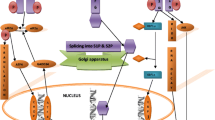
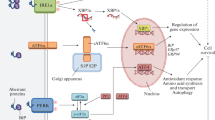
Abstract
Proteins destined for the secretory pathway are processed in the endoplasmic reticulum (ER) where a delicate balance exists between protein folding and degradation of terminally misfolded proteins. Different physiological as well as pathological stress conditions however, can lead to an imbalance between the ER protein folding capacity and protein load, giving rise to an accumulation of misfolded proteins in the ER lumen, a condition dubbed as ‘ER stress’. In an attempt to meet the increased folding demand, cells utilize a conserved signaling pathway, the unfolded protein response (UPR), which is initially charged to re-establish ER homeostasis and support survival. If this mechanism fails, persistent ER stress will eventually cause this cytoprotective UPR to switch into a cell death pathway that can activate mitochondrial apoptosis. As such, the dual function of the UPR in controlling cell fate may play a part in disease development and response to stress signals in conflicting ways. The lethal arm of the UPR may contribute to pathologies that are linked to unscheduled cell death, like diabetes and certain neurodegenerative diseases such as Alzheimer's and Parkinson’s, or be utilized by certain anticancer drugs to kill cancer cells. On the other hand, activation of the pro-survival function of the UPR may assist processes like tumorigenesis and chemoresistance, by endowing cancer cells with an increased capability to adapt to their hostile environment and to cope with cellular damage. Therefore, a better understanding of the different signaling pathways that emanate from the stressed ER and how their integration modulates cell fate decisions represents a crucial requirement to develop new strategies aimed at targeting the UPR for therapeutic purposes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- Akt:
-
Protein Kinase B
- ATF4/6:
-
Activating Transcription Factor 4/6
- ATP:
-
Adenosine Triphosphate
- Bax:
-
Bcl-2 associated X protein
- Bak:
-
Bcl-2 antagonist/killer
- Bcl-2:
-
B-cell lymphoma 2
- BH3:
-
Bcl-2 Homology 3
- BI-1:
-
Bax inhibitor 1
- Bim:
-
Bcl2-interacting mediator of cell death
- BiP:
-
immunoglobulin heavy chain-Binding Protein
- Ca2+ :
-
Calcium
- CHOP:
-
C/EBP Homologous Protein
- DKO:
-
Double Knock Out
- eIF2α:
-
eukaryotic Initiation Factor-2α
- ER:
-
Endoplasmic Reticulum
- ERAD:
-
ER Associated Degradation
- ERO1:
-
Endoplasmic Reticulum Oxidoreductin 1
- GADD34:
-
Growth Arrest and DNA Damage protein 34
- GRP78/94:
-
Glucose Regulated protein 78/94P
- IP3R:
-
Inositol 1,4,5-trisphosphate Receptor
- IP3:
-
Inositol trisphosphate
- IRE1:
-
Inositol Requiring Enzyme 1
- JNK:
-
c-Jun N-terminal Kinase
- KEAP1:
-
Kelch-like Ech-Associated Protein 1
- MAMs:
-
Mitochondria Associated ER Membranes
- MAPK:
-
Mitogen Activated Protein Kinase
- MOMP:
-
Mitochondrial Outer Membrane Permeabilization
- Nrf2:
-
Nuclear factor-E2-related factor 2
- MEF:
-
Murine Embryonic Fibroblast
- MOMP:
-
Mitochondrial Outer Membrane Permeabilization
- MFN2:
-
Mitofusin 2
- PDI:
-
Protein Disulphide Isomerase
- PERK:
-
double stranded RNA-activated protein kinase (PKR)—like ER Kinase
- PI3K:
-
Phosphatidylinositol-3-Kinase
- RIDD:
-
Regulated IRE1 Dependent Decay
- ROS:
-
Reactive Oxygen Species
- S1/2P:
-
Site 1/2 Protease
- SERCA:
-
Sarco/Endoplasmic Reticulum Ca2+ ATPase
- S1T:
-
Truncated SERCA1
- Sig-1R:
-
Sigma-1 receptor
- TG:
-
Thapsigargin
- TM:
-
Tunicamycin
- TRAF2:
-
Tumor necrosis factor receptor (TNFR) Associated Factor 2
- UPR:
-
Unfolded Protein Response
- XBP1u/s:
-
XBP1 unspliced/spliced
References
Gorlach A, Klappa P, Kietzmann T (2006) The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal 8(9–10):1391–1418. doi:10.1089/ars.2006.8.1391
Anelli T, Sitia R (2008) Protein quality control in the early secretory pathway. EMBO J 27(2):315–327. doi:7601974 [pii] 10.1038/sj.emboj.7601974
Sevier CS, Kaiser CA (2008) Ero1 and redox homeostasis in the endoplasmic reticulum. Biochim Biophys Acta 1783(4):549–556. doi:S0167-4889(07)00315-1 [pii] 10.1016/j.bbamcr.2007.12.011
Kim I, Xu W, Reed JC (2008) Cell death and endoplasmic reticulum stress: disease relevance and therapeutic opportunities. Nat Rev Drug Discov 7(12):1013–1030. doi:nrd2755 [pii] 10.1038/nrd2755
Tabas I, Ron D (2011) Integrating the mechanisms of apoptosis induced by endoplasmic reticulum stress. Nat Cell Biol 13(3):184–190. doi:ncb0311-184 [pii] 10.1038/ncb0311–184
