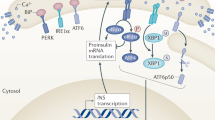
Abstract
Diabetes mellitus is a global chronic disease, major cause of morbidity and mortality, and significantly decreases both quality of life and life expectancy. The reduction in functional β cell mass due to increased β cell apoptosis and decreased β cell proliferation is a crucial factor in the pathogenesis of diabetes mellitus. Mounting clinical and experimental research findings suggest that endoplasmic reticulum (ER) stress and activation of the unfolded protein response (UPR) play fundamental roles in the diminution of functional β cell mass during the prediabetic phase. In this chapter, the physiological purpose of ER stress in the pancreatic β cell is reviewed and the pathological features of chronic ER stress and unwarranted UPR activation in the progression to β cell dysfunction and diabetes progression are described. The molecular pathways activated during the transition from physiological to pathological UPR and proapoptotic signaling in a variety of environmental and genetic diabetic conditions are addressed. In addition, therapeutic approaches to modulate the level of β cell ER stress and mitigate UPR activation are discussed. Finally, we propose that the identification of clinical biomarkers for detection of overt ER stress and UPR activation would herald ER stress and the UPR as viable targets in the prevention of diabetes progression or treatment of established diabetes.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- ADA:
-
American Diabetes Association
- ATF4/6:
-
Activating Transcription Factor 4/6
- ATP/ADP:
-
Adenosine Triphosphate/Adenosine Diphosphate
- BMI:
-
Body Mass Index
- CHOP:
-
C/EBP Homologous Protein
- CREBH:
-
cyclic AMP response element-binding protein H
- eIF2α:
-
eukaryotic Initiation Factor-2α
- ER:
-
Endoplasmic Reticulum
- FAD:
-
flavin adenine dinucleotide
- FBG:
-
Fasting Blood Glucose
- FFAs:
-
Free Fatty Acids
- GRP78:
-
Glucose Regulated protein 78
- GSK3β:
-
glycogen synthase kinase 3β
- GWAS:
-
genome-wide association studies
- IAPP:
-
islet amyloid polypeptide
- IFN-γ:
-
interferon-γ
- IKK:
-
inhibitor of κB
- IL-1β:
-
interleukin-1β
- IRE1:
-
Inositol Requiring Enzyme 1
- JNK:
-
c-Jun N-terminal Kinase
- K+:
-
Potassium
- MIDYyouth:
-
mutant INS-gene-induced diabetes of
- NF-κB:
-
Nuclear Factor κ-light-chain enhancer of activated B cells
- Nrf2:
-
Nuclear factor-E2-related factor 2
- OGTT:
-
oral glucose tolerance test
- PDI:
-
Protein Disulphide Isomerase
- PERK:
-
double stranded RNA-activated protein kinase (PKR)–like ER Kinase
- PND:
-
Permanent Neonatal Diabetes
- RIDD:
-
Regulated IRE1 Dependent Decay
- ROS:
-
Reactive Oxygen Species
- SERCA:
-
Sarco/Endoplasmic Reticulum Ca2+ ATPase
- TRAF2:
-
Tumor necrosis factor receptor (TNFR) Associated Factor 2
- TUDCA:
-
tauro-ursodeoxycholic acid
- UPR:
-
Unfolded Protein Response
- WFS1:
-
Wolframin 1
- WHO:
-
World Health Organization
- XBP1:
-
X-box Binding Protein 1
References
Diabetes Statistics—American Diabetes Association http://www.diabetes.org/diabetes-basics/diabetes-statistics
