2012). Further research is needed to clarify the role of HDL-C as a risk factor. It is possible that other characteristics of HDL particles may be more important in this respect than the total cholesterol concentration.
In patients with CHD, the protective role of HDL-C is controversial (Silbernagel et al. 2013). Some studies have shown that low HDL-C is associated with atherosclerotic progression in myocardial infarction survivors (Johansson et al. 1991; Duffy et al. 2012; Liosis et al. 2013), provides additional prognostic value also in patients with acute coronary syndrome (Correia et al. 2009), and reduces the risk after coronary interventions (Sattler et al. 2009), whereas some studies have shown that HDL-C has no protective role in the secondary prevention of CHD after bypass operation (Angeloni et al. 2013).
3 HDL Cholesterol as a Risk Factor for Other Diseases
The low level of HDL-C has been shown to be a risk factor for other diseases not related to atherosclerosis. Recent reports have shown that HDL-C may be a biomarker for diseases like psoriasis (Holzer et al. 2012), rheumatoid arthritis (Raterman et al. 2013), and liver fibrosis in hepatitis C patients (Gangadharan et al. 2012). Alterations in HDL composition have been observed also in hemodialysis patients (Mangé et al. 2012).
Special interest has been recently focused on HDL in cancer patients. Several epidemiological studies have shown that low HDL-C level may be a risk and/or prognostic factor of biliary tract cancers (Andreotti et al. 2008), prostate cancer (Mondul et al. 2011; Kotani et al. 2013), colon cancer (van Duijnhoven et al. 2011), breast cancer (Furberg et al. 2005, Melvin et al. 2012) and gastric cancer (Tamura et al. 2012), but not that of endometrial cancer (Cust et al. 2007; Esposito et al. 2014) or rectal cancer (van Duijnhoven et al. 2011). Confounding factors related to obesity and metabolic syndrome may be more strongly associated with the latter cancer types (Kotani et al. 2013).
The association with low HDL-C levels is shared among many types of cancer, and it is mainly linked to obesity and inflammation, suggesting a common pathway (Melvin et al. 2013; Vílchez et al. 2014). The mechanism of cancer protection is not known. Lipoprotein particles may carry cancerogenic molecules but at least one small study has shown that persistent organogenic pollutants in HDL particles are more associated with CVD than cancer (Ljunggren et al. 2014).
The potential importance of HDL particles in cancer protection has recently led to attempts to develop cancer treatments by HDL-mimetic synthetic nanoparticles (Zheng et al. 2013; Yang et al. 2013). These interesting approaches need to be followed closely in the future.
4 Total HDL-C in Various Populations
Racial differences. Several studies have shown differences in HDL-C between various ethnic groups (Thelle et al. 1982; Heiss et al. 1984; Haffner et al. 1986; Saha 1987). The differences may in part be due to genetic factors but the role of behavioral and anthropometric variables seem to affect the HDL-C (Thelle et al. 1982; Haffner et al. 1986). Environmental factors such as diet, smoking, and alcohol use may confound the differences observed between ethnic groups.
In the USA, the African-American population has higher HDL-C than Caucasians after adjustment for weight, smoking, and alcohol consumption than Caucasians (Heiss et al. 1984), while people originating from India have lower HDL-C than Europeans or many other populations (Saha 1987; de Munter et al. 2011). Differences in HDL-C and other lipoproteins between various ethnic groups can be observed already in children (van Vliet et al. 2011). Even at an early age, the HDL-C levels are confounded by other cardiovascular risk factors including blood pressure, overweight, and obesity.
Gender differences. Women have higher HDL-C levels than men (Heiss et al. 1980; Seidell et al. 1991), and significantly increased CHD risk is defined at levels below 50 mg/dl (1.29 mmol/l) and 40 mg/dl (1.03 mmol/l), respectively. The reason for the gender difference may mainly be sex hormones but the fat distribution seems to play a central role since adjustment for waist/thigh ratio almost totally removed the gender difference in HDL cholesterol (Seidell et al. 1991). In women HDL-C levels decline after menopause (Heiss et al. 1980) but the sex difference remains significant even in the seventh decade of life (Ostlund et al. 1990).
Age-related differences. Lipoprotein levels are very low at birth and then increase during childhood. HDL-C levels decrease in males during puberty and early adulthood and thereafter remain lower than those in women (Kreisberg and Kasim 1987; Walter 2009). HDL-C levels decline with age and low HDL cholesterol remains a powerful risk predictor into old age (Kreisberg and Kasim 1987; Walter 2009). A selection bias by HDL-lowering genetic variation may explain why HDL deficiency is rare among very old people (Kervinen et al. 1994; Baggio et al. 1998).
5 Total HDL-C Modulated by Environmental Factors
Alcohol consumption in moderation is associated with a reduced risk for cardiovascular diseases. Alcohol consumption increases the concentration of HDL-C, possibly secondary to an inhibition of CETP (cholesteryl ester transfer protein) (Savolainen et al. 1990; Hannuksela et al. 1992). A common polymorphism in the CETP gene (TaqIB2) modifies the relationship of alcohol intake with HDL-C suggesting a gene-environment interaction on the risk of CHD (Jensen et al. 2008). However, it has not been definitely shown whether the HDL-C elevation associated with alcohol consumption is cardioprotective.
The quantity of alcohol needed to increase HDL-C by 0.1 mmol/l (3.87 mg/dl) is about 30 g/d (Brien et al. 2011). Therefore, alcohol drinking cannot be recommended as a method to raise low HDL-C levels. The alcohol-induced increase in HDL-C occurs commonly without significant changes in other lipids although alcohol may increase triglyceride levels especially in subjects with elevated triglyceride concentration.
The death rate from CHD among moderate alcohol users is lower than in total abstainers or heavy drinkers (Rimm et al. 1999; Brien et al. 2011; Bergmann et al. 2013). Even very low alcohol consumption, e.g., a couple of drinks per week, seems to protect from CHD even though it does not have any significant effect on HDL-C. To explain this fact, it has been suggested that ethanol metabolism may produce specific bioactive lipids, e.g., phosphatidylethanol, that could serve as a memory molecule in the body (Liisanantti et al. 2004). If HDL particles of alcohol drinkers contain this bioactive lipid, it could circulate for several days even without daily alcohol drinking, and when HDL enters into the endothelial cell, it could then exert its positive effects on the vascular endothelium (Liisanantti and Savolainen 2005). However, the concentration of phosphatidylethanol in HDL may be in the low nanomolar range which makes the analysis challenging and may hamper its determination of epidemiological studies.
Smoking reduces HDL-C level and smoking cessation is associated with an increase in the plasma concentration of HDL-C (Maeda et al. 2003). The mechanism by which smoking reduces HDL-C is not known. It is noteworthy that in many cases smoking is associated with alcohol drinking and therefore smoking could attenuate the alcohol-induced increase in HDL-C.
Physical activity is associated with high HDL-C (Marti 1991). Low level of physical activity is very common in the developed countries, and therefore, increasing the level of exercise might be more beneficial for HDL-C than any other preventive measure.
Education and socioeconomic status. HDL-C levels increase with income and educational attainment after controlling diet, exercise, and other risk factors for elevated cholesterol (Muennig et al. 2007). The mechanisms are not clear. It has been suggested that stress differences by social class may play a role (Muennig et al. 2007). However, several lifestyle factors including leisure-time physical activity, smoking, alcohol drinking, and dietary habits correlate with the socioeconomic status, classified as the degree of educational level (Schröder et al. 2004).
Dietary carbohydrates affect the lipoprotein profile. The epidemiological studies can be divided into three categories. First, the type of carbohydrate modulates the impact of carbohydrates on plasma lipoproteins. The intake of refined carbohydrates has increased in Western societies, and they have more deleterious effects on abdominal obesity and consequently on insulin resistance and hepatic lipogenesis in comparison with complex carbohydrates or starches (Ma et al. 2012; Stanhope et al. 2009). The end result of high intake of refined carbohydrates is a low HDL-C level (Heiss et al. 1980; Sonestedt et al. 2012).
Second, trials focusing on dietary carbohydrate restriction have shown modulation of atherogenic dyslipidemia. The effect on HDL-C is modest but commonly greater than that on total cholesterol, and thus, the atherogenic burden is improved. Results from epidemiological studies are difficult to interpret due to differences in diets.
The third type of studies involves the replacement of carbohydrate with different fats in order to maintain isocaloric intake of macronutrients. A classic meta-analysis of 60 trials (Mensink et al. 2003) showed that replacement of 10 % of energy from carbohydrate with saturated fat, monounsaturated fat, and polyunsaturated fat increased HDL-C by 4.7, 3.4, and 2.8 mg/dl (0.12, 0.09, and 0.07 mmol/l), respectively. However, LDL cholesterol increased by 13 mg/dl (0.34 mmol/l) with saturated fat substitution and decreased by 3.3 mg/dl (0.09 mmol/l) with polyunsaturated fat substitution while the substitution with monounsaturated fat had no effect on LDL-C. Thus, the atherogenicity of the plasma lipoprotein profile improved with the unsaturated fat substitution of carbohydrate.
