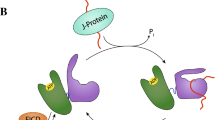
Abstract
Molecular chaperones assist the folding of nascent chains in the cell. Chaperones also aid in quality control decisions as persistent chaperone binding can help to sort terminal misfolded proteins for degradation. There are two major molecular chaperone families in the endoplasmic reticulum (ER) that assist proteins in reaching their native structure and evaluating the fidelity of the maturation process. The ER Hsp70 chaperone, BiP, supports adenine nucleotide-regulated binding to non-native proteins that possess exposed hydrophobic regions. In contrast, the carbohydrate-dependent chaperone system involving the membrane protein calnexin and its soluble paralogue calreticulin recognize a specific glycoform of an exposed hydrophilic protein modification for which the composition is controlled by a series of glycosidases and transferases. Here, we compare and contrast the properties, mechanisms of action and functions of these different chaperones systems that work in parallel, as well as together, to assist a large variety of substrates that traverse the eukaryotic secretory pathway.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
Abbreviations
- AMP:
-
Adenosine 5′-monophosphate
- ATP:
-
Adenosine triphosphate
- BiP:
-
Binding-immunoglobulin protein
- CNX:
-
Calnexin
- CRT:
-
Calreticulin
- CypB:
-
Cyclophilin B
- ER:
-
Endoplasmic reticulum
- ERdj:
-
ER-localized DNAJ
- NBD:
-
Nucleotide binding domain
- PDIA3:
-
Protein disulfide-isomerase A3
- PDIA9:
-
Protein disulfide-isomerase A9
- SBD:
-
Substrate binding domain
- Sep15:
-
Selenoprotein F
- TPR:
-
Tetratrico peptide repeat
- UGGT1(2):
-
UDP-glucose: glycoprotein glucosyltransferase I (II)
References
Adams BM, Ke H, Gierash LM, Gershenson A, Hebert DN (2019a) Proper secretion of the serpin antithrombin relies strictly on thiol-dependent quality control. J Biol Chem 294(50):18992–18911. https://doi.org/10.1074/jbc.RA119.010450
Adams BM, Oster ME, Hebert DN (2019b) Protein quality control in the endoplasmic reticulum. Protein J 38(3):317–329. https://doi.org/10.1007/s10930-019-09831-w
Amin-Wetzel N, Saunders RA, Kamphuis MJ, Rato C, Preissler S, Harding HP, Ron D (2017) A J-protein co-chaperone recruits BiP to monomerize IRE1 and repress the unfolded protein response. Cell 171(7):1625–1637.e13. https://doi.org/10.1016/j.cell.2017.10.040
Andréasson C, Rampelt H, Fiaux J, Druffel-Augustin S, Bukau B (2010) The endoplasmic reticulum Grp170 acts as a nucleotide exchange factor of Hsp70 via a mechanism similar to that of the cytosolic Hsp110. J Biol Chem 285(16):12445–12453. https://doi.org/10.1074/jbc.M109.096735
Anfinsen CB (1973) Principles that govern the folding of protein chains. Science 167(3919):886–887. https://doi.org/10.1126/science.181.4096.223
Avezov E, Frenkel Z, Ehrlich M, Herscovics A, Lederkremer GZ (2008) Endoplasmic reticulum (ER) mannosidase i is compartmentalized and required for N-glycan trimming to Man5–6GlcNAc2 in glycoprotein ER-associated degradation. Mol Biol Cell 19(1):216–225. https://doi.org/10.1091/mbc.E07-05-0505
Balchin D, Hayer-Hartl M, Ulrich Hartl F (2020) Recent advances in understanding catalysis of protein folding by molecular chaperones. FEBS Lett 594(17):2770–2781. https://doi.org/10.1002/1873-3468.13844
Behnke J, Hendershot LM (2014) The large Hsp70 Grp170 binds to unfolded protein substrates in vivo with a regulation distinct from conventional Hsp70s. J Biol Chem 289(5):2899–2907. https://doi.org/10.1074/jbc.M113.507491
Behnke J, Feige MJ, Hendershot LM (2015) BiP and its nucleotide exchange factors Grp170 and Sil1: mechanisms of action and biological functions. J Mol Biol 427(7):1589–1608. https://doi.org/10.1016/j.jmb.2015.02.011