WHO | Diabetes http://www.who.int/mediacentre/factsheets/fs312/en/index.html
Oslowski CM, Urano F (2011) The binary switch that controls the life and death decisions of ER stressed beta cells. Curr Opin Cell Biol 23(2):207–215
Fonseca SG, Gromada J, Urano F (2011) Endoplasmic reticulum stress and pancreatic beta-cell death. Trends Endocrinol Metab 22(7):266–274
Ron D, Walter P (2007) Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8(7):519–529
Tillmar L, Carlsson C, Welsh N (2002) Control of insulin mRNA stability in rat pancreatic islets. Regulatory role of a 3’-untranslated region pyrimidine-rich sequence. J Biol Chem 277(2):1099–1106
Schuit FC, In’t Veld PA, Pipeleers DG (1988) Glucose stimulates proinsulin biosynthesis by a dose-dependent recruitment of pancreatic beta cells. Proc Natl Acad Sci U S A 85(11):3865–3869
Scheuner DJ, Kaufman RJ (2008) The unfolded protein response: a pathway that links insulin demand with β-cell failure and diabetes. Endocr Rev 29(3):317–333
Blundell TL, Dodson GG, Dodson E, Hodgkin DC, Vijayan M (1971) X-ray analysis and the structure of insulin. Recent Prog Horm Res 27:1–40
MacDonald PE, Joseph JW, Rorsman P (2005) Glucose-sensing mechanisms in pancreatic β-cells. Philos Trans R Soc B Biol Sci 360(1464):2211–2225
Joslin EP, Kahn CR (2005) Joslin’s diabetes mellitus. Lippincott Williams & Wilkins, 9780781727969
Cardozo AK, Ortis F, Storling J, Feng Y-M, Rasschaert J, Tonnesen M, Van Eylen F, Mandrup-Poulsen T, Herchuelz A, Eizirik DL (2005) Cytokines downregulate the sarcoendoplasmic reticulum pump Ca2+ ATPase 2b and deplete endoplasmic reticulum Ca2+, leading to induction of endoplasmic reticulum stress in pancreatic beta-cells. Diabetes 54(2):452–461
Cunha DA, Hekerman P, Ladriere L, Bazarra-Castro A, Ortis F, Wakeham MC, Moore F, Rasschaert J, Cardozo AK, Bellomo E, Hai T, Herchuelz A, Marchetti P, Rutter GA, Eizirik DL, Cnop M (2008) Initiation and execution of lipotoxic ER stress in pancreatic β-cells. J Cell Sci 121:2308–2318
Kharroubi I, Ladriere L, Dogusan Z, Cnop M, Eizirik DcL (2004) Free fatty acids and cytokines induce pancreatic beta-cell apoptosis by different mechanisms: role of nuclear factor-kappaB and endoplasmic reticulum stress. Endocrinology 145(11):5087–5096
Lipson KL, Ghosh R, Urano F (2008) The role of IRE1alpha in the degradation of insulin mRNA in pancreatic beta-cells. PloS One 3(2):e1648
Lipson KL, Fonseca SG, Ishigaki S, Nguyen LX, Foss E, Bortell R, Rossini AA, Urano F (2006) Regulation of insulin biosynthesis in pancreatic beta cells by an endoplasmic reticulum-resident protein kinase IRE1. Cell Metab 4(3):245–254
Pirot P, Eizirik DL, Cardozo AK (2006) Interferon-gamma potentiates endoplasmic reticulum stress-induced death by reducing pancreatic beta cell defence mechanisms. Diabetologia 49(6):1229–1236
Han D, Lerner AG, Vande Walle L, Upton J-P, Xu W, Hagen A, Backes BJ, Oakes SA, Papa FR (2009) IRE1alpha Kinase Activation Modes Control Alternate Endoribonuclease Outputs to Determine Divergent Cell Fates. Cell 138(3):562–575.