The effects of dietary carbohydrates on HDL-C and CHD risk have been analyzed also on the basis of their glycemic index (GI, the effect on blood glucose level) or glycemic load (GL, including carbohydrate content and intake of foods in addition to GI). High GL and GI were associated with significant increased risk of CVDs, specifically for women. Several cross-sectional studies have reported inverse associations of low GI and GL diets with HDL-C, but meta-analyses (Kelly et al. 2004; Goff et al. 2013) have not found any effect on HDL-C.
Fatty acids and especially omega-3 fatty acids have been the focus of many epidemiological studies. The most important omega-3 fatty acids in this respect are eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) that increase HDL-C by 1–3 % (Balk et al. 2006).
6 HDL-C in Diseases and Conditions
Type 2 diabetes. Patients with type 2 diabetes may have various types of dyslipidemias that usually are accompanied with a low concentration of HDL-C (Chehade et al. 2013; Morgantini et al. 2014). Low HDL-C contributes to diabetes-related CHD risk more in women than in men, the diabetes-related hazard ratio for a major CHD event being 3 times higher in women after adjustment for other cardiovascular risk factors (Juutilainen et al. 2004). On the other hand, lipid composition of HDL particles is associated with the development of metabolic syndrome (Abbasi et al. 2013; Onat et al. 2013).
Dyslipidemias. Traditionally, dyslipidemia has been characterized by an elevation in plasma triglycerides and total or LDL cholesterol and reduction in HDL-C. This is commonly referred to as the atherogenic triad. LDL particles are small and dense (Mooradian 2009). Genetic studies have indicated linkage of apoB gene to peak LDL size and plasma triglycerides, HDL-C, and apoB levels.
Obesity. Overweight and obese subjects usually have low HDL-C concentrations (Seidell et al. 1991). The underlying cause may be insulin resistance that promotes free fatty acid flux to the liver, stimulates hepatic lipogenesis, and finally enhances the secretion of triglyceride-rich apoB-containing lipoproteins from the liver. The excess triglyceride-rich particles also enhance the CETP-mediated exchange of cholesteryl esters from HDL to apoB-containing lipoprotein particles and simultaneous transfer of triglycerides into HDL (Mann et al. 1991; Liinamaa et al. 1997). This may further reduce HDL-C levels.
Weight reduction is an important modulator of the lipoprotein profile and long-term weight reduction increases HDL-C levels especially in subjects with type 2 diabetes. It is noteworthy that the effect of weight reduction on HDL-C is different in the initial weight loss period compared with the weight maintenance phase. A meta-analysis has shown that HDL-C is increased by 0.35 mg/dl (0.009 mmol/l) per kilogram weight lost during the stable weight reduction. During active weight loss, however, HDL-C is reduced by 0.27 mg/dl (0.007 mmol/l) for every kilogram of weight lost (Browning et al. 2011). Theoretically, an increase in HDL-C of 2.45 mg/dl (0.06 mmol/l) could be expected during the stable weight maintenance stage after a weight reduction of 7 % in an individual with an initial weight of 100 kg. Furthermore, this would translate into 7.4 % reduction in CHD risk in women.
7 High HDL Levels Do Not Add to the Protection
While low levels of HDL-C are associated with increased CHD risk, high HDL-C levels are not uniformly atheroprotective. At higher concentrations the curve of CHD risk gradually tapers off as depicted in Fig. 1 which is based on the results from the Framingham Heart Study (Gordon et al. 1977), and there is no additional effect of higher HDL-C levels compared with average HDL-C concentrations.
Potential differences in the functions of HDL particles. HDLaa depicts a theoretical subclass of particles comprising the bulk of plasma HDL-C concentration but without any significant role in reverse cholesterol transport or pleiotropic effects of HDL, whereas HDLbb may be a smaller fraction but more active in reverse cholesterol transport. HDLcc could be a small subclass without any significant contribution to the HDL-C quantity. These particles may, however, have many pleiotropic effects on cytokines, growth factors, etc. [Modified from Hannuksela et al. (2004)]
8 Effect of HDL on Stroke
In contrast to the role of HDL-C as a major risk factor of CHD, the role of HDL-C in the pathogenesis of ischemic stroke is less clear. Epidemiological studies of carotid intima media thickness and stroke protection have provided conflicting results. Even in the studies reporting positive results, the effect of HDL-C on protection of stroke is modest (a 10 mg/dl or 0.38 mmol/l increase in HDL-C reduces stroke risk by 11–15 %) compared with its protective effect on CHD. The discordant results in prospective cohort studies as well as in case-control studies may be due to the heterogeneity of stroke, since dyslipidemia including low HDL-C levels may not be involved in the pathogenesis of some subtypes such as lacunar and cardioembolic strokes (Amarenco et al. 2008).
9 Time Trends in Total HDL-C
During the last decades, favorable trends in HDL-C levels have occurred in US adults and youths aged 6–19 years despite changes in physical activity, obesity, and diabetes (Carroll et al. 2012; Kit et al. 2012). Similar trends have been observed also in Europe and India (Muntoni et al. 2009; Gupta et al. 2012).
10 Are There Other Biomarkers than the Total HDL-C?
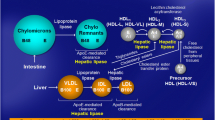
The cholesterol in HDL (i.e., HDL-C) may reflect the rate of reverse cholesterol transport from the peripheral tissues to liver. However, it may be that the bulk of HDL cholesterol does not necessarily represent the particles that are involved in the reverse cholesterol transport or any other potentially antiatherogenic activity of HDL (Fig. 1).
HDL particles comprise a heterogeneous fraction of lipoprotein which vary by the apolipoprotein and lipid content and consequently also in density, shape, size, and charge. Various subfractions can be analyzed, e.g., by ultracentrifugation (density), electron microscopy (shape), nondenaturing gel electrophoresis, gel filtration or nuclear magnetic resonance (NMR) spectroscopy (size), NMR or immunoaffinity (composition), and 2-dimensional electrophoresis (charge).
11 HDL Fractions
Analytic ultracentrifugation was first used to separate subclasses of HDL (DeLalla et al. 1954). Later, the sequential ultracentrifugation and various precipitation methods enabled the analysis of larger series in epidemiological studies.
Sequential ultracentrifugation methods have enabled separation of two major HDL subfractions, namely, HDL2 and HDL3, while the isolation of further subclasses has required more tedious methods such as gradient ultracentrifugation. Nuclear magnetic resonance (NMR) spectroscopy has simplified the differentiation into five subclasses. However, the nomenclature has varied and it is generally quite difficult to compare HDL subclasses separated by different methods. Recently, a unified nomenclature for a simplified differentiation into 5 subclasses according to particle size (very small, small, medium, large, and very large HDL) has been proposed (Rosenson et al. 2011).
Controversial results have been obtained in studies aimed at distinguishing cardiovascular differences between HDL subclasses separated by density (Superko et al. 2012; Pirillo et al. 2013). A majority of the studies, however, have found HDL2-C to be more predictive of CHD risk than total HDL-C or HDL3-C (Johansson et al. 1991; Drexel et al. 1992; Lamarche et al. 1997). A recent study with 29-year follow-up of the Gofman’s Livermore cohort showed that HDL2 and HDL3 are independently related to CHD risk (Williams and Feldman 2011).
12 HDL Particle Size
Further characterization of HDL subfractions by nondenaturing polyacrylamide gels has identified three HDL3 and two HDL2 subclasses. Very large HDL particles (HDL2b subfraction with diameter between 9.7 and 12.9 nm) are strongly correlated with the total HDL-C concentration and most strongly inversely related to CHD risk in normotriglyceridemic subjects (Johansson et al. 1991). On the other hand, an increased concentration small HDL particles (HDL3b subfraction, 7.8–8.2 nm) is associated with an atherogenic lipoprotein profile characterized by low HDL2b levels, high plasma triglyceride concentration, and increased level of small, dense LDL particles (Berneis and Krauss 2002). Low concentration of HDL2b subclass has also been shown in patients with T2DM (Xian et al. 2009).
13 HDL Particle Number
The complexity of HDL metabolism leads to the formation of multiple HDL subpopulations with varying density, size, charge, and chemical composition. Two individuals with the same HDL-C concentration may have HDL particles of different size distribution, and consequently, the particle number is different. Recently, NMR methods have enabled high-throughput determination of HDL particle concentration. Plasma levels of large particles and low particle number are consistently associated with low CHD prevalence (Mora et al. 2012).