Behnke J, Mann MJ, Scruggs F-L, Feige MJ, Hendershot LM (2016) Members of the Hsp70 family recognize distinct types of sequences to execute ER quality control. Mol Cell 63(5):739–752. https://doi.org/10.1016/j.molcel.2016.07.012
Blees A, Januliene D, Hofmann T, Koller N, Schmidt C, Trowitzsch S, Moeller A, Tampé R (2017) Structure of the human MHC-I peptide-loading complex. Nature 551(7681):525–528. https://doi.org/10.1038/nature24627
Blond-Elguindi S, Cwirla SE, Dower WJ, Lipshutz RJ, Sprang SR, Sambrook JF, Gething M-J (1993) Affinity panning of a library of peptides displayed on bacteriophages reveals the binding specificity of BiP. Cell 75(4):717–728. https://doi.org/10.1016/0092-8674(93)90492-9
Broncel M, Serwa RA, Bunney TD, Katan M, Tate EW (2016) Global profiling of huntingtin-associated protein E (HYPE)-mediated AMPylation through a chemical proteomic approach. Mol Cell Proteomics 15(2):715–725. https://doi.org/10.1074/mcp.O115.054429
Cameron PH, Chevet E, Pluquet O, Thomas DY, Bergeron JJM (2009) Calnexin phosphorylation attenuates the release of partially misfolded 1-antitrypsin to the secretory pathway. J Biol Chem 284(50):34570–34579. https://doi.org/10.1074/jbc.M109.053165
Caramelo JJ, Castro OA, de Prat-Gay G, Parodi AJ (2004) The endoplasmic reticulum glucosyltransferase recognizes nearly native glycoprotein folding intermediates. J Biol Chem 279(44):46280–46285. https://doi.org/10.1074/jbc.M408404200
Chang Y-W, Sun Y-J, Chung W, Hsiao C-D (2008) Crystal structures of the 70-KDa heat shock proteins in domain disjoining conformation. J Biol Chem 283(22):15502–15511. https://doi.org/10.1074/jbc.M708992200
Chen K-C, Qu S, Chowdhury S, Noxon IC, Schonhoft JD, Plate L, Powers ET, Kelly JW, Lander GC, Wiseman RL (2017) The endoplasmic reticulum HSP40 co-chaperone ERdj3/DNAJB11 assembles and functions as a tetramer. EMBO J 36(15):2296–2309. https://doi.org/10.15252/embj.201695616
Cherepanova NA, Venev SV, Leszyk JD, Shaffer SA, Gilmore R (2019) Quantitative proteomics reveals new classes of STT3A- and STT3B-dependent N-glycosylation sites. J Cell Biol 218(8):2782–2796. https://doi.org/10.1083/jcb.201904004
Chevet E, Smirle J, Cameron PH, Thomas DY, Bergeron JJM (2010) Calnexin phosphorylation: linking cytoplasmic signalling to endoplasmic reticulum lumenal functions. Semin Cell Dev Biol 21(5):486–490. https://doi.org/10.1016/j.semcdb.2009.12.005
Chung KT, Shen Y, Hendershot LM (2002) BAP, a mammalian BiP-associated protein, is a nucleotide exchange factor that regulates the ATPase activity of BiP. J Biol Chem 277(49):47557–47563. https://doi.org/10.1074/jbc.M208377200
Craven RA, Egerton M, Stirling CJ (1996) A novel Hsp70 of the yeast ER lumen is required for the efficient translocation of a number of protein precursors. EMBO J 15(11):2640–2650. https://doi.org/10.1002/j.1460-2075.1996.tb00624.x
Cunnea PM, Miranda-Vizuete A, Bertoli G, Simmen T, Damdimopoulos AE, Hermann S, Leinonen S et al (2003) ERdj5, an endoplasmic reticulum (ER)-resident protein containing DnaJ and thioredoxin domains, is expressed in secretory cells or following ER stress. J Biol Chem 278(2):1059–1066. https://doi.org/10.1074/jbc.M206995200
Daniels R, Kurowski B, Johnson AE, Hebert DN (2003) N-linked glycans direct the cotranslational folding pathway of influenza hemagglutinin. Mol Cell 11(1):79–90. https://doi.org/10.1016/s1097-2765(02)00821-3
Danilcyk UG, Williams DB (2001) The lectin chaperone calnexin utilizes polypeptide-based interactions to associate with many of its substrates in vivo. J Biol Chem 276(27):25532–25540. https://doi.org/10.1074/jbc.M100270200
Deprez P, Gautschi M, Helenius A (2005) More than one glycan is needed for ER glucosidase II to allow entry of glycoproteins into the calnexin/calreticulin cycle. Mol Cell 19(2):183–195. https://doi.org/10.1016/j.molcel.2005.05.029