Urano F, Wang X, Bertolotti A, Zhang Y, Chung P, Harding HP, Ron D (2000) Coupling of stress in the ER to activation of JNK protein kinases by transmembrane protein kinase IRE1. Science 287(5453):664–666 (New York, NY)
Nishitoh H, Matsuzawa A, Tobiume K, Saegusa K, Takeda K, Inoue K, Hori S, Kakizuka A, Ichijo H (2002) ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev 16(11):1345–1355
Giacca A, Xiao C, Oprescu AI, Carpentier AC, Lewis GF (2011) Lipid-induced pancreatic β-cell dysfunction: focus on in vivo studies. Am J Physiol Endocrinol Metab 300(2):E255–262
Lai E, Bikopoulos G, Wheeler MB, Rozakis-Adcock M, Volchuk A (2008) Differential activation of ER stress and apoptosis in response to chronically elevated free fatty acids in pancreatic beta-cells. Am J Physiol Metab 294(3):E540–550
Cnop M, Ladriere L, Hekerman P, Ortis F, Cardozo AK, Dogusan Z, Flamez D, Boyce M, Yuan J, Eizirik DL (2007) Selective inhibition of eukaryotic translation initiation factor 2 alpha dephosphorylation potentiates fatty acid-induced endoplasmic reticulum stress and causes pancreatic beta-cell dysfunction and apoptosis. J Biol Chem 282(6):3989–3997
Karaskov E, Scott C, Zhang L, Teodoro T, Ravazzola M, Volchuk A (2006) Chronic palmitate but not oleate exposure induces endoplasmic reticulum stress, which may contribute to INS-1 pancreatic beta-cell apoptosis. Endocrinology 147(7):3398–3407
Laybutt DR, Preston AM, Akerfeldt MC, Kench JG, Busch AK, Biankin AV, Biden TJ (2007) Endoplasmic reticulum stress contributes to beta cell apoptosis in type 2 diabetes. Diabetologia 50(4):752–763
Tanabe K, Liu Y, Hasan SD, Martinez SC, Cras-Meneur C, Welling CM, Bernal-Mizrachi E, Tanizawa Y, Rhodes CJ, Zmuda E, Abumrad NA, Permutt MA (2011) Glucose and fatty acids synergize to promote B-cell apoptosis through activation of glycogen synthase kinase 3β independent of JNK Activation. PloS One 6(4):e18146
Edghill EL, Flanagan SE, Patch A-M, Boustred C, Parrish A, Shields B, Shepherd MH, Hussain K, Kapoor RR, Malecki M, MacDonald MJ, Støy J, Steiner DF, Philipson LH, Bell GI, Hattersley AT, Ellard S (2008) Insulin mutation screening in 1,044 patients with diabetes: mutations in the INS gene are a common cause of neonatal diabetes but a rare cause of diabetes diagnosed in childhood or adulthood. Diabetes 57(4):1034–1042
Stoy J, Edghill EL, Flanagan SE, Paz VP, Pluzhnikov A, Below JE, Hayes MG, Cox NJ, Lipkind GM, Lipton RB, Greeley SAW, Patch A-M, Ellard S, Steiner DF, Hattersley AT, Philipson LH, Bell GI, Neonatal Diabetes International Collaboative G (2007) Insulin gene mutations as a cause of permanent neonatal diabetes. PNAS 104(38):15040–15044
Wang J, Takeuchi T, Tanaka S, Kubo SK, Kayo T, Lu D, Takata K, Koizumi A, Izumi T (1999) A mutation in the insulin 2 gene induces diabetes with severe pancreatic beta-cell dysfunction in the Mody mouse. J Clin Invest 103(1):27–37
Herbach N, Rathkolb B, Kemter E, Pichl L, Klaften M, de Angelis MH, Halban PA, Wolf E, Aigner B, Wanke R (2007) Dominant-negative effects of a novel mutated Ins2 allele causes early-onset diabetes and severe beta-cell loss in Munich Ins2C95S mutant mice. Diabetes 56(5):1268–1276
Liu M, Hodish I, Haataja L, Lara-Lemus R, Rajpal G, Wright J, Arvan P (2010) Proinsulin misfolding and diabetes: mutant INS gene-induced diabetes of youth. Trends Endocrinol Metab 21(11):652–659
Liu M, Haataja L, Wright J, Wickramasinghe NP, Hua QX, Phillips NF, Barbetti F, Weiss MA, Arvan P (2011) Mutant INS-gene induced diabetes of youth: proinsulin cysteine residues impose dominant-negative inhibition on wild-type proinsulin transport. PLoS One 5(10):e13333.