14 HDL Lipids
Some of the effects of HDL on cellular functions are mediated by sphingomyelin-1-phosphate (S1P) that is a bioactive lipid in HDL. Alterations in S1P content in HDL particles could explain the dysfunction of HDL since the HDL-associated S1P seems to be responsible for many of the pleiotropic effects of HDL by activating special S1P receptors. In epidemiological studies low levels of S1P in HDL has been associated with coronary heart disease (Argraves et al. 2011). Sphingomyelin content in HDL particles has been associated with coronary heart disease in postmenopausal women (Horter et al. 2002). Sphingomyelin has also been associated with kidney disease in patients with type 1 diabetes (Mäkinen et al. 2012).
15 HDL Apolipoproteins
The structure of HDL particles is very complex, apoA-I and apoA-II being the major protein components. ApoA-I accounts for about two-thirds of the protein content of HDL and is also functionally important since it is the acceptor in the efflux of phospholipids and free cholesterol from peripheral cells. The measurement of apolipoprotein levels is more expensive and time-consuming than that of lipid concentrations. To circumvent this, data from large epidemiological cohorts was recently used for the development of computer software that enables accurate estimation of apolipoprotein levels on the basis of lipid measurements (Raitakari et al. 2013).
High plasma levels of apoA-I are protective against atherosclerosis in some studies (Arsenault et al. 2011; Emerging Risk Factor Collaboration 2012). It has been proposed that apoA-I concentration could even be a better predictor of atherosclerosis development than HDL-C. A recent study reported that adjustment for apoA-I changes the direction of the association between HDL-C and the severity of atherosclerotic lesions as determined with coronary artery calcium score (Sung et al. 2013). Among patients treated with statin therapy, apoA-I levels are strongly associated with a reduced cardiovascular risk, even among those achieving very low LDL-C level (Boekholdt et al. 2013).
ApoA-II is the second major apolipoprotein in HDL particles. It is present in most but not all particles and the clinical significance of apoA-II-containing and apoA-II-free particles is controversial (Rosenson et al. 2011).
Some studies have reported enrichment with apoC-III in the HDL of patients with CHD (Vaisar et al. 2010; Kavo et al. 2012; Jensen et al. 2012). Interestingly, Jensen and coworkers found that HDL particles without apoC-III were inversely associated with the risk of CHD while apoC-III-containing HDL particles were directly associated with increased risk of CHD (Jensen et al. 2012). HDL with apoC-III comprised about 13 % of the total HDL-C.
16 HDL Proteomics
Several proteins circulate attached to HDL particles although most of them in much lower numbers than apolipoproteins mentioned above. The protein content of HDL particles has been characterized recently using various methods commonly described as proteomics. It has been shown that HDL2 fraction of CHD patients carries a distinct protein cargo (Vaisar et al. 2010; Gordon et al. 2010; Kavo et al. 2012; Alwaili et al. 2012).
Proteomics as a new promising approach for detecting biomarkers and mechanisms could potentially explain the beneficial characteristics of HDL particles unrelated to their cholesterol content. To this end, novel sophisticated methods have been recently developed (Gordon et al. 2010; Mazur et al. 2010; Burillo et al. 2013; Hoofnagle et al. 2012; Mazur and Cardasis 2013; Riwanto et al. 2013). High-throughput methods are urgently needed for large-scale epidemiological studies.
17 HDL Function
Cholesterol efflux is the first step of reverse cholesterol transport. Excess cellular cholesterol from peripheral tissues is effluxed to extracellular HDL-based acceptor particles through the action of active transporters and passive diffusion. Recently, it was reported that the cholesterol efflux measured from cultured macrophages enriched with free cholesterol to apoB-depleted serum as cholesterol acceptor was inversely associated with the history of CHD independent of HDL-C levels (Khera et al. 2011). However, more recently, enhanced efflux has been associated with high cardiovascular risk (Li et al. 2013). Further studies are urgently needed to resolve the issue with conflicting results.
Antioxidative properties of HDL are mainly attributed to apoA-I although paraoxonase 1 (PON1) may also play an important role. Antioxidative activity of HDL subfractions increases with increment in density as follows: HDL2b < HDL2a < HDL3a < HDL3b < HDL3c (Kontush et al. 2003). The determination of the antioxidative capacity of HDL particles is technically challenging, and therefore, indirect approaches measuring arylesterase or paraoxonase activities have been used in larger cohorts.
Molecules carried by HDL particles. Recent studies have shown that short noncoding RNAs (microRNAs, miRNAs) are present in the circulation as a result of cellular damage or secretion (Laterza et al. 2009). miRNAs are ideal biomarkers since they are stable and their sequences can be easily amplified. Alterations in circulating miRNA profiles have been associated with cardiovascular risk factors such as hypertension, diabetes, and dyslipidemias as well as with cardiovascular diseases such as coronary heart disease, myocardial infarction, and heart failure (Fichtlscherer et al. 2011; de Rosa et al. 2011). However, this association has not been found in all studies (Wagner et al. 2013).
Several proteins are also attached to HDL particles. Paraoxonase 1 (PON1) is an HDL-associated enzyme that has been suggested to mediate many antiatherogenic and cardioprotective effects of HDL particles (Mackness et al. 2004; Aviram and Vaya 2013).
18 Pleiotropy
In addition to the antioxidative properties HDL particles have also other functions independent of the effects of HDL particles on cholesterol homeostasis. These include anti-inflammatory, anti-infective, antithrombotic, and endotoxin-neutralizing effects as well as effects on endothelial function. These pleiotropic effects have been recently reviewed (Annema and von Eckardstein 2013). The laboratory assays used for analyzing the pleiotropic effects of HDL are demanding and need to be standardized before they can be used in large epidemiological studies in order to improve the CHD risk assessment.
19 Future Approaches of Epidemiological Studies
New approaches have been used in order to resolve the complex effects of diseases, conditions, environmental factors, and genes in relation to the protective role of HDL. On one hand, there have been efforts to dissect the HDL fraction into as many well-defined subfractions and individual molecules of HDL particles as possible. On the other hand, as discussed above, the focus has been shifted from the structure and composition to the function of HDL particles (Fig. 2). Moreover, recent development in the field of “omics” (e.g., metabonomics, lipidomics, proteomics, etc.) has enabled the analysis of more holistic patterns on lipoproteins and subfractions and their relation to the risk of CHD (Ala-Korpela 2008; Rosenson et al. 2011; Würtz et al. 2012).
Relation between HDL-C and risk of myocardial infarction. The solid curve shows the average risk level by HDL-C concentration. The risk of individuals, however, may vary substantially, and even at the same HDL-C level, the CHD risk could be anywhere between the dotted lines. Furthermore, the nearly horizontal arrow depicts how previous approaches have attempted to improve the atheroprotective capacity of HDL by increasing the quantity of HDL-C. Nowadays, several studies have suggested that it might be more important to change the quality of HDL particles as shown by the arrow down. Various biomarkers and function assays have been commonly used to determine the improved quality that ultimately could lead to lower risk of myocardial infarction
Dietary patterns have been used as a complementary approach to the traditional single-nutrient analysis (Randall et al. 1990; Bogl et al. 2013). Dietary patterns are, e.g., “fruit and vegetables,” “meat,” “sweets and desserts,” “junk food,” and “fish.” The “junk food” pattern is characterized by higher intakes of energy-dense nutrient-poor foods, such as hamburger, pizza, French fries, salty snacks, and liquorices and lower intakes of porridge, rye bread, and fruit. The “junk food” pattern distinguishes from the commonly used definition of “Western pattern” in that “junk food” does not contain high amounts of meat, eggs, or high-fat dairy. The healthy diet pattern has been associated with higher levels of HDL-C, whereas the fast-food dietary pattern, high in saturated fat, did not have this effect (Hamer and Mishra 2010).
The idea of analyzing the whole diet through dietary patterns is based on the fact that people do not eat single purified nutrients or simple foods, but mixed meals consisting of several foods and nutrients at a time. The term “nutritional epidemiology” depicting food patterns was coined already in the 1970s (Krehl 1977) but has only recently used more frequently.
The Mediterranean diet refers to a dietary profile commonly available in the early 1960s in the Mediterranean regions and characterized by a high consumption of fruit, vegetables, legumes, and complex carbohydrates, with a moderate consumption of fish, and the consumption of olive oil as the main source of fats and a low-to-moderate amount of red wine during meals (Sofi et al. 2010). A recent meta-analysis of 50 studies summarized the impact of the Mediterranean diet on CHD risk factors (Kastorini et al. 2011). The diet is associated with a 3 % increase in HDL-C, but many other lipid and non-lipid risk factors such as hypertriglyceridemia, blood pressure, glucose, insulin resistance, and abdominal obesity are also affected.
20 Conclusion
HDL-C is a strong and independent predictor of major cardiovascular events in a wide range of populations, in men and women with or without preceding CHD. As molecular biology and related approaches have revealed new biomarkers or profiles in HDL structure and function, the epidemiological research is also moving from the classical determination of total HDL-C to structure-function analyses. Recent methodological breakthroughs in HDL structure analyses have enabled their use also in large cohorts. In the future, further investigations are urgently needed for validation and clinical applications of HDL function assays.