Ellgaard L, Riek R, Herrmann T, Güntert P, Braun D, Helenius A, Wüthrich K (2001) NMR structure of the calreticulin P-domain. Proc Natl Acad Sci U S A 98(6):3133–3138. https://doi.org/10.1073/pnas.051630098
Frickel E-M, Riek R, Jelesarov I, Helenius A, Wüthrich K, Ellgaard L (2002) TROSY-NMR reveals interaction between ERp57 and the tip of the calreticulin Pdomain. Proc Natl Acad Sci U S A 99(4):1954–1959. https://doi.org/10.1073/pnas.042699099
Frickel E-M, Frei P, Bouvier M, Stafford WF, Helenius A, Ellgaard L (2004) ERp57 is a multifunctional thiol-disulfide oxidoreductase. J Biol Chem 279(18):18277–18287. https://doi.org/10.1074/jbc.M314089200
Hagiwara M, Maegawa K-i, Suzuki M, Ushioda R, Araki K, Matsumoto Y, Hoseki J, Nagata K, Inaba K (2011) Structural basis of an ERAD pathway mediated by the ER-resident protein disulfide reductase ERdj5. Mol Cell 41(4):432–444. https://doi.org/10.1016/j.molcel.2011.01.021
Hebert DN, Lamriben L, Powers ET, Kelly JW (2014) The intrinsic and extrinsic effects of N-linked glycans on glycoproteostasis. Nat Chem Biol 10(11):902–910. https://doi.org/10.1038/nchembio.1651
Hetz C, Zhang K, Kaufman RJ (2020) Mechanisms, regulation and functions of the unfolded protein response. Nat Rev Mol Cell Biol 21(8):421–438. https://doi.org/10.1038/s41580-020-0250-z
Itzhak DN, Tyanova S, Cox J, Borner GHH (2016) Global, quantitative and dynamic mapping of protein subcellular localization. elife 5:e16950. https://doi.org/10.7554/eLife.16950
Jin Y, Awad W, Petrova K, Hendershot LM (2008) Regulated release of ERdj3 from unfolded proteins by BiP. EMBO J 27(21):2873–2882. https://doi.org/10.1038/emboj.2008.207
Kapoor M, Srinivas H, Kandiah E, Gemma E, Ellgaard L, Oscarson S, Helenius A, Surolia A (2003) Interactions of substrate with calreticulin, an endoplasmic reticulum chaperone. J Biol Chem 278(8):6194–6200. https://doi.org/10.1074/jbc.M209132200
Kasaikina MV, Fomenko DE, Labunskyy VM, Lachke SA, Qiu W, Moncaster JA, Zhang J et al (2011) Roles of the 15-KDa selenoprotein (Sep15) in redox homeostasis and cataract development revealed by the analysis of Sep 15 knockout mice. J Biol Chem 286(38):33203–33212. https://doi.org/10.1074/jbc.M111.259218
Korotkov KV, Kumaraswamy E, Zhou Y, Hatfield DL, Gladyshev VN (2001) Association between the 15-KDa selenoprotein and UDP-glucose: glycoprotein glucosyltransferase in the endoplasmic reticulum of mammalian cells. J Biol Chem 276(18):15330–15336. https://doi.org/10.1074/jbc.M009861200
Kozlov G, Gehring K (2020) Calnexin cycle - structural features of the ER chaperone system. FEBS J 287(20):4322–4340. https://doi.org/10.1111/febs.15330
Kozlov G, Määttänen P, Schrag JD, Pollock S, Cygler M, Nagar B, Thomas DY, Gehring K (2006) Crystal structure of the Bb’ domains of the protein disulfide isomerase ERp57. Structure 14(8):1331–1339. https://doi.org/10.1016/j.str.2006.06.019
Kozlov G, Pocanschi CL, Rosenauer A, Bastos-Aristizabal S, Gorelik A, Williams DB, Gehring K (2010) Structural basis of carbohydrate recognition by calreticulin. J Biol Chem 285(49):38612–38620. https://doi.org/10.1074/jbc.M110.168294
Kozlov G, Muñoz-Escobar J, Castro K, Gehring K (2017) Mapping the ER interactome: the P domains of calnexin and calreticulin as plurivalent adapters for foldases and chaperones. Structure 25(9):1415–1422. https://doi.org/10.1016/j.str.2017.07.010
Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zhetab O, Guigó R, Gladyshev VN (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443. https://doi.org/10.1126/science.1083516