Riggs AC, Bernal-Mizrachi E, Ohsugi M, Wasson J, Fatrai S, Welling C, Murray J, Schmidt RE, Herrera PL, Permutt MA (2005) Mice conditionally lacking the Wolfram gene in pancreatic islet beta cells exhibit diabetes as a result of enhanced endoplasmic reticulum stress and apoptosis. Diabetologia 48(11):2313–2321
Sandhu MS, Weedon MN, Fawcett KA, Wasson J, Debenham SL, Daly A, Lango H, Frayling TM, Neumann RJ, Sherva R, Blech I, Pharoah PD, Palmer CNA, Kimber C, Tavendale R, Morris AD, McCarthy MI, Walker M, Hitman G, Glaser B, Permutt MA, Hattersley AT, Wareham NJ, Barroso I (2007) Common variants in WFS1 confer risk of type 2 diabetes. Nat Genet 39(8):951–953
Wasson J, Permutt MA (2008) Candidate gene studies reveal that the WFS1 gene joins the expanding list of novel type 2 diabetes genes. Diabetologia 51(3):391–393
Harding HP, Zeng H, Zhang Y, Jungries R, Chung P, Plesken H, Sabatini DD, Ron D (2001) Diabetes mellitus and exocrine pancreatic dysfunction in perk-/- mice reveals a role for translational control in secretory cell survival. Mol Cell 7(6):1153–1163
Zhang W, Feng D, Li Y, Iida K, McGrath B, Cavener DR (2006) PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metab 4(6):491–497
Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ (2001) Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7(6):1165–1176
Ladiges WC, Knoblaugh SE, Morton JF, Korth MJ, Sopher BL, Baskin CR, MacAuley A, Goodman AG, LeBoeuf RC, Katze MG (2005) Pancreatic beta-cell failure and diabetes in mice with a deletion mutation of the endoplasmic reticulum molecular chaperone gene P58IPK. Diabetes 54(4):1074–1081
Ritzel RA, Meier JJ, Lin CY, Veldhuis JD, Butler PC (2007) Human islet amyloid polypeptide oligomers disrupt cell coupling, induce apoptosis, and impair insulin secretion in isolated human islets. Diabetes 56(1):65–71
Sawaya MR, Sambashivan S, Nelson R, Ivanova MI, Sievers SA, Apostol MI, Thompson MJ, Balbirnie M, Wiltzius JJ, McFarlane HT, Madsen AO, Riekel C, Eisenberg D (2007) Atomic structures of amyloid cross-beta spines reveal varied steric zippers. Nature 447(7143):453–457
Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC (2007) High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes 56(8):2016–2027
Matveyenko AV, Gurlo T, Daval M, Butler AE, Butler PC (2009) Successful versus failed adaptation to high-fat diet-induced insulin resistance: the role of IAPP-induced beta-cell endoplasmic reticulum stress. Diabetes 58(4):906–916.