Abbreviations
- ApoA-I:
-
Apolipoprotein A-I
- ApoA-II:
-
Apolipoprotein A-II
- ApoC-III:
-
Apolipoprotein C-III
- CHD:
-
Coronary heart disease
- CETP:
-
Cholesteryl ester transfer protein
- CVD:
-
Cardiovascular disease
- EPA:
-
Eicosapentaenoic acid
- DHA:
-
Docosahexaenoic acid
- GI:
-
Glycemic index
- GL:
-
Glycemic load
- HDL:
-
High-density lipoproteins
- HDL-C:
-
High-density lipoprotein cholesterol
- HDL2:
-
High-density lipoprotein fraction 2
- LDL:
-
Low-density lipoprotein
- LDL-C:
-
Low-density lipoprotein cholesterol
- NMR:
-
Nuclear magnetic resonance
- PON1:
-
Paraoxonase 1
- S1P:
-
Sphingosine-1-phosphate
- T2DM:
-
Type 2 diabetes mellitus
References
Abbasi A, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, Stolk RP, Navis G, Bakker SJ, Dullaart RP (2013) Role of HDL cholesterol and estimates of HDL particle composition in future development of type 2 diabetes in the general population: the PREVEND study. J Clin Endocrinol Metab 98:E1352–E1359. doi:10.1210/jc.2013-1680
Ala-Korpela M (2008) Critical evaluation of 1H NMR metabonomics of serum as a methodology for disease risk assessment and diagnostics. Clin Chem Lab Med 46:27–42
Alwaili K, Bailey D, Awan Z, Bailey SD, Ruel I, Hafiane A, Krimbou L, Laboissiere S, Genest J (2012) The HDL proteome in acute coronary syndromes shifts to an inflammatory profile. Biochim Biophys Acta 1821:405–415. doi:10.1016/j.bbalip.2011.07.013
Amarenco P, Labreuche J, Touboul PJ (2008) High-density lipoprotein-cholesterol and risk of stroke and carotid atherosclerosis: a systematic review. Atherosclerosis 196:489–496
Andreotti G, Chen J, Gao YT, Rashid A, Chang SC, Shen MC, Wang BS, Han TQ, Zhang BH, Danforth KN, Althuis MD, Hsing AW (2008) Serum lipid levels and the risk of biliary tract cancers and biliary stones: a population-based study in China. Int J Cancer 122:2322–2329
Angeloni E, Paneni F, Landmesser U, Benedetto U, Melina G, Lüscher TF, Volpe M, Sinatra R, Cosentino F (2013) Lack of protective role of HDL-C in patients with coronary artery disease undergoing elective coronary artery bypass grafting. Eur Heart J 34:3557–3562. doi:10.1093/eurheartj/eht163
Annema W, von Eckardstein A (2013) High-density lipoproteins. Multifunctional but vulnerable protections from atherosclerosis. Circ J 77:2432–2448
Argraves KM, Sethi AA, Gazzolo PJ, Wilkerson BA, Remaley AT, Tybjaerg-Hansen A, Nordestgaard BG, Yeatts SD, Nicholas KS, Barth JL, Argraves WS (2011) S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis 10:70. doi:10.1186/1476-511X-10-70
Arsenault BJ, Boekholdt SM, Kastelein JJ (2011) Lipid parameters for measuring risk of cardiovascular disease. Nat Rev Cardiol 8:197–206. doi:10.1038/nrcardio.2010.223
Assmann G, Schulte H, von Eckardstein A, Huang Y (1996) High-density lipoprotein cholesterol as a predictor of coronary heart disease risk. The PROCAM experience and pathophysiological implications for reverse cholesterol transport. Atherosclerosis 124(Suppl):S11–S20
Aviram M, Vaya J (2013) Paraoxonase 1 activities, regulation, and interactions with atherosclerotic lesion. Curr Opin Lipidol 24:339–344. doi:10.1097/MOL.0b013e32835ffcfd
Baggio G, Donazzan S, Monti D, Mari D, Martini S, Gabelli C, Dalla Vestra M, Previato L, Guido M, Pigozzo S, Cortella I, Crepaldi G, Franceschi C (1998) Lipoprotein(a) and lipoprotein profile in healthy centenarians: a reappraisal of vascular risk factors. FASEB J 12:433–437
Balk EM, Lichtenstein AH, Chung M, Kupelnick B, Chew P, Lau J (2006) Effects of omega-3 fatty acids on serum markers of cardiovascular disease risk: a systematic review. Atherosclerosis 189:19–30
Barr DP, Russ EM, Eder HA (1951) Protein-lipid relationships in human plasma. II. In atherosclerosis and related conditions. Am J Med 11:480–493
Barter P, Gotto AM, LaRosa JC, Maroni J, Szarek M, Grundy SM, Kastelein JJ, Bittner V, Fruchart JC (2007) Treating to new targets investigators. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med 357:1301–1310
Bergmann MM, Rehm J, Klipstein-Grobusch K, Boeing H, Schütze M, Drogan D, Overvad K, Tjønneland A, Halkjær J, Fagherazzi G, Boutron-Ruault MC, Clavel-Chapelon F, Teucher B, Kaaks R, Trichopoulou A, Benetou V, Trichopoulos D, Palli D, Pala V, Tumino R, Vineis P, Beulens JW, Redondo ML, Duell EJ, Molina-Montes E, Navarro C, Barricarte A, Arriola L, Allen NE, Crowe FL, Khaw KT, Wareham N, Romaguera D, Wark PA, Romieu I, Nunes L, Riboli E, Ferrari P (2013) The association of pattern of lifetime alcohol use and cause of death in the European prospective investigation into cancer and nutrition (EPIC) study. Int J Epidemiol 42:1772–1790. doi:10.1093/ije/dyt154
Berneis KK, Krauss RM (2002) Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res 43:1363–1379
Boekholdt SM, Arsenault BJ, Hovingh GK, Mora S, Pedersen TR, Larosa JC, Welch KM, Amarenco P, Demicco DA, Tonkin AM, Sullivan DR, Kirby A, Colhoun HM, Hitman GA, Betteridge DJ, Durrington PN, Clearfield MB, Downs JR, Gotto AM Jr, Ridker PM, Kastelein JJ (2013) Levels and changes of HDL cholesterol and apolipoprotein A-I in relation to risk of cardiovascular events among statin-treated patients: a meta-analysis. Circulation 128:1504–1512
Bogl LH, Pietiläinen KH, Rissanen A, Kangas AJ, Soininen P, Rose RJ, Ala-Korpela M, Kaprio J (2013) Association between habitual dietary intake and lipoprotein subclass profile in healthy young adults. Nutr Metab Cardiovasc Dis 23:1071–1078. doi:10.1016/j.numecd.2012.11.007
Brien SE, Ronksley PE, Turner BJ, Mukamal KJ, Ghali WA (2011) Effect of alcohol consumption on biological markers associated with risk of coronary heart disease: systematic review and meta-analysis of interventional studies. BMJ 342:d636. doi:10.1136/bmj.d636
Browning JD, Baker JA, Rogers T, Davis J, Satapati S, Burgess SC (2011) Short-term weight loss and hepatic triglyceride reduction: evidence of a metabolic advantage with dietary carbohydrate restriction. Am J Clin Nutr 93:1048–1052. doi:10.3945/ajcn.110.007674
Burillo E, Vazquez J, Jorge I (2013) Quantitative proteomics analysis of high-density lipoproteins by stable 18O-isotope labeling. Methods Mol Biol 1000:139–156. doi:10.1007/978-1-62703-405-0_11
Carroll MD, Kit BK, Lacher DA, Shero ST, Mussolino ME (2012) Trends in lipids and lipoproteins in US adults, 1988–2010. JAMA 308:1545–1554. doi:10.1001/jama.2012.13260
Chehade JM, Gladysz M, Mooradian AD (2013) Dyslipidemia in type 2 diabetes: prevalence, pathophysiology, and management. Drugs 73:327–339. doi:10.1007/s40265-013-0023-5
Chirovsky DR, Fedirko V, Cui Y, Sazonov V, Barter P (2009) Prospective studies on the relationship between high-density lipoprotein cholesterol and cardiovascular risk: a systematic review. Eur J Cardiovasc Prev Rehabil 16:404–423. doi:10.1097/HJR.0b013e32832c8891
Cooper JA, Miller GJ, Humphries SE (2005) A comparison of the PROCAM and Framingham point-scoring systems for estimation of individual risk of coronary heart disease in the Second Northwick Park Heart Study. Atherosclerosis 181:93–100
Correia LC, Rocha MS, Esteves JP (2009) HDL-cholesterol level provides additional prognosis in acute coronary syndromes. Int J Cardiol 136:307–314. doi:10.1016/j.ijcard.2008.05.067
Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Tjønneland A, Olsen A, Overvad K, Jakobsen MU, Chajès V, Clavel-Chapelon F, Boutron-Ruault MC, Linseisen J, Lukanova A, Boeing H, Pischon T, Trichopoulou A, Christina B, Trichopoulos D, Palli D, Berrino F, Panico S, Tumino R, Sacerdote C, Gram IT, Lund E, Quirós JR, Travier N, Martínez-García C, Larrañaga N, Chirlaque MD, Ardanaz E, Berglund G, Lundin E, Bueno-de-Mesquita HB, van Duijnhoven FJ, Peeters PH, Bingham S, Khaw KT, Allen N, Key T, Ferrari P, Rinaldi S, Slimani N, Riboli E (2007) Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European prospective investigation into cancer and nutrition (EPIC). Endocr Relat Cancer 14:755–767
de Munter JS, van Valkengoed IG, Stronks K, Agyemang C (2011) Total physical activity might not be a good measure in the relationship with HDL cholesterol and triglycerides in a multi-ethnic population: a cross-sectional study. Lipids Health Dis 10:223. doi:10.1186/1476-511X-10-223
de Rosa S, Fichtlscherer S, Lehmann R, Assmus B, Dimmeler S, Zeiher AM (2011) Transcoronary concentration gradients of circulating microRNAs. Circulation 124(18):1936–1944. doi:10.1161/CIRCULATIONAHA.111.037572
DeLalla OF, Elliott HA, Gofman JW (1954) Ultracentrifugal studies of high density serum lipoproteins in clinically healthy adults. Am J Physiol 179:333–337
Drexel H, Amann FW, Rentsch K, Neuenschwander C, Luethy A, Khan SI, Follath F (1992) Relation of the level of high-density lipoprotein subfractions to the presence and extent of coronary artery disease. Am J Cardiol 70:436–440
Duffy D, Holmes DN, Roe MT, Peterson ED (2012) The impact of high-density lipoprotein cholesterol levels on long-term outcomes after non-ST-elevation myocardial infarction. Am Heart J 163:705–713. doi:10.1016/j.ahj.2012.01.029
Emerging Risk Factors Collaboration, Di Angelantonio E, Gao P, Pennells L, Kaptoge S, Caslake M, Thompson A, Butterworth AS, Sarwar N, Wormser D, Saleheen D, Ballantyne CM, Psaty BM, Sundström J, Ridker PM, Nagel D, Gillum RF, Ford I, Ducimetiere P, Kiechl S, Koenig W, Dullaart RP, Assmann G, D'Agostino RB Sr, Dagenais GR, Cooper JA, Kromhout D, Onat A, Tipping RW, Gómez-de-la-Cámara A, Rosengren A, Sutherland SE, Gallacher J, Fowkes FG, Casiglia E, Hofman A, Salomaa V, Barrett-Connor E, Clarke R, Brunner E, Jukema JW, Simons LA, Sandhu M, Wareham NJ, Khaw KT, Kauhanen J, Salonen JT, Howard WJ, Nordestgaard BG, Wood AM, Thompson SG, Boekholdt SM, Sattar N, Packard C, Gudnason V, Danesh J (2012) Lipid-related markers and cardiovascular disease prediction. JAMA 307:2499–2506. doi:10.1001/jama.2012.6571
Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Giugliano D (2014) Metabolic syndrome and endometrial cancer: a meta-analysis. Endocrine 45:28–36. doi:10.1007/s12020-013-9973-3
Fichtlscherer S, Zeiher AM, Dimmeler S (2011) Circulating microRNAs: biomarkers or mediators of cardiovascular diseases? Arterioscler Thromb Vasc Biol 31:2383–2390. doi:10.1161/ATVBAHA.111.226696
Furberg AS, Jasienska G, Bjurstam N, Torjesen PA, Emaus A, Lipson SF, Ellison PT, Thune I (2005) Metabolic and hormonal profiles: HDL cholesterol as a plausible biomarker of breast cancer risk. The Norwegian EBBA study. Cancer Epidemiol Biomarkers Prev 14:33–40
Gangadharan B, Bapat M, Rossa J, Antrobus R, Chittenden D, Kampa B, Barnes E, Klenerman P, Dwek RA, Zitzmann N (2012) Discovery of novel biomarker candidates for liver fibrosis in hepatitis C patients: a preliminary study. PLoS ONE 7:e39603. doi:10.1371/journal.pone.0039603
Glomset JA, Janssen ET, Kennedy R, Dobbins J (1966) Role of plasma lecithin:cholesterol acyltransferase in the metabolism of high density lipoproteins. J Lipid Res 7:638–648
Goff LM, Cowland DE, Hooper L, Frost GS (2013) Low glycaemic index diets and blood lipids: a systematic review and meta-analysis of randomised controlled trials. Nutr Metab Cardiovasc Dis 23:1–10. doi:10.1016/j.numecd.2012.06.002
Gofman JW, Young W, Tandy R (1966) Ischemic heart disease, atherosclerosis, and longevity. Circulation 34:679–697
Goldbourt U, Yaari S, Medalie JH (1997) Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21-year follow-up of 8000 men. Arterioscler Thromb Vasc Biol 17:107–113
Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR (1977) High density lipoprotein as a protective factor against coronary heart disease. The Framingham study. Am J Med 62:707–714
Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA (1989) High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 79:8–15
Gordon SM, Deng J, Lu LJ, Davidson WS (2010) Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res 9:5239–5249. doi:10.1021/pr100520x
Gupta R, Guptha S, Gupta VP, Agrawal A, Gaur K, Deedwania PC (2012) Twenty-year trends in cardiovascular risk factors in India and influence of educational status. Eur J Prev Cardiol 19(6):1258–1271. doi:10.1177/1741826711424567
Haffner SM, Stern MP, Hazuda HP, Rosenthal M, Knapp JA (1986) The role of behavioral variables and fat patterning in explaining ethnic differences in serum lipids and lipoproteins. Am J Epidemiol 123:830–839
Halcox JP, Tubach F, Sazova O, Sweet S, Medina J, on behalf of the EURIKA steering committee (2013) Reclassification of European patients’ cardiovascular risk using the updated systematic coronary risk evaluation algorithm. Eur J Prev Cardiol (in press)
Hamer M, Mishra GD (2010) Dietary patterns and cardiovascular risk markers in the UK. Low Income Diet and Nutrition Survey. Nutr Metab Cardiovasc Dis 20(7):491–497. doi:10.1016/j.numecd.2009.05.002
Hannuksela M, Marcel YL, Kesäniemi YA, Savolainen MJ (1992) Reduction in the concentration and activity of plasma cholesteryl ester transfer protein by alcohol. J Lipid Res 33:737–744
Hannuksela ML, Rämet ME, Nissinen AE, Liisanantti MK, Savolainen MJ (2004) Effects of ethanol on lipids and atherosclerosis. Pathophysiology 10:93–103
Heiss G, Johnson NJ, Reiland S, Davis CE, Tyroler HA (1980) The epidemiology of plasma high-density lipoprotein cholesterol levels. The lipid research clinics program prevalence study. Summary. Circulation 62(4 Pt 2):IV116–IV136
Heiss G, Schonfeld G, Johnson JL, Heyden S, Hames CG, Tyroler HA (1984) Black-white differences in plasma levels of apolipoproteins: the Evans County heart study. Am Heart J 108:807–814
Hippisley-Cox J, Coupland C, Brindle P (2013) Derivation and validation of QStroke score for predicting risk of ischaemic stroke in primary care and comparison with other risk scores: a prospective open cohort study. BMJ 346:f2573. doi:10.1136/bmj.f2573
Holmes MV, Asselbergs FW, Palmer TM, Drenos F, Lanktree MB, Nelson CP, Dale CE, Padmanabhan S, Finan C, Swerdlow DI, Tragante V, van Iperen EP, Sivapalaratnam S, Shah S, Elbers CC, Shah T, Engmann J, Giambartolomei C, White J, Zabaneh D, Sofat R, McLachlan S, Doevendans PA, Balmforth AJ, Hall AS, North KE, Almoguera B, Hoogeveen RC, Cushman M, Fornage M, Patel SR, Redline S, Siscovick DS, Tsai MY, Karczewski KJ, Hofker MH, Verschuren WM, Bots ML, van der Schouw YT, Melander O, Dominiczak AF, Morris R, Ben-Shlomo Y, Price J, Kumari M, Baumert J, Peters A, Thorand B, Koenig W, Gaunt TR, Humphries SE, Clarke R, Watkins H, Farrall M, Wilson JG, Rich SS, de Bakker PI, Lange LA, Davey Smith G, Reiner AP, Talmud PJ, Kivimäki M, Lawlor DA, Dudbridge F, Samani NJ, Keating BJ, Hingorani AD, Casas JP, on behalf of the UCLEB consortium (2014) Mendelian randomization of blood lipids for coronary heart disease. Eur Heart J (in press)