Kang SW, Rane NS, Kim SJ, Garrison JL, Taunton J, Hegde RS (2006) Substrate-specific translocational attenuation during ER stress defines a pre-emptive quality control pathway. Cell 127(5):999–1013. doi:S0092-8674(06)01410-3 [pii] 10.1016/j.cell.2006.10.032
Cox JS, Shamu CE, Walter P (1993) Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73(6):1197–1206. doi:0092-8674(93)90648-A [pii]
Mori K, Ma W, Gething MJ, Sambrook J (1993) A transmembrane protein with a cdc2+ /CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74(4):743–756. doi:0092-8674(93)90521-Q [pii]
Wang XZ, Harding HP, Zhang Y, Jolicoeur EM, Kuroda M, Ron D (1998) Cloning of mammalian Ire1 reveals diversity in the ER stress responses. Embo J 17(19):5708–5717
Tirasophon W, Welihinda AA, Kaufman RJ (1998) A stress response pathway from the endoplasmic reticulum to the nucleus requires a novel bifunctional protein kinase/endoribonuclease (Ire1p) in mammalian cells. Genes Dev 12(12):1812–1824
Kimata Y, Kohno K (2011) Endoplasmic reticulum stress-sensing mechanisms in yeast and mammalian cells. Curr Opin Cell Biol 23(2):135–142. doi:S0955-0674(10)00179-1 [pii] 10.1016/j.ceb.2010.10.008
Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P (2009) The unfolded protein response signals through high-order assembly of Ire1. Nature 457(7230):687–693. doi:nature07661 [pii] 10.1038/nature07661
Kimata Y, Oikawa D, Shimizu Y, Ishiwata-Kimata Y, Kohno K (2004) A role for BiP as an adjustor for the endoplasmic reticulum stress-sensing protein Ire1. J Cell Biol 167(3):445–456. doi:jcb.200405153 [pii] 10.1083/jcb.200405153
Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P (2005) On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci U S A 102(52):18773–18784. doi:0509487102 [pii] 10.1073/pnas.0509487102
Gardner BM, Walter P (2011) Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science 333(6051):1891–1894. doi:science.1209126 [pii] 10.1126/science.1209126
Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ (2006) The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci U S A 103(39):14343–14348. doi:0606480103 [pii] 10.1073/pnas.0606480103
Oikawa D, Kimata Y, Kohno K, Iwawaki T (2009) Activation of mammalian IRE1alpha upon ER stress depends on dissociation of BiP rather than on direct interaction with unfolded proteins. Exp Cell Res 315(15):2496–2504. doi:S0014-4827(09)00270-5 [pii] 10.1016/j.yexcr.2009.06.009
Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K (2001) XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107(7):881–891
Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D (2002) IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415(6867):92–96. doi:10.1038/415092a 415092a [pii]
Lee AH, Iwakoshi NN, Glimcher LH (2003) XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23(21):7448–7459
Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, Lee AH, Qian SB, Zhao H, Yu X, Yang L, Tan BK, Rosenwald A, Hurt EM, Petroulakis E, Sonenberg N, Yewdell JW, Calame K, Glimcher LH, Staudt LM (2004) XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity 21(1):81–93. doi:10.1016/j.immuni.2004.06.010 S1074761304001670 [pii]
Yamamoto K, Yoshida H, Kokame K, Kaufman RJ, Mori K (2004) Differential contributions of ATF6 and XBP1 to the activation of endoplasmic reticulum stress-responsive cis-acting elements ERSE, UPRE and ERSE-II. J Biochem 136(3):343–350. doi:136/3/343 [pii] 10.1093/jb/mvh122
Acosta-Alvear D, Zhou Y, Blais A, Tsikitis M, Lents NH, Arias C, Lennon CJ, Kluger Y, Dynlacht BD (2007) XBP1 controls diverse cell type- and condition-specific transcriptional regulatory networks. Mol Cell 27(1):53–66. doi:S1097-2765(07)00400-5 [pii] 10.1016/j.molcel.2007.06.011
Liu Y, Adachi M, Zhao S, Hareyama M, Koong AC, Luo D, Rando TA, Imai K, Shinomura Y (2009) Preventing oxidative stress: a new role for XBP1. Cell Death Differ 16(6):847–857. doi:cdd200914 [pii] 10.1038/cdd.2009.14
Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ (2002) IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16(4):452–466. doi:10.1101/gad.964702
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666. doi:8218 [pii]
Reimold AM, Etkin A, Clauss I, Perkins A, Friend DS, Zhang J, Horton HF, Scott A, Orkin SH, Byrne MC, Grusby MJ, Glimcher LH (2000) An essential role in liver development for transcription factor XBP-1. Genes Dev 14(2):152–157
Kakiuchi C, Ishiwata M, Hayashi A, Kato T (2006) XBP1 induces WFS1 through an endoplasmic reticulum stress response element-like motif in SH-SY5Y cells. J Neurochem 97(2):545–555. doi:JNC3772 [pii] 10.1111/j.1471-4159.2006.03772.x
Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K (2007) Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6alpha and XBP1. Dev Cell 13(3):365–376. doi:S1534-5807(07)00300-0 [pii] 10.1016/j.devcel.2007.07.018
Park SW, Zhou Y, Lee J, Lu A, Sun C, Chung J, Ueki K, Ozcan U (2010) The regulatory subunits of PI3 K, p85alpha and p85beta, interact with XBP-1 and increase its nuclear translocation. Nat Med 16(4):429–437. doi:nm.2099 [pii] 10.1038/nm.2099
Winnay JN, Boucher J, Mori MA, Ueki K, Kahn CR (2010) A regulatory subunit of phosphoinositide 3-kinase increases the nuclear accumulation of X-box-binding protein-1 to modulate the unfolded protein response. Nat Med 16(4):438–445. doi:nm.2121 [pii] 10.1038/nm.2121
Wang FM, Chen YJ, Ouyang HJ (2010) Regulation of unfolded protein response modulator XBP1s by acetylation and deacetylation. Biochem J 433(1):245–252. doi:BJ20101293 [pii] 10.1042/BJ20101293
Ma K, Vattem KM, Wek RC (2002) Dimerization and release of molecular chaperone inhibition facilitate activation of eukaryotic initiation factor-2 kinase in response to endoplasmic reticulum stress. J Biol Chem 277(21):18728–18735. doi:10.1074/jbc.M200903200 [pii]
Marciniak SJ, Garcia-Bonilla L, Hu J, Harding HP, Ron D (2006) Activation-dependent substrate recruitment by the eukaryotic translation initiation factor 2 kinase PERK. J Cell Biol 172(2):201–209. doi:jcb.200508099 [pii] 10.1083/jcb.200508099
DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M (2009) Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol 29(15):4295–4307. doi:MCB.00260-09 [pii] 10.1128/MCB.00260-09
Harding HP, Calfon M, Urano F, Novoa I, Ron D (2002) Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol 18:575–599. doi:10.1146/annurev.cellbio.18.011402.160624 011402.160624 [pii]
Marciniak SJ, Yun CY, Oyadomari S, Novoa I, Zhang Y, Jungreis R, Nagata K, Harding HP, Ron D (2004) CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Dev 18(24):3066–3077
Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D (2003) An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11(3):619–633
Cullinan SB, Diehl JA (2006) Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38(3):317–332. doi:S1357-2725(05)00305-5 [pii] 10.1016/j.biocel.2005.09.018
Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M (2000) Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275(21):16023–16029. doi:275/21/16023 [pii]
Cullinan SB, Zhang D, Hannink M, Arvisais E, Kaufman RJ, Diehl JA (2003) Nrf2 is a direct PERK substrate and effector of PERK-dependent cell survival. Mol Cell Biol 23(20):7198–7209
Shen J, Chen X, Hendershot L, Prywes R (2002) ER stress regulation of ATF6 localization by dissociation of BiP/GRP78 binding and unmasking of Golgi localization signals. Dev Cell 3(1):99–111. doi:S1534580702002034 [pii]
Haze K, Yoshida H, Yanagi H, Yura T, Mori K (1999) Mammalian transcription factor ATF6 is synthesized as a transmembrane protein and activated by proteolysis in response to endoplasmic reticulum stress. Mol Biol Cell 10(11):3787–3799
Sakai J, Duncan EA, Rawson RB, Hua X, Brown MS, Goldstein JL (1996) Sterol-regulated release of SREBP-2 from cell membranes requires two sequential cleavages, one within a transmembrane segment. Cell 85(7):1037–1046. doi:S0092-8674(00)81304-5 [pii]
Thuerauf DJ, Marcinko M, Belmont PJ, Glembotski CC (2007) Effects of the isoform-specific characteristics of ATF6 alpha and ATF6 beta on endoplasmic reticulum stress response gene expression and cell viability. J Biol Chem 282(31):22865–22878. doi:M701213200 [pii] 10.1074/jbc.M701213200
Yoshida H, Uemura A, Mori K (2009) pXBP1(U), a negative regulator of the unfolded protein response activator pXBP1(S), targets ATF6 but not ATF4 in proteasome-mediated degradation. Cell Struct Funct 34(1):1–10. doi:JST.JSTAGE/csf/06028 [pii]
Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ (2007) ATF6alpha optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13(3):351–364. doi:S1534-5807(07)00266-3 [pii] 10.1016/j.devcel.2007.07.005
Bommiasamy H, Back SH, Fagone P, Lee K, Meshinchi S, Vink E, Sriburi R, Frank M, Jackowski S, Kaufman RJ, Brewer JW (2009) ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. J Cell Sci 122(Pt 10):1626–1636. doi:122/10/1626 [pii] 10.1242/jcs.045625
Rodriguez D, Rojas-Rivera D, Hetz C (2011) Integrating stress signals at the endoplasmic reticulum: The BCL-2 protein family rheostat. Biochim Biophys Acta 1813(4):564–574. doi:S0167-4889(10)00297-1 [pii] 10.1016/j.bbamcr.2010.11.012