Malhotra JD, Kaufman RJ (2007) Endoplasmic reticulum stress and oxidative stress: a vicious cycle or a double-edged sword? Antioxid Redox Signal 9(12):2277–2293
Haynes CM, Titus EA, Cooper AA (2004) Degradation of misfolded proteins prevents ER-derived oxidative stress and cell death. Mol Cell 15(5):767–776
Cullinan SB, Diehl JA (2006) Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol 38(3):317–332
Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GkS (2006) Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci U S A 103(28):10741–10746
Deng J, Lu PD, Zhang Y, Scheuner D, Kaufman RJ, Sonenberg N, Harding HP, Ron D (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24(23):10161–10168
Hu P, Han Z, Couvillon AD, Exton JH (2004) Critical Role of Endogenous Akt/IAPs and MEK1/ERK Pathways in Counteracting Endoplasmic Reticulum Stress-induced Cell Death. J Biol Chem 279(47):49420–49429
Shoelson SE, Lee J, Goldfine AB (2006) Inflammation and insulin resistance. J Clin Invest 116(7):1793–1801
Jiang H-Y, Wek SA, McGrath BC, Scheuner D, Kaufman RJ, Cavener DR, Wek RC (2003) Phosphorylation of the alpha subunit of eukaryotic initiation factor 2 is required for activation of NF-kappaB in response to diverse cellular stresses. Mol Cell Biol 23(16):5651–5663
Yamazaki H, Hiramatsu N, Hayakawa K, Tagawa Y, Okamura M, Ogata R, Huang T, Nakajima S, Yao J, Paton AW, Paton JC, Kitamura M (2009) Activation of the Akt-NF-kappaB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. J Immunol 183(2):1480–1487 (Baltimore, Md: 1950)
Zhang K, Shen X, Wu J, Sakaki K, Saunders T, Rutkowski DT, Back SH, Kaufman RJ (2006) Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell 124(3):587–599
Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, Mullooly N, Mielke LA, Harris J, Coll RC, Mills KHG, Mok KH, Newsholme P, Nuñez G, Yodoi J, Kahn SE, Lavelle EC, O’Neill LAJ (2010) Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1β in type 2 diabetes. Nat Immunol 11(10):897–904
Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgün CZ, Hotamisligil GS (2006) Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313(5790):1137–1140 (New York, NY)
Malo A, Kruger B, Seyhun E, Schafer C, Hoffmann RT, Goke B, Kubisch CH (2010) Tauroursodeoxycholic acid reduces endoplasmic reticulum stress, trypsin activation, and acinar cell apoptosis while increasing secretion in rat pancreatic acini. Am J Physiol Gastrointest Liver Physiol 299(4):G877–886
Lee YY, Hong SH, Lee YJ, Chung SS, Jung HS, Park SG, Park KS (2010) Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem Biophys Res Commun 397(4):735–739
Bouchecareilh M, Higa A, Fribourg S, Moenner M, Chevet E (2011) Peptides derived from the bifunctional kinase/RNase enzyme IRE1α modulate IRE1α activity and protect cells from endoplasmic reticulum stress. Faseb J 25(9):3115–3129
Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS (2009) Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186(3):323–331
Fonseca SG, Fukuma M, Lipson KL, Nguyen LX, Allen JR, Oka Y, Urano F (2005) WFS1 is a novel component of the unfolded protein response and maintains homeostasis of the endoplasmic reticulum in pancreatic beta-cells. J Biol Chem 280(47):39609–39615
Hatanaka M, Tanabe K, Yanai A, Ohta Y, Kondo M, Akiyama M, Shinoda K, Oka Y, Tanizawa Y (2011) Wolfram syndrome 1 gene (WFS1) product localizes to secretory granules and determines granule acidification in pancreatic beta-cells. Hum Mol Genet 20(7):1274–1284
Acknowledgements
Work in the laboratory of F. Urano is supported by grants from NIH-NIDDK (R01DK067493), the Diabetes and Endocrinology Research Center at the University of Massachusetts Medical School (5 P30 DK32520), and the Juvenile Diabetes Research Foundation International (1-2008-593 and 40-2011-14).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2012 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Urano, F., O’Sullivan-Murphy, B. (2012). Pathological ER Stress in β Cells. In: Agostinis, P., Afshin, S. (eds) Endoplasmic Reticulum Stress in Health and Disease. Springer, Dordrecht. https://doi.org/10.1007/978-94-007-4351-9_9
Download citation
DOI: https://doi.org/10.1007/978-94-007-4351-9_9
Published:
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-007-4350-2
Online ISBN: 978-94-007-4351-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)