Holzer M, Wolf P, Curcic S, Birner-Gruenberger R, Weger W, Inzinger M, El-Gamal D, Wadsack C, Heinemann A, Marsche G (2012) Psoriasis alters HDL composition and cholesterol efflux capacity. J Lipid Res 53:1618–1624. doi:10.1194/jlr.M027367
Hoofnagle AN, Becker JO, Oda MN, Cavigiolio G, Mayer P, Vaisar T (2012) Multiple-reaction monitoring-mass spectrometric assays can accurately measure the relative protein abundance in complex mixtures. Clin Chem 58:777–781. doi:10.1373/clinchem.2011.173856
Horter MJ, Sondermann S, Reinecke H, Bogdanski J, Woltering A, Kerber S, Breithardt G, Assmann G, Von Eckardstein A (2002) Associations of HDL phospholipids and paraoxonase activity with coronary heart disease in postmenopausal women. Acta Physiol Scand 176:123–130
Jacobs DR Jr, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA (1990) High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow-up study of the lipid research clinics prevalence study. Am J Epidemiol 131:32–47
Jensen MK, Mukamal KJ, Overvad K, Rimm EB (2008) Alcohol consumption, TaqIB polymorphism of cholesteryl ester transfer protein, high-density lipoprotein cholesterol, and risk of coronary heart disease in men and women. Eur Heart J 29:104–112
Jensen MK, Rimm EB, Furtado JD, Sacks FM (2012) Apolipoprotein C-III as a potential modulator of the association between HDL-cholesterol and incident coronary heart disease. J Am Heart Assoc 1. pii:jah3-e000232. doi:10.1161/JAHA.111.000232
Johansson J, Carlson LA, Landou C, Hamsten A (1991) High density lipoproteins and coronary atherosclerosis. A strong inverse relation with the largest particles is confined to normotriglyceridemic patients. Arterioscler Thromb 11:174–182
Juutilainen A, Kortelainen S, Lehto S, Rönnemaa T, Pyörälä K, Laakso M (2004) Gender difference in the impact of type 2 diabetes on coronary heart disease risk. Diabetes Care 27:2898–2904
Kastorini CM, Milionis HJ, Ioannidi A, Kalantzi K, Nikolaou V, Vemmos KN, Goudevenos JA, Panagiotakos DB (2011) Adherence to the Mediterranean diet in relation to acute coronary syndrome or stroke nonfatal events: a comparative analysis of a case/case–control study. Am Heart J 162(4):717–724. doi:10.1016/j.ahj.2011.07.012
Kavo AE, Rallidis LS, Sakellaropoulos GC, Lehr S, Hartwig S, Eckel J, Bozatzi PI, Anastasiou-Nana M, Tsikrika P, Kypreos KE (2012) Qualitative characteristics of HDL in young patients of an acute myocardial infarction. Atherosclerosis 220:257–264. doi:10.1016/j.atherosclerosis.2011.10.017
Kelly S, Frost G, Whittaker V, Summerbell C (2004) Low glycaemic index diets for coronary heart disease. Cochrane Database Syst Rev 4, CD004467
Kervinen K, Savolainen MJ, Salokannel J, Hynninen A, Heikkinen J, Ehnholm C, Koistinen MJ, Kesäniemi YA (1994) Apolipoprotein E and B polymorphisms – longevity factors assessed in nonagenarians. Atherosclerosis 105:89–95
Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ (2011) Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N Engl J Med 364:127–135
Kit BK, Carroll MD, Lacher DA, Sorlie PD, DeJesus JM, Ogden C (2012) Trends in serum lipids among US youths aged 6 to 19 years, 1988–2010. JAMA 308(6):591–600. doi:10.1001/jama.2012.9136
Kontush A, Chantepie S, Chapman MJ (2003) Small, dense HDL particles exert potent protection of atherogenic LDL against oxidative stress. Arterioscler Thromb Vasc Biol 23:1881–1888
Kotani K, Sekine Y, Ishikawa S, Ikpot IZ, Suzuki K, Remaley AT (2013) High-density lipoprotein and prostate cancer: an overview. J Epidemiol 23:313–319
Krehl WA (1977) The nutritional epidemiology of cardiovascular disease. Ann N Y Acad Sci 300:335–359
Kreisberg RA, Kasim S (1987) Cholesterol metabolism and aging. Am J Med 82(1B):54–60
Lamarche B, Moorjani S, Cantin B, Dagenais GR, Lupien PJ, Després JP (1997) Associations of HDL2 and HDL3 subfractions with ischemic heart disease in men. Prospective results from the Québec cardiovascular study. Arterioscler Thromb Vasc Biol 17:1098–1105
Laterza OF, Lim L, Garrett-Engele PW, Vlasakova K, Muniappa N, Tanaka WK, Johnson JM, Sina JF, Fare TL, Sistare FD, Glaab WE (2009) Plasma microRNAs as sensitive and specific biomarkers of tissue injury. Clin Chem 55:1977–1983. doi:10.1373/clinchem.2009.131797
Li XM, Tang WH, Mosior MK, Huang Y, Wu Y, Matter W, Gao V, Schmitt D, Didonato JA, Fisher EA, Smith JD, Hazen SL (2013) Paradoxical association of enhanced cholesterol efflux with increased incident cardiovascular risks. Arterioscler Thromb Vasc Biol 33:1696–1705. doi:10.1161/ATVBAHA.113.301373
Liinamaa MJ, Hannuksela ML, Kesäniemi YA, Savolainen MJ (1997) Altered transfer of cholesteryl esters and phospholipids in plasma from alcohol abusers. Arterioscler Thromb Vasc Biol 17:2940–2947
Liisanantti MK, Savolainen MJ (2005) Phosphatidylethanol in high density lipoproteins increases the vascular endothelial growth factor in smooth muscle cells. Atherosclerosis 180:263–269
Liisanantti MK, Hannuksela ML, Rämet ME, Savolainen MJ (2004) Lipoprotein-associated phosphatidylethanol increases the plasma concentration of vascular endothelial growth factor. Arterioscler Thromb Vasc Biol 24:1037–1042
Liosis S, Bauer T, Schiele R, Gohlke H, Gottwik M, Katus H, Sabin G, Zahn R, Schneider S, Rauch B, Senges J, Zeymer U (2013) Predictors of 1-year mortality in patients with contemporary guideline-adherent therapy after acute myocardial infarction: results from the OMEGA study. Clin Res Cardiol 102:671–677. doi:10.1007/s00392-013-0581-2
Ljunggren SA, Helmfrid I, Salihovic S, van Bavel B, Wingren G, Lindahl M, Karlsson H (2014) Persistent organic pollutants distribution in lipoprotein fractions in relation to cardiovascular disease and cancer. Environ Int 65:93–99. doi:10.1016/j.envint.2013.12.017
Ma XY, Liu JP, Song ZY (2012) Glycemic load, glycemic index and risk of cardiovascular diseases: meta-analyses of prospective studies. Atherosclerosis 223:491–496. doi:10.1016/j.atherosclerosis.2012.05.028
Mackness M, Durrington P, Mackness B (2004) Paraoxonase 1 activity, concentration and genotype in cardiovascular disease. Curr Opin Lipidol 15:399–404
Maeda K, Noguchi Y, Fukui T (2003) The effects of cessation from cigarette smoking on the lipid and lipoprotein profiles: a meta-analysis. Prev Med 37:283–290
Mäkinen VP, Tynkkynen T, Soininen P, Forsblom C, Peltola T, Kangas AJ, Groop PH, Ala-Korpela M (2012) Sphingomyelin is associated with kidney disease in type 1 diabetes (The FinnDiane study). Metabolomics 8:369–375
Mangé A, Goux A, Badiou S, Patrier L, Canaud B, Maudelonde T, Cristol JP, Solassol J (2012) HDL proteome in hemodialysis patients: a quantitative nanoflow liquid chromatography-tandem mass spectrometry approach. PLoS ONE 7:e34107. doi:10.1371/journal.pone.0034107
Mann CJ, Yen FT, Grant AM, Bihain BE (1991) Mechanism for plasma cholesteryl ester transfer in hyperlipidemia. J Clin Invest 88:2059–2066
Marti B (1991) Health effects of recreational running in women. Some epidemiological and preventive aspects. Sports Med 11:20–51
Mazur MT, Cardasis HL (2013) Quantitative analysis of apolipoproteins in human HDL by top-down differential mass spectrometry. Methods Mol Biol 1000:115–137. doi:10.1007/978-1-62703-405-0_10
Mazur MT, Cardasis HL, Spellman DS, Liaw A, Yates NA, Hendrickson RC (2010) Quantitative analysis of intact apolipoproteins in human HDL by top-down differential mass spectrometry. Proc Natl Acad Sci U S A 107:7728–7733. doi:10.1073/pnas.0910776107