Szegezdi E, Macdonald DC, Ni Chonghaile T, Gupta S, Samali A (2009) Bcl-2 family on guard at the ER. Am J Physiol Cell Physiol 296(5):C941–C953. doi:00612.2008 [pii] 10.1152/ajpcell.00612.2008
Chipuk JE, Green DR (2008) How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18(4):157–164. doi:S0962-8924(08)00066-4 [pii] 10.1016/j.tcb.2008.01.007
Kuwana T, Newmeyer DD (2003) Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol 15(6):691–699. doi:S0955067403001315 [pii]
Chen R, Valencia I, Zhong F, McColl KS, Roderick HL, Bootman MD, Berridge MJ, Conway SJ, Holmes AB, Mignery GA, Velez P, Distelhorst CW (2004) Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J Cell Biol 166(2):193–203. doi:10.1083/jcb.200309146 jcb.200309146 [pii]
White C, Li C, Yang J, Petrenko NB, Madesh M, Thompson CB, Foskett JK (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-X(L) modulation of the InsP3R. Nat Cell Biol 7(10):1021–1028. doi:ncb1302 [pii] 10.1038/ncb1302
Zong WX, Li C, Hatzivassiliou G, Lindsten T, Yu QC, Yuan J, Thompson CB (2003) Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J Cell Biol 162(1):59–69. doi:10.1083/jcb.200302084 jcb.200302084 [pii]
Orrenius S, Zhivotovsky B, Nicotera P (2003) Regulation of cell death: the calcium-apoptosis link. Nat Rev Mol Cell Biol 4(7):552–565. doi:10.1038/nrm1150 nrm1150 [pii]
Pinton P, Ferrari D, Magalhaes P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R (2000) Reduced loading of intracellular Ca(2+) stores and downregulation of capacitative Ca(2+) influx in Bcl-2-overexpressing cells. J Cell Biol 148(5):857–862
Hanson CJ, Bootman MD, Distelhorst CW, Wojcikiewicz RJ, Roderick HL (2008) Bcl-2 suppresses Ca2+ release through inositol 1,4,5-trisphosphate receptors and inhibits Ca2+ uptake by mitochondria without affecting ER calcium store content. Cell Calcium 44(3):324–338. doi:S0143-4160(08)00014-6 [pii] 10.1016/j.ceca.2008.01.003
Dremina ES, Sharov VS, Kumar K, Zaidi A, Michaelis EK, Schoneich C (2004) Anti-apoptotic protein Bcl-2 interacts with and destabilizes the sarcoplasmic/endoplasmic reticulum Ca2+ -ATPase (SERCA). Biochem J 383(Pt 2):361–370. doi:10.1042/BJ20040187 BJ20040187 [pii]
Chae HJ, Kim HR, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, Banares S, Cui J, Digicaylioglu M, Ke N, Kitada S, Monosov E, Thomas M, Kress CL, Babendure JR, Tsien RY, Lipton SA, Reed JC (2004) BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell 15(3):355–366. doi:10.1016/j.molcel.2004.06.038 S109727650400382X [pii]
Bailly-Maitre B, Fondevila C, Kaldas F, Droin N, Luciano F, Ricci JE, Croxton R, Krajewska M, Zapata JM, Kupiec-Weglinski JW, Farmer D, Reed JC (2006) Cytoprotective gene bi-1 is required for intrinsic protection from endoplasmic reticulum stress and ischemia-reperfusion injury. Proc Natl Acad Sci U S A 103(8):2809–2814. doi:0506854103 [pii] 10.1073/pnas.0506854103
Xu C, Xu W, Palmer AE, Reed JC (2008) BI-1 regulates endoplasmic reticulum Ca2+ homeostasis downstream of Bcl-2 family proteins. J Biol Chem 283(17):11477–11484. doi:M708385200 [pii] 10.1074/jbc.M708385200
Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ (2005) Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci U S A 102(1):105–110. doi:0408352102 [pii] 10.1073/pnas.0408352102
Wang X, Olberding KE, White C, Li C (2010) Bcl-2 proteins regulate ER membrane permeability to luminal proteins during ER stress-induced apoptosis. Cell Death Differ 18(1):38–47. doi:cdd201068 [pii] 10.1038/cdd.2010.68
Ullman E, Fan Y, Stawowczyk M, Chen HM, Yue Z, Zong WX (2008) Autophagy promotes necrosis in apoptosis-deficient cells in response to ER stress. Cell Death Differ 15(2):422–425. doi:4402234 [pii] 10.1038/sj.cdd.4402234
Buytaert E, Callewaert G, Vandenheede JR, Agostinis P (2006) Deficiency in apoptotic effectors Bax and Bak reveals an autophagic cell death pathway initiated by photodamage to the endoplasmic reticulum. Autophagy 2(3):238–240. doi:2730 [pii]
Scorrano L, Oakes SA, Opferman JT, Cheng EH, Sorcinelli MD, Pozzan T, Korsmeyer SJ (2003) BAX and BAK regulation of endoplasmic reticulum Ca2+ : a control point for apoptosis. Science 300(5616):135–139. doi:10.1126/science.1081208 1081208 [pii]
Zhang D, Armstrong JS (2007) Bax and the mitochondrial permeability transition cooperate in the release of cytochrome c during endoplasmic reticulum-stress-induced apoptosis. Cell Death Differ 14(4):703–715. doi:4402072 [pii] 10.1038/sj.cdd.4402072