Labunskyy VM, Ferguson AD, Fomenko DE, Chelliah Y, Hatfield DL, Gladyshev VN (2005) A novel cysteine-rich domain of Sep15 mediates the interaction with UDP-glucose: glycoprotein glucosyltransferase. J Biol Chem 280(45):37839–37845. https://doi.org/10.1074/jbc.M508685200
Lakkaraju AKK, Abrami L, Lemmin T, Blaskovic S, Kunz B, Kihara A, Peraro MD, van der Goot F (2012) Palmitoylated calnexin is a key component of the ribosome–translocon complex. Eur Mol Biol Organ J 31(7):1823–1835. https://doi.org/10.1038/emboj.2012.15
Lamriben L, Graham JB, Adams BM, Hebert DN (2016) N-glycan-based ER molecular chaperone and protein quality control system: the calnexin binding cycle. Traffic (Copenhagen, Denmark) 17(4):308–326. https://doi.org/10.1111/tra.12358
Lee D, Kraus A, Prins D, Groenendyk J, Aubry I, Liu W-X, Li H-D et al (2015) UBC9-dependent association between calnexin and protein tyrosine phosphatase 1B (PTP1B) at the endoplasmic reticulum. J Biol Chem 290(9):5725–5738. https://doi.org/10.1074/jbc.M114.635474
Liepinsh E, Baryshev M, Sharipo A, Ingelman-Sundberg M, Otting G, Mkrtchian S (2001) Thioredoxin fold as homodimerization module in the putative chaperone ERp29: NMR structures of the domains and experimental model of the 51 KDa dimer. Structure 9(6):457–571. https://doi.org/10.1016/s0969-2126(01)00607-4
Lum R, Ahmad S, Hong SJ, Chapman DC, Kozlov G, Williams DB (2016) Contributions of the lectin and polypeptide binding sites of calreticulin to its chaperone functions in vitro and in cells. J Biol Chem 291(37):19631–19641. https://doi.org/10.1074/jbc.M116.746321
Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T (2012) Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. Eur Mol Biol Organ J 31(2):457–470. https://doi.org/10.1038/emboj.2011.384
Marcinowski M, Höller M, Feige MJ, Baerend D, Lamb DC, Buchner J (2011) Substrate discrimination of the chaperone BiP by autonomous and cochaperone-regulated conformational transitions. Nat Struct Mol Biol 18(2):150–158. https://doi.org/10.1038/nsmb.1970
Mayer MP, Gierasch LM (2019) Recent advances in the structural and mechanistic aspects of Hsp70 molecular chaperones. J Biol Chem 294(6):2085–2097. https://doi.org/10.1074/jbc.REV118.002810
Melville MW, Tan S-L, Wambach M, Song J, Morimoto RI, Katze MG (1999) The cellular inhibitor of the PKR protein kinase, P58IPK, is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J Biol Chem 274(6):3797–3803. https://doi.org/10.1074/jbc.274.6.3797
Molinari M, Helenius A (2000) Chaperone selection during glycoprotein translocation into the endoplasmic reticulum. Science 288(5464):331–333. https://doi.org/10.1126/science.288.5464.331
Molinari M, Calanca V, Galli C, Lucca P, Paganetti P (2003) Role of EDEM in the release of misfolded glycoproteins from the calnexin cycle. Science 299(5611):1397–1400. https://doi.org/10.1126/science.1079474
Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T (2008) The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19(7):2777–2788. https://doi.org/10.1091/mbc.e07-10-0995
Oda Y, Hosokawa N, Wada I, Nagata K (2003) EDEM as an acceptor of terminally misfolded glycoproteins released from calnexin. Science 299(5611):1394–1397. https://doi.org/10.1126/science.1079181
Ou W-J, Bergeron JJM, Li Y, Kang YC, Thomas DY (1995) Conformational changes induced in the endoplasmic reticulum luminal domain of calnexin by Mg-ATP and Ca2+. J Biol Chem 270(30):18051–18059. https://doi.org/10.1074/jbc.270.30.18051
Perera LA, Rato C, Yan Y, Neidhardt L, McLaughlin SH, Read RJ, Preissler S, Ron D (2019) An oligomeric state-dependent switch in the ER enzyme FICD regulates AMPylation and DeAMPylation of BiP. EMBO J 38(21):e102177–e102177. https://doi.org/10.15252/embj.2019102177
Petrova K, Oyadomari S, Hendershot LM, Ron D (2008) Regulated association of misfolded endoplasmic reticulum lumenal proteins with P58/DNAJc3. EMBO J 27(21):2862–2872. https://doi.org/10.1038/emboj.2008.199