Melvin JC, Seth D, Holmberg L, Garmo H, Hammar N, Jungner I, Walldius G, Lambe M, Wigertz A, Van Hemelrijck M (2012) Lipid profiles and risk of breast and ovarian cancer in the Swedish AMORIS study. Cancer Epidemiol Biomarkers Prev 21:1381–1384. doi:10.1158/1055-9965.EPI-12-0188
Melvin JC, Holmberg L, Rohrmann S, Loda M, Van Hemelrijck M (2013) Serum lipid profiles and cancer risk in the context of obesity: four meta-analyses. J Cancer Epidemiol 2013:823849. doi:10.1155/2013/823849
Mensink RP, Zock PL, Kester AD, Katan MB (2003) Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta-analysis of 60 controlled trials. Am J Clin Nutr 77(5):1146–1155
Miller GJ, Miller NE (1975) Plasma-high-density-lipoprotein concentration and development of ischaemic heart-disease. Lancet 1:16–19
Miller NE, Thelle DS, Forde OH, Mjos OD (1977) The Tromsø heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet 8019:965–968
Mondul AM, Weinstein SJ, Virtamo J, Albanes D (2011) Serum total and HDL cholesterol and risk of prostate cancer. Cancer Causes Control 22:1545–1552. doi:10.1007/s10552-011-9831-7
Mooradian AD (2009) Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab 5:150–159. doi:10.1038/ncpendmet1066
Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, Deedwania P, Kastelein JJ, Waters DD (2012) Determinants of residual risk in secondary prevention patients treated with high- versus low-dose statin therapy: the treating to new targets (TNT) study. Circulation 125:1979–1987. doi:10.1161/CIRCULATIONAHA.111.088591
Morgantini C, Meriwether D, Baldi S, Venturi E, Pinnola S, Wagner AC, Fogelman AM, Ferrannini E, Natali A, Reddy ST (2014) HDL lipid composition is profoundly altered in patients with type 2 diabetes and atherosclerotic vascular disease. Nutr Metab Cardiovasc Dis. pii:S0939-4753(14)00004-0. doi:10.1016/j.numecd.2013.12.011
Muennig P, Sohler N, Mahato B (2007) Socioeconomic status as an independent predictor of physiological biomarkers of cardiovascular disease: evidence from NHANES. Prev Med 45:35–40
Muntoni S, Atzori L, Mereu R, Manca A, Satta G, Gentilini A, Bianco P, Baule A, Baule GM, Muntoni S (2009) Risk factors for cardiovascular disease in Sardinia from 1978 to 2001: a comparative study with Italian mainland. Eur J Intern Med 20(4):373–377. doi:10.1016/j.ejim.2008.10.007
Nikkilä EA (1953) Studies on the lipid-protein relationships in normal and pathological sera and the effect of heparin on serum lipoproteins. Scand J Clin Lab Invest 5(Suppl 8):1–101
Onat A, Altuğ Çakmak H, Can G, Yüksel M, Köroğlu B, Yüksel H (2013) Serum total and high-density lipoprotein phospholipids: independent predictive value for cardiometabolic risk. Clin Nutr. pii:S0261-5614(13)00279-3. doi:10.1016/j.clnu.2013.10.020
Ostlund RE Jr, Staten M, Kohrt WM, Schultz J, Malley M (1990) The ratio of waist-to-hip circumference, plasma insulin level, and glucose intolerance as independent predictors of the HDL2 cholesterol level in older adults. N Engl J Med 322(4):229–234
Pirillo A, Norata GD, Catapano AL (2013) High-density lipoprotein subfractions–what the clinicians need to know. Cardiology 124:116–125
Raitakari OT, Mäkinen VP, McQueen MJ, Niemi J, Juonala M, Jauhiainen M, Salomaa V, Hannuksela ML, Savolainen MJ, Kesäniemi YA, Kovanen PT, Sundvall J, Solakivi T, Loo BM, Marniemi J, Hernesniemi J, Lehtimäki T, Kähönen M, Peltonen M, Leiviskä J, Jula A, Anand SS, Miller R, Yusuf S, Viikari JS, Ala-Korpela M (2013) Computationally estimated apolipoproteins B and A1 in predicting cardiovascular risk. Atherosclerosis 226:245–251. doi:10.1016/j.atherosclerosis.2012.10.049
Randall E, Marshall JR, Graham S, Brasure J (1990) Patterns in food use and their associations with nutrient intakes. Am J Clin Nutr 52:739–745
Raterman HG, Levels H, Voskuyl AE, Lems WF, Dijkmans BA, Nurmohamed MT (2013) HDL protein composition alters from proatherogenic into less atherogenic and proinflammatory in rheumatoid arthritis patients responding to rituximab. Ann Rheum Dis 72:560–565. doi:10.1136/annrheumdis-2011-201228
Rhoads GG, Gulbrandsen CL, Kagan A (1976) Serum lipoproteins and coronary heart disease in a population study of Hawaii Japanese men. N Engl J Med 294:293–298
Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, Nordestgaard BG, Mora S, MacFadyen JG, Glynn RJ, Kastelein JJ, JUPITER Trial Study Group (2010) HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet 376:333–339. doi:10.1016/S0140-6736(10)60713-1
Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, Perisa D, Heinrich K, Altwegg L, von Eckardstein A, Lüscher TF, Landmesser U (2013) Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation 127:891–904. doi:10.1161/CIRCULATIONAHA.112.108753
Rosenson RS, Brewer HB Jr, Chapman MJ, Fazio S, Hussain MM, Kontush A, Krauss RM, Otvos JD, Remaley AT, Schaefer EJ (2011) HDL measures, particle heterogeneity, proposed nomenclature, and relation to atherosclerotic cardiovascular events. Clin Chem 57:392–410. doi:10.1373/clinchem.2010.155333
Saha N (1987) Serum high density lipoprotein cholesterol, apolipoprotein A-I, A-II and B levels in Singapore ethnic groups. Atherosclerosis 68:117–121
Sattler KJ, Herrmann J, Yün S, Lehmann N, Wang Z, Heusch G, Sack S, Erbel R, Levkau B (2009) High high-density lipoprotein-cholesterol reduces risk and extent of percutaneous coronary intervention-related myocardial infarction and improves long-term outcome in patients undergoing elective percutaneous coronary intervention. Eur Heart J 30:1894–1902. doi:10.1093/eurheartj/ehp183
Savolainen MJ, Hannuksela M, Seppänen S, Kervinen K, Kesäniemi YA (1990) Increased high-density lipoprotein cholesterol concentration in alcoholics is related to low cholesteryl ester transfer protein activity. Eur J Clin Invest 20:593–599
Schröder H, Rohlfs I, Schmelz EM, Marrugat J, REGICOR investigators (2004) Relationship of socioeconomic status with cardiovascular risk factors and lifestyle in a Mediterranean population. Eur J Nutr 43:77–85
Seidell JC, Cigolini M, Charzewska J, Ellsinger BM, Björntorp P, Hautvast JG, Szostak W (1991) Fat distribution and gender differences in serum lipids in men and women from four European communities. Atherosclerosis 87:203–210
Silbernagel G, Schöttker B, Appelbaum S, Scharnagl H, Kleber ME, Grammer TB, Ritsch A, Mons U, Holleczek B, Goliasch G, Niessner A, Boehm BO, Schnabel RB, Brenner H, Blankenberg S, Landmesser U, März W (2013) High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. Eur Heart J 34:3563–3571. doi:10.1093/eurheartj/eht343
Sirtori CR, Calabresi L, Franceschini G, Baldassarre D, Amato M, Johansson J, Salvetti M, Monteduro C, Zulli R, Muiesan ML, Agabiti-Rosei E (2001) Cardiovascular status of carriers of the apolipoprotein A-I(Milano) mutant: the Limone sul Garda study. Circulation 103:1949–1954
Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, McGahan JP, Seibert A, Krauss RM, Chiu S, Schaefer EJ, Ai M, Otokozawa S, Nakajima K, Nakano T, Beysen C, Hellerstein MK, Berglund L, Havel PJ (2009) Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 119(5):1322–1334
Sofi F, Abbate R, Gensini GF, Casini A (2010) Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr 92:1189–1196. doi:10.3945/ajcn.2010.29673
Sonestedt E, Wirfält E, Wallström P, Gullberg B, Drake I, Hlebowicz J, Nordin Fredrikson G, Hedblad B, Nilsson J, Krauss RM, Orho-Melander M (2012) High disaccharide intake associates with atherogenic lipoprotein profile. Br J Nutr 107:1062–1069. doi:10.1017/S0007114511003783