Mathai JP, Germain M, Shore GC (2005) BH3-only BIK regulates BAX, BAK-dependent release of Ca2+ from endoplasmic reticulum stores and mitochondrial apoptosis during stress-induced cell death. J Biol Chem 280(25):23829–23836. doi:M500800200 [pii] 10.1074/jbc.M500800200
Luo X, He Q, Huang Y, Sheikh MS (2005) Transcriptional upregulation of PUMA modulates endoplasmic reticulum calcium pool depletion-induced apoptosis via Bax activation. Cell Death Differ 12(10):1310–1318. doi:4401659 [pii] 10.1038/sj.cdd.4401659
Zhang L, Li L, Liu H, Borowitz JL, Isom GE (2009) BNIP3 mediates cell death by different pathways following localization to endoplasmic reticulum and mitochondrion. FASEB J 23(10):3405–3414. doi:fj.08-124354 [pii] 10.1096/fj.08-124354
Klee M, Pallauf K, Alcala S, Fleischer A, Pimentel-Muinos FX (2009) Mitochondrial apoptosis induced by BH3-only molecules in the exclusive presence of endoplasmic reticular Bak. Embo J 28(12):1757–1768. doi:emboj200990 [pii] 10.1038/emboj.2009.90
Breckenridge DG, Stojanovic M, Marcellus RC, Shore GC (2003) Caspase cleavage product of BAP31 induces mitochondrial fission through endoplasmic reticulum calcium signals, enhancing cytochrome c release to the cytosol. J Cell Biol 160(7):1115–1127. doi:10.1083/jcb.200212059 jcb.200212059 [pii]
Ng FW, Nguyen M, Kwan T, Branton PE, Nicholson DW, Cromlish JA, Shore GC (1997) p28 Bap31, a Bcl-2/Bcl-XL- and procaspase-8-associated protein in the endoplasmic reticulum. J Cell Biol 139(2):327–338
Ullman E, Pan JA, Zong WX (2011) Squamous cell carcinoma antigen 1 promotes caspase-8-mediated apoptosis in response to endoplasmic reticulum stress while inhibiting necrosis induced by lysosomal injury. Mol Cell Biol 31(14):2902–2919. doi:MCB.05452-11 [pii] 10.1128/MCB.05452-11
Pinton P, Brini M, Bastianutto C, Tuft RA, Pozzan T, Rizzuto R (1998) New light on mitochondrial calcium. Biofactors 8(3–4):243–253
Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T (1998) Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280(5370):1763–1766
Vance JE (1990) Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265(13):7248–7256
Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, Zollo M, Borsani G, Ballabio A, Renieri A (1998) FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics 47(3):350–358. doi:S0888-7543(97)95104-1 [pii] 10.1006/geno.1997.5104
Stone SJ, Vance JE (2000) Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem 275(44):34534–34540. doi:10.1074/jbc.M002865200 [pii]
Rizzuto R, Pozzan T (2006) Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev 86(1):369–408. doi:86/1/369 [pii] 10.1152/physrev.00004.2005
Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol 175(6):901–911. doi:jcb.200608073 [pii] 10.1083/jcb.200608073
Hayashi T, Su TP (2007) Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell 131(3):596–610. doi:S0092-8674(07)01099-9 [pii] 10.1016/j.cell.2007.08.036
Chami M, Oules B, Szabadkai G, Tacine R, Rizzuto R, Paterlini-Brechot P (2008) Role of SERCA1 truncated isoform in the proapoptotic calcium transfer from ER to mitochondria during ER stress. Mol Cell 32(5):641–651. doi:S1097-2765(08)00809-5 [pii] 10.1016/j.molcel.2008.11.014
Verfaillie et al (2012) Cell death and differentiation. doi:10.1038/cdd.2012.74 (In press)
Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, Forte M, d’Azzo A (2009) GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell 36(3):500–511. doi:S1097-2765(09)00786-2 [pii] 10.1016/j.molcel.2009.10.021
Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D (2000) Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5(5):897–904
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7(6):1165–1176
Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR (2006) PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4(6):491–497. doi:S1550-4131(06)00364-0 [pii] 10.1016/j.cmet.2006.11.002
Bi M, Naczki C, Koritzinsky M, Fels D, Blais J, Hu N, Harding H, Novoa I, Varia M, Raleigh J, Scheuner D, Kaufman RJ, Bell J, Ron D, Wouters BG, Koumenis C (2005) ER stress-regulated translation increases tolerance to extreme hypoxia and promotes tumor growth. EMBO J 24(19):3470–3481. doi:7600777 [pii] 10.1038/sj.emboj.7600777
Lu PD, Jousse C, Marciniak SJ, Zhang Y, Novoa I, Scheuner D, Kaufman RJ, Ron D, Harding HP (2004) Cytoprotection by pre-emptive conditional phosphorylation of translation initiation factor 2. Embo J 23(1):169–179. doi:10.1038/sj.emboj.7600030 7600030 [pii]
Lin JH, Li H, Zhang Y, Ron D, Walter P (2009) Divergent effects of PERK and IRE1 signaling on cell viability. PLoS One 4 (1):e4170. doi:10.1371/journal.pone.0004170