Pobre KFR, Poet GJ, Hendershot LM (2019) The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: getting by with a little help from ERdj friends. J Biol Chem 294(6):2098–2108. https://doi.org/10.1074/jbc.REV118.002804
Preissler S, Chambers JE, Crespillo-Casado A, Avezov E, Miranda E, Perez J, Hendershot LM, Harding HP, Ron D (2015) Physiological modulation of BiP activity by trans-protomer engagement of the interdomain linker. elife 4(Oct):e08961. https://doi.org/10.7554/eLife.08961
Preissler S, Rato C, Perera L, Saudek V, Ron D (2017a) FICD acts bifunctionally to AMPylate and De-AMPylate the endoplasmic reticulum chaperone BiP. Nat Struct Mol Biol 24(1):23–29. https://doi.org/10.1038/nsmb.3337
Preissler S, Rohland L, Yan Y, Chen R, Read RJ, Ron D (2017b) AMPylation targets the rate-limiting step of BiP’s ATPase cycle for its functional inactivation. elife 6(Oct):e29428. https://doi.org/10.7554/eLife.29428
Rajagopalan S, Xu Y, Brenner MB (1994) Retention of unassembled components of integral membrane proteins by calnexin. Science 263(5145):387–390. https://doi.org/10.1126/science.8278814
Rosam M, Krader D, Nickels C, Hochmair J, Back KC, Agam G, Barth A et al (2018) bap (sil1) regulates the molecular chaperone BiP by coupling release of nucleotide and substrate. Nat Struct Mol Biol 25(1):90–100. https://doi.org/10.1038/s41594-017-0012-6
Roth J, Zuber C (2017) Quality control of glycoprotein folding and ERAD: the role of N-glycan handling, EDEM1 and OS-9. Histochem Cell Biol 147:269–284. https://doi.org/10.1007/s00418-016-1513-9
Roversi P, Marti L, Caputo AT, Alonzi DS, Hill JC, Dent KC, Kumar A et al (2017) Interdomain conformational flexibility underpins the activity of UGGT, the eukaryotic glycoprotein secretion checkpoint. Proc Natl Acad Sci U S A 114(32):8544–8549. https://doi.org/10.1073/pnas.1703682114
Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J 16(7):1501–1507. https://doi.org/10.1093/emboj/16.7.1501
Rutkowski DT, Kang S-W, Goodman AG, Garrison JL, Taunton J, Katze MG, Kaufman RJ, Hegde RS (2007) The role of P58IPK in protecting the stressed endoplasmic reticulum. Mol Biol Cell 18(9):3681–3691. https://doi.org/10.1091/mbc.e07-03-0272
Satoh T, Song C, Zhu T, Toshimori T, Murata K, Hayashi Y, Kamikubo H, Uchihashi T, Kato K (2017) Visualization of a flexible modular structure of the ER folding-sensor enzyme UGGT. Sci Rep 7(1):12142. https://doi.org/10.1038/s41598-017-12283-w
Schrag JD, Bergeron JJM, Li Y, Borisova S, Hahn M, Thomas DY, Cygler M (2001) The structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol Cell 8(3):633–644. https://doi.org/10.1016/s1097-2765(01)00318-5
Shen Y, Hendershot LM (2005) ERdj3, a stress-inducible endoplasmic reticulum DnaJ homologue, serves as a cofactor for BiP’s interactions with unfolded substrates. Mol Biol Cell 16(1):40–50. https://doi.org/10.1091/mbc.e04-05-0434
Shrimal S, Cherepanova NA, Mandon ES, Venev SV, Gilmore R (2019) Asparagine-linked glycosylation is not directly coupled to protein translocation across the endoplasmic reticulum in Saccharomyces Cerevisiae. Mol Biol Cell 30(21):2626–2638. https://doi.org/10.1091/mbc.E19-06-0330
Sousa MC, Ferrero-Garcia MA, Parodi AJ (1992) Recognition of the oligosaccharide and protein moieties of glycoproteins by the UDP-Glc:glycoprotein glucosyltransferase. Biochemistry 31(1):97–105. https://doi.org/10.1021/bi00116a015
Spiro RG, Zhu Q, Bhoyroo V, Söling H-D (1996) Definition of the lectin-like properties of the molecular chaperone, calreticulin, and demonstration of its copurification with endomannosidase from rat liver golgi. J Biol Chem 271(19):11588–11594. https://doi.org/10.1074/jbc.271.19.11588