Sung KC, Wild SH, Byrne CD (2013) Controlling for apolipoprotein A-I concentrations changes the inverse direction of the relationship between high HDL-C concentration and a measure of pre-clinical atherosclerosis. Atherosclerosis 231:181–186. doi:10.1016/j.atherosclerosis.2013.09.009
Superko HR, Pendyala L, Williams PT, Momary KM, King SB 3rd, Garrett BC (2012) High-density lipoprotein subclasses and their relationship to cardiovascular disease. J Clin Lipidol 6:496–523
Tamura T, Inagawa S, Hisakura K, Enomoto T, Ohkohchi N (2012) Evaluation of serum high-density lipoprotein cholesterol levels as a prognostic factor in gastric cancer patients. J Gastroenterol Hepatol 27:1635–1640. doi:10.1111/j.1440-1746.2012.07189.x
Tehrani DM, Gardin JM, Yanez D, Hirsch CH, Lloyd-Jones DM, Stein PK, Wong ND (2013) Impact of inflammatory biomarkers on relation of high density lipoprotein-cholesterol with incident coronary heart disease: cardiovascular Health Study. Atherosclerosis 231:246–251. doi:10.1016/j.atherosclerosis.2013.08.036
Thelle DS, Førde OH, Arnesen E (1982) Distribution of high-density lipoprotein cholesterol according to age, sex, and ethnic origin: cardiovascular disease study in Finnmark 1977. J Epidemiol Community Health 36:243–247
Toth PP, Barter PJ, Rosenson RS, Boden WE, Chapman MJ, Cuchel M, D'Agostino RB Sr, Davidson MH, Davidson WS, Heinecke JW, Karas RH, Kontush A, Krauss RM, Miller M, Rader DJ (2013) High-density lipoproteins: a consensus statement from the National Lipid Association. J Clin Lipidol 7:484–525. doi:10.1016/j.jacl.2013.08.001
Touboul PJ, Labreuche J, Bruckert E, Schargrodsky H, Prati P, Tosetto A, Hernandez-Hernandez R, Woo KS, Silva H, Vicaut E, Amarenco P (2014) HDL-C, triglycerides and carotid IMT: a meta-analysis of 21,000 patients with automated edge detection IMT measurement. Atherosclerosis 232:65–71. doi:10.1016/j.atherosclerosis.2013.10.011
Vaisar T, Mayer P, Nilsson E, Zhao XQ, Knopp R, Prazen BJ (2010) HDL in humans with cardiovascular disease exhibits a proteomic signature. Clin Chim Acta 411:972–979. doi:10.1016/j.cca.2010.03.023
van Capelleveen JC, Bochem AE, Motazacker MM, Hovingh GK, Kastelein JJ (2013) Genetics of HDL-C: a causal link to atherosclerosis? Curr Atheroscler Rep 15:326. doi:10.1007/s11883-013-0326-8
van Duijnhoven FJ, Bueno-De-Mesquita HB, Calligaro M, Jenab M, Pischon T, Jansen EH, Frohlich J, Ayyobi A, Overvad K, Toft-Petersen AP, Tjønneland A, Hansen L, Boutron-Ruault MC, Clavel-Chapelon F, Cottet V, Palli D, Tagliabue G, Panico S, Tumino R, Vineis P, Kaaks R, Teucher B, Boeing H, Drogan D, Trichopoulou A, Lagiou P, Dilis V, Peeters PH, Siersema PD, Rodríguez L, González CA, Molina-Montes E, Dorronsoro M, Tormo MJ, Barricarte A, Palmqvist R, Hallmans G, Khaw KT, Tsilidis KK, Crowe FL, Chajes V, Fedirko V, Rinaldi S, Norat T, Riboli E (2011) Blood lipid and lipoprotein concentrations and colorectal cancer risk in the European prospective investigation into cancer and nutrition. Gut 60:1094–1102. doi:10.1136/gut.2010.225011
van Vliet M, Heymans MW, von Rosenstiel IA, Brandjes DP, Beijnen JH, Diamant M (2011) Cardiometabolic risk variables in overweight and obese children: a worldwide comparison. Cardiovasc Diabetol 10:106. doi:10.1186/1475-2840-10-106
Vílchez JA, Martínez-Ruiz A, Sancho-Rodríguez N, Martínez-Hernández P, Noguera-Velasco JA (2014) The real role of prediagnostic high-density lipoprotein cholesterol and the cancer risk: a concise review. Eur J Clin Invest 44:103–114. doi:10.1111/eci.12185
Voight BF, Peloso GM, Orho-Melander M, Frikke-Schmidt R, Barbalic M, Jensen MK, Hindy G, Hólm H, Ding EL, Johnson T, Schunkert H, Samani NJ, Clarke R, Hopewell JC, Thompson JF, Li M, Thorleifsson G, Newton-Cheh C, Musunuru K, Pirruccello JP, Saleheen D, Chen L, Stewart A, Schillert A, Thorsteinsdottir U, Thorgeirsson G, Anand S, Engert JC, Morgan T, Spertus J, Stoll M, Berger K, Martinelli N, Girelli D, McKeown PP, Patterson CC, Epstein SE, Devaney J, Burnett MS, Mooser V, Ripatti S, Surakka I, Nieminen MS, Sinisalo J, Lokki ML, Perola M, Havulinna A, de Faire U, Gigante B, Ingelsson E, Zeller T, Wild P, de Bakker PI, Klungel OH, Maitland-van der Zee AH, Peters BJ, de Boer A, Grobbee DE, Kamphuisen PW, Deneer VH, Elbers CC, Onland-Moret NC, Hofker MH, Wijmenga C, Verschuren WM, Boer JM, van der Schouw YT, Rasheed A, Frossard P, Demissie S, Willer C, Do R, Ordovas JM, Abecasis GR, Boehnke M, Mohlke KL, Daly MJ, Guiducci C, Burtt NP, Surti A, Gonzalez E, Purcell S, Gabriel S, Marrugat J, Peden J, Erdmann J, Diemert P, Willenborg C, König IR, Fischer M, Hengstenberg C, Ziegler A, Buysschaert I, Lambrechts D, Van de Werf F, Fox KA, El Mokhtari NE, Rubin D, Schrezenmeir J, Schreiber S, Schäfer A, Danesh J, Blankenberg S, Roberts R, McPherson R, Watkins H, Hall AS, Overvad K, Rimm E, Boerwinkle E, Tybjaerg-Hansen A, Cupples LA, Reilly MP, Melander O, Mannucci PM, Ardissino D, Siscovick D, Elosua R, Stefansson K, O'Donnell CJ, Salomaa V, Rader DJ, Peltonen L, Schwartz SM, Altshuler D, Kathiresan S (2012) Plasma HDL cholesterol and risk of myocardial infarction: a mendelian randomisation study. Lancet 380:572–580. doi:10.1016/S0140-6736(12)60312-2
Wagner J, Riwanto M, Besler C, Knau A, Fichtlscherer S, Röxe T, Zeiher AM, Landmesser U, Dimmeler S (2013) Characterization of levels and cellular transfer of circulating lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol 33:1392–1400. doi:10.1161/ATVBAHA.112.300741
Walter M (2009) Interrelationships among HDL metabolism, aging, and atherosclerosis. Arterioscler Thromb Vasc Biol 29:1244–1250. doi:10.1161/ATVBAHA.108.181438
Williams PT, Feldman DE (2011) Prospective study of coronary heart disease vs. HDL2, HDL3, and other lipoproteins in Gofman's Livermore Cohort. Atherosclerosis 214:196–202. doi:10.1016/j.atherosclerosis.2010.10.024 (Epub 2010 Oct 23)
Würtz P, Raiko JR, Magnussen CG, Soininen P, Kangas AJ, Tynkkynen T, Thomson R, Laatikainen R, Savolainen MJ, Laurikka J, Kuukasjärvi P, Tarkka M, Karhunen PJ, Jula A, Viikari JS, Kähönen M, Lehtimäki T, Juonala M, Ala-Korpela M, Raitakari OT (2012) High-throughput quantification of circulating metabolites improves prediction of subclinical atherosclerosis. Eur Heart J 33:2307–2316. doi:10.1093/eurheartj/ehs020
Yang S, Damiano MG, Zhang H, Tripathy S, Luthi AJ, Rink JS, Ugolkov AV, Singh AT, Dave SS, Gordon LI, Thaxton CS (2013) Biomimetic, synthetic HDL nanostructures for lymphoma. Proc Natl Acad Sci U S A 110:2511–2516. doi:10.1073/pnas.1213657110
Zheng Y, Liu Y, Jin H, Pan S, Qian Y, Huang C, Zeng Y, Luo Q, Zeng M, Zhang Z (2013) Scavenger receptor B1 is a potential biomarker of human nasopharyngeal carcinoma and its growth is inhibited by HDL-mimetic nanoparticles. Theranostics 3:477–486. doi:10.7150/thno.6617
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this chapter
Cite this chapter
Savolainen, M.J. (2015). Epidemiology: Disease Associations and Modulators of HDL-Related Biomarkers. In: von Eckardstein, A., Kardassis, D. (eds) High Density Lipoproteins. Handbook of Experimental Pharmacology, vol 224. Springer, Cham. https://doi.org/10.1007/978-3-319-09665-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-09665-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-09664-3
Online ISBN: 978-3-319-09665-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)