Mounir Z, Krishnamoorthy JL, Wang S, Papadopoulou B, Campbell S, Muller WJ, Hatzoglou M, Koromilas AE (2011) Akt Determines Cell Fate Through Inhibition of the PERK-eIF2{alpha} Phosphorylation Pathway. Sci Signal 4 (192):ra62. doi:4/192/ra62 [pii] 10.1126/scisignal.2001630
Okada T, Yoshida H, Akazawa R, Negishi M, Mori K (2002) Distinct roles of activating transcription factor 6 (ATF6) and double-stranded RNA-activated protein kinase-like endoplasmic reticulum kinase (PERK) in transcription during the mammalian unfolded protein response. Biochem J 366(Pt 2):585–594. doi:10.1042/BJ20020391 BJ20020391 [pii]
Ma Y, Brewer JW, Diehl JA, Hendershot LM (2002) Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318(5):1351–1365
Song B, Scheuner D, Ron D, Pennathur S, Kaufman RJ (2008) Chop deletion reduces oxidative stress, improves beta cell function, and promotes cell survival in multiple mouse models of diabetes. J Clin Invest 118(10):3378–3389. doi:10.1172/JCI34587
Zinszner H, Kuroda M, Wang X, Batchvarova N, Lightfoot RT, Remotti H, Stevens JL, Ron D (1998) CHOP is implicated in programmed cell death in response to impaired function of the endoplasmic reticulum. Genes Dev 12(7):982–995
Silva RM, Ries V, Oo TF, Yarygina O, Jackson-Lewis V, Ryu EJ, Lu PD, Marciniak SJ, Ron D, Przedborski S, Kholodilov N, Greene LA, Burke RE (2005) CHOP/GADD153 is a mediator of apoptotic death in substantia nigra dopamine neurons in an in vivo neurotoxin model of parkinsonism. J Neuroche 95(4):974–986
Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ (2006) Adaptation to ER stress is mediated by differential stabilities of pro-survival and pro-apoptotic mRNAs and proteins. PLoS Biol 4(11):e374. doi:06-PLBI-RA-0091R3 [pii] 10.1371/journal.pbio.0040374
Palam LR, Baird TD, Wek RC (2011) Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem 286(13):10939–10949. doi:M110.216093 [pii] 10.1074/jbc.M110.216093
Maytin EV, Ubeda M, Lin JC, Habener JF (2001) Stress-inducible transcription factor CHOP/gadd153 induces apoptosis in mammalian cells via p38 kinase-dependent and -independent mechanisms. Exp Cell Res 267(2):193–204. doi:10.1006/excr.2001.5248 S0014-4827(01)95248-6 [pii]
Youle RJ, Strasser A (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9(1):47–59. doi:nrm2308 [pii] 10.1038/nrm2308
McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ (2001) Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21(4):1249–1259
Chiribau CB, Gaccioli F, Huang CC, Yuan CL, Hatzoglou M (2010) Molecular symbiosis of CHOP and C/EBP beta isoform LIP contributes to endoplasmic reticulum stress-induced apoptosis. Mol Cell Biol 30(14):3722–3731. doi:MCB.01507-09 [pii] 10.1128/MCB.01507-09
Fu HY, Okada K, Liao Y, Tsukamoto O, Isomura T, Asai M, Sawada T, Okuda K, Asano Y, Sanada S, Asanuma H, Asakura M, Takashima S, Komuro I, Kitakaze M, Minamino T (2010) Ablation of C/EBP homologous protein attenuates endoplasmic reticulum-mediated apoptosis and cardiac dysfunction induced by pressure overload. Circulation 122(4):361–369. doi:CIRCULATIONAHA.109.917914 [pii] 10.1161/CIRCULATIONAHA.109.917914
Puthalakath H, O’Reilly LA, Gunn P, Lee L, Kelly PN, Huntington ND, Hughes PD, Michalak EM, McKimm-Breschkin J, Motoyama N, Gotoh T, Akira S, Bouillet P, Strasser A (2007) ER stress triggers apoptosis by activating BH3-only protein Bim. Cell 129(7):1337–1349. doi:S0092-8674(07)00537-5 [pii] 10.1016/j.cell.2007.04.027
Gupta et al (2012) Cell death and disease. (In Press)
Cazanave SC, Elmi NA, Akazawa Y, Bronk SF, Mott JL, Gores GJ (2010) CHOP and AP-1 cooperatively mediate PUMA expression during lipoapoptosis. Am J Physiol Gastrointest Liver Physiol 299(1):G236–G243. doi:ajpgi.00091.2010 [pii] 10.1152/ajpgi.00091.2010
Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ (2009) Translation attenuation through eIF2alpha phosphorylation prevents oxidative stress and maintains the differentiated state in beta cells. Cell Metab 10(1):13–26. doi:S1550-4131(09)00160-0 [pii] 10.1016/j.cmet.2009.06.002
Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15(5):767–776. doi:10.1016/j.molcel.2004.08.025 S1097276504005118 [pii]
Santos CX, Tanaka LY, Wosniak J, Laurindo FR (2009) Mechanisms and implications of reactive oxygen species generation during the unfolded protein response: roles of endoplasmic reticulum oxidoreductases, mitochondrial electron transport, and NADPH oxidase. Antioxid Redox Signal 11(10):2409–2427. doi:10.1089/ARS.2009.2625