Svärd M, Biterova EI, Bourhis J-M, Guy JE (2011) The crystal structure of the human co-chaperone P58(IPK). PLoS One 6(7):e22337–e22337. https://doi.org/10.1371/journal.pone.0022337
Takeda Y, Seko A, Hachisu M, Daikoku S, Izumi M, Koizumi A, Fujikawa K, Kajihara Y, Ito Y (2014) Both isoforms of human UDP-glucose:glycoprotein glucosyltransferase are enzymatically active. Glycobiology 24(4):344–350. https://doi.org/10.1093/glycob/cwt163
Terajima M, Taga Y, Cabral WA, Nagasawa M, Sumida N, Hattor S, Marini JC, Yamauchi M (2017) Cyclophilin B deficiency causes abnormal dentin collagen matrix. J Proteome Res 16(8):2914–2293. https://doi.org/10.1021/acs.jproteome.7b00190
Ushioda R, Hoseki J, Araki K, Jansen G, Thomas DY, Nagata K (2008) ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science 321(5888):569. https://doi.org/10.1126/science.1159293
Ushioda R, Hoseki J, Nagata K (2013) Glycosylation-independent ERAD pathway serves as a backup system under ER stress. Mol Biol Cell 24(20):3155–3163. https://doi.org/10.1091/mbc.E13-03-0138
Vassilakos A, Michalak M, Lehrman MA, Williams DB (1998) Oligosaccharide binding characteristics of the molecular chaperones calnexin and calreticulin. Biochemistry 37(10):3480–3490. https://doi.org/10.1021/bi972465g
Viviano J, Brecker M, Ferrara-Cook C, Suaud L, Rubenstein RC (2020) ERp29 as a regulator of insulin biosynthesis. PLoS One 15(5):e0233502. https://doi.org/10.1371/journal.pone.0233502
Wang N, Glidden EJ, Murphy SR, Pearse BR, Hebert DN (2008) The cotranslational maturation program for the type II membrane glycoprotein influenza neuraminidase. J Biol Chem 283(49):33826–33837. https://doi.org/10.1074/jbc.M806897200
Wei J, Gaut JR, Hendershot LM (1995) In vitro dissociation of BiP-peptide complexes requires a conformational change in BiP after ATP binding but does not require ATP hydrolysis. J Biol Chem 270(44):26677–26682. https://doi.org/10.1074/jbc.270.44.26677
Wijeyesakere SJ, Gagnon JK, Arora K, Brooks CL III, Raghavan M (2015) Regulation of calreticulin–major histocompatibility complex (MHC) class I interactions by ATP. Proc Natl Acad Sci USA 112(41):E5608–E5617. https://doi.org/10.1073/pnas.1510132112
Yan M, Li J, Sha B (2011) Structural analysis of the Sil1–Bip complex reveals the mechanism for Sil1 to function as a nucleotide-exchange factor. Biochem J 438(3):447–455. https://doi.org/10.1042/BJ20110500
Yang J, Nune M, Zong Y, Zhou L, Liu Q (2015) Close and allosteric opening of the polypeptide-binding site in a human Hsp70 chaperone BiP. Structure (London, England: 1993) 23(12):2191–2203. https://doi.org/10.1016/j.str.2015.10.012
Zapun A, Darby NJ, Tessier DC, Michalak M, Bergeron JJM, Thomas DY (1998) enhanced catalysis of ribonuclease B folding by the interaction of calnexin or calreticulin with ERp57. J Biol Chem 273(11):6009–6012. https://doi.org/10.1074/jbc.273.11.6009
Acknowledgements
This work was supported by the National Institutes of Health ((GM086874 to D.N.H.) and a Chemistry-Biology Interface program training grant (T32GM008515 to B.M.A. and N.P.C.)).
Conflict-of-Interest Disclosure Statement
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Adams, B.M., Canniff, N.P., Guay, K.P., Hebert, D.N. (2021). The Role of Endoplasmic Reticulum Chaperones in Protein Folding and Quality Control. In: Agellon, L.B., Michalak, M. (eds) Cellular Biology of the Endoplasmic Reticulum . Progress in Molecular and Subcellular Biology, vol 59. Springer, Cham. https://doi.org/10.1007/978-3-030-67696-4_3
Download citation
DOI: https://doi.org/10.1007/978-3-030-67696-4_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-67695-7
Online ISBN: 978-3-030-67696-4
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)