Malhotra JD, Miao H, Zhang K, Wolfson A, Pennathur S, Pipe SW, Kaufman RJ (2008) Antioxidants reduce endoplasmic reticulum stress and improve protein secretion. Proc Natl Acad Sci U S A 105(47):18525–18530. doi:0809677105 [pii] 10.1073/pnas.0809677105
Li G, Mongillo M, Chin KT, Harding H, Ron D, Marks AR, Tabas I (2009) Role of ERO1-alpha-mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress-induced apoptosis. J Cell Biol 186(6):783–792. doi:jcb.200904060 [pii] 10.1083/jcb.200904060
Timmins JM, Ozcan L, Seimon TA, Li G, Malagelada C, Backs J, Backs T, Bassel-Duby R, Olson EN, Anderson ME, Tabas I (2009) Calcium/calmodulin-dependent protein kinase II links ER stress with Fas and mitochondrial apoptosis pathways. J Clin Invest 119(10):2925–2941. doi:38857 [pii] 10.1172/JCI38857
Brush MH, Weiser DC, Shenolikar S (2003) Growth arrest and DNA damage-inducible protein GADD34 targets protein phosphatase 1 alpha to the endoplasmic reticulum and promotes dephosphorylation of the alpha subunit of eukaryotic translation initiation factor 2. Mol Cell Biol 23(4):1292–1303
Zhou W, Brush MH, Choy MS, Shenolikar S (2011) Association with endoplasmic reticulum promotes proteasomal degradation of GADD34 protein. J Biol Chem 286(24):21687–21696. doi:M110.212787 [pii] 10.1074/jbc.M110.212787
Ohoka N, Yoshii S, Hattori T, Onozaki K, Hayashi H (2005) TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. Embo J 24(6):1243–1255
Jousse C, Deval C, Maurin AC, Parry L, Cherasse Y, Chaveroux C, Lefloch R, Lenormand P, Bruhat A, Fafournoux P (2007) TRB3 inhibits the transcriptional activation of stress-regulated genes by a negative feedback on the ATF4 pathway. J Biol Chem 282(21):15851–15861. doi:M611723200 [pii] 10.1074/jbc.M611723200
Salazar M, Carracedo A, Salanueva IJ, Hernandez-Tiedra S, Lorente M, Egia A, Vazquez P, Blazquez C, Torres S, Garcia S, Nowak J, Fimia GM, Piacentini M, Cecconi F, Pandolfi PP, Gonzalez-Feria L, Iovanna JL, Guzman M, Boya P, Velasco G (2009) Cannabinoid action induces autophagy-mediated cell death through stimulation of ER stress in human glioma cells. J Clin Invest 119(5):1359–1372
Zou CG, Cao XZ, Zhao YS, Gao SY, Li SD, Liu XY, Zhang Y, Zhang KQ (2009) The molecular mechanism of endoplasmic reticulum stress-induced apoptosis in PC-12 neuronal cells: the protective effect of insulin-like growth factor I. Endocrinology 150(1):277–285. doi:en.2008-0794 [pii] 10.1210/en.2008-0794
Lin JH, Li H, Yasumura D, Cohen HR, Zhang C, Panning B, Shokat KM, Lavail MM, Walter P (2007) IRE1 signaling affects cell fate during the unfolded protein response. Science 318(5852):944–949. doi:318/5852/944 [pii] 10.1126/science.1146361
Woehlbier U, Hetz C (2011) Modulating stress responses by the UPRosome: a matter of life and death. Trends Biochem Sci 36(6):329–337. doi:S0968-0004(11)00045-4 [pii] 10.1016/j.tibs.2011.03.001
Yoshida H, Nadanaka S, Sato R, Mori K (2006) XBP1 is critical to protect cells from endoplasmic reticulum stress: evidence from Site-2 protease-deficient Chinese hamster ovary cells. Cell Struct Funct 31(2):117–125. doi:JST.JSTAGE/csf/06016 [pii]
Lee AH, Iwakoshi NN, Anderson KC, Glimcher LH (2003) Proteasome inhibitors disrupt the unfolded protein response in myeloma cells. Proc Natl Acad Sci U S A 100(17):9946–9951. doi:10.1073/pnas.1334037100 [pii]
Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186(3):323–331. doi:jcb.200903014 [pii] 10.1083/jcb.200903014
Han D, Lerner AG, Vande Walle L, Upton JP, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR (2009) IRE1alpha kinase activation modes control alternate endoribonuclease outputs to determine divergent cell fates. Cell 138(3):562–575. doi:S0092-8674(09)00892-7 [pii] 10.1016/j.cell.2009.07.017
Bouchecareilh M, Higa A, Fribourg S, Moenner M, Chevet E (2011) Peptides derived from the bifunctional kinase/RNase enzyme IRE1{alpha} modulate IRE1{alpha} activity and protect cells from endoplasmic reticulum stress. FASEB J. doi:fj.11-182931 [pii] 10.1096/fj.11-182931
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16(11):1345–1355
Yoneda T, Imaizumi K, Oono K, Yui D, Gomi F, Katayama T, Tohyama M (2001) Activation of caspase-12, an endoplastic reticulum (ER) resident caspase, through tumor necrosis factor receptor-associated factor 2-dependent mechanism in response to the ER stress. J Biol Chem 276(17):13935–13940. doi:10.1074/jbc.M010677200 [pii]
Wang XZ, Ron D (1996) Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 272(5266):1347–1349
Lei K, Davis RJ (2003) JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc Natl Acad Sci U S A 100(5):2432–2437. doi:10.1073/pnas.0438011100 [pii]
Yamamoto K, Ichijo H, Korsmeyer SJ (1999) BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol 19(12):8469–8478
Rao RV, Castro-Obregon S, Frankowski H, Schuler M, Stoka V, del Rio G, Bredesen DE, Ellerby HM (2002) Coupling endoplasmic reticulum stress to the cell death program. An Apaf-1-independent intrinsic pathway. J Biol Chem 277(24):21836–21842. doi:10.1074/jbc.M202726200 [pii]
Morishima N, Nakanishi K, Takenouchi H, Shibata T, Yasuhiko Y (2002) An endoplasmic reticulum stress-specific caspase cascade in apoptosis. Cytochrome c-independent activation of caspase-9 by caspase-12. J Biol Chem 277(37):34287–34294. doi:10.1074/jbc.M204973200 [pii]
Saleh M, Vaillancourt JP, Graham RK, Huyck M, Srinivasula SM, Alnemri ES, Steinberg MH, Nolan V, Baldwin CT, Hotchkiss RS, Buchman TG, Zehnbauer BA, Hayden MR, Farrer LA, Roy S, Nicholson DW (2004) Differential modulation of endotoxin responsiveness by human caspase-12 polymorphisms. Nature 429(6987):75–79. doi:10.1038/nature02451 [pii]
Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y, Manabe T, Yamagishi S, Bando Y, Imaizumi K, Tsujimoto Y, Tohyama M (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Abeta-induced cell death. J Cell Biol 165(3):347–356. doi:10.1083/jcb.200310015 [pii]
Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH (2006) Autocrine tumor necrosis factor alpha links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1alpha-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26(8):3071–3084. doi:26/8/3071 [pii] 10.1128/MCB.26.8.3071-3084.2006
Gu F, Nguyen DT, Stuible M, Dube N, Tremblay ML, Chevet E (2004) Protein-tyrosine phosphatase 1B potentiates IRE1 signaling during endoplasmic reticulum stress. J Biol Chem 279(48):49689–49693. doi:C400261200 [pii] 10.1074/jbc.C400261200
Luo D, He Y, Zhang H, Yu L, Chen H, Xu Z, Tang S, Urano F, Min W (2008) AIP1 is critical in transducing IRE1-mediated endoplasmic reticulum stress response. J Biol Chem 283(18):11905–11912. doi:M710557200 [pii] 10.1074/jbc.M710557200
Gupta S, Deepti A, Deegan S, Lisbona F, Hetz C, Samali A (2010) HSP72 protects cells from ER stress-induced apoptosis via enhancement of IRE1alpha-XBP1 signaling through a physical interaction. PLoS Biol 8 (7):e1000410. doi:10.1371/journal.pbio.1000410
Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, Korsmeyer SJ (2006) Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1alpha. Science 312(5773):572–576
Xu Q, Reed JC (1998) Bax inhibitor-1, a mammalian apoptosis suppressor identified by functional screening in yeast. Mol Cell 1(3):337–346. doi:S1097-2765(00)80034-9 [pii]
Lisbona F, Rojas-Rivera D, Thielen P, Zamorano S, Todd D, Martinon F, Glavic A, Kress C, Lin JH, Walter P, Reed JC, Glimcher LH, Hetz C (2009) BAX inhibitor-1 is a negative regulator of the ER stress sensor IRE1alpha. Mol Cell 33(6):679–691. doi:S1097-2765(09)00133-6 [pii] 10.1016/j.molcel.2009.02.017
Teske BF, Wek SA, Bunpo P, Cundiff JK, McClintick JN, Anthony TG, Wek RC (2011) The eIF2 kinase PERK and the integrated stress response facilitate activation of ATF6 during endoplasmic reticulum stress. Mol Biol Cell. doi:mbc.E11-06-0510 [pii] 10.1091/mbc.E11-06-0510
Morishima N, Nakanishi K, Nakano A (2011) Activating transcription factor-6 (ATF6) mediates apoptosis with reduction of myeloid cell leukemia sequence 1 (Mcl-1) via induction of WW domain binding protein 1. J Biol Chem. doi:M111.233502 [pii] 10.1074/jbc.M111.233502
Belmont PJ, Tadimalla A, Chen WJ, Martindale JJ, Thuerauf DJ, Marcinko M, Gude N, Sussman MA, Glembotski CC (2008) Coordination of growth and endoplasmic reticulum stress signaling by regulator of calcineurin 1 (RCAN1), a novel ATF6-inducible gene. J Biol Chem 283(20):14012–14021. doi:M709776200 [pii] 10.1074/jbc.M709776200
Wang HG, Pathan N, Ethell IM, Krajewski S, Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF, Reed JC (1999) Ca2+ -induced apoptosis through calcineurin dephosphorylation of BAD. Science 284(5412):339–343
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Verfaillie, T., Jäger, R., Samali, A., Agostinis, P. (2012). ER Stress Signaling Pathways in Cell Survival and Death. In: Agostinis, P., Afshin, S. (eds) Endoplasmic Reticulum Stress in Health and Disease. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4351-9_3
Download citation
DOI: https://doi.org/10.1007/978-94-007-4351-9_3
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4350-2
Online ISBN: 978-94-007-4351-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)