Abstract
The species of genus Calophyllum have been reported for several ethnomedicinal uses in the traditional systems of medicine. The scientific study of the genus Calophyllum revealed that it is a rich source of bioactive secondary metabolites. These phytochemicals have shown a wide range of biological activities. Some of these have reached to the clinical developmental stage. The Calophyllum inophyllum seed oil has been proved to be an acceptable sustainable source of biodiesel. Few species of the genus are endangered and have been included in the red list of threatened species by the IUCN Red List. Owing to the importance of the genus a review of its ethnomedicinal uses, phytochemistry, and pharmacology has been carried out. It will further help to explore the molecular mechanism of phytochemicals for health benefits.
You have full access to this open access chapter, Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plant-based natural products have been a potential source of lead compounds for the discovery and development of drugs. Antimalarial drugs such as artemisinin and quinine and anticancer drugs such as paclitaxel and vinblastine are some of the well-known natural products which are used for the effective treatment of the disease. The major problem associated with the natural products is poor bioavailability and limited yield. These problems are being overcome by medicinal chemists by synthesizing the natural products in good yield and by preparing their analogues with good bioavailability. The genus Calophyllum has also proved to be a potential source of lead compounds for the drug discovery and development. Calanolide A, a non-nucleoside reverse transcriptase inhibitor isolated from the genus, is being evaluated under clinical trial. The genus is a rich source of several medicinally active compounds falling under various chemical classes. The genus includes 190 species and is classified under Calophyllaceae family. The species of the genus are identified with various distinguishing characters like red-coloured outer bark and drupe fruit. The species of the genus such as Calophyllum inophyllum is also known to be used traditionally to alleviate disease and is used in the management of leprosy. Owing to the medicinal importance of the genus Calophyllum, this chapter presents a review of its ethnomedicinal uses, phytochemistry and pharmacology.
2 Morphology and Taxonomy
The genus Calophyllum was previously included in the Guttiferae family (Group AP 2009). Now, the APG III (Angiosperm Phylogeny Group) system of flowering plant classification classifies it under Calophyllaceae family. There are 190 species in the genus, of which 179 were identified in the Old World (Africa, Asia and Europe) and only 10 species in the New World (the Americas and Oceania) (Eckenwalder 1980). These species are distributed mainly from eastern Africa to the Pacific in the Old World (Stevens 1980). The multivariate analysis by Diaz et al. showed that other American species of the genus Calophyllum are originated from this taxon (Díaz 2013). The species in genus Calophyllum are difficult to classify due to the challenge of establishing distinct boundaries (Watt 2014).
The species under the Calophyllum genus range from very high trees to shrubs. However, most of the species are medium-sized trees. The habitat of the species ranges from wet tropical rainforest of the lowlands to drier areas at higher altitudes. Some of the species are also found in flooded areas. The genus has several distinguishing taxonomical characteristics like red-coloured outer bark with diamond-shaped fissures and presence of opposite leaves with closely and alternating parallel veins. Other characteristics of the species include axillary, terminal and raceme inflorescences. The fruits of the genus Calophyllum are drupe possessing very thin layers of flesh along with a large seed. The sepals and petals in the genus are arranged in hermaphrodite flowers. These also secrete latex which is yellow or white in colour (Eckenwalder 1980). Several species of the genus such as C. apetalum, C. bracteatum, C. caudatum, C. cordato-oblongum and C. mooni have been included in the red list of threatened species by the IUCN RedList. Furthermore, 18 species are categorized as vulnerable (viz. C. apetalum, C. caudatum, C. cordato-oblongum), 5 species as endangered (viz. C. insularum, C. morobense, C. nubicola, C. trapezifolium and C. waliense) and 3 species as critically endangered (viz. C. acutiputamen, C. africanum and C. cuneifolium) (Stevens 1980).
3 Ethnomedicinal Uses
A number of plants of the genus Calophyllum are used as traditional medicine for the treatment of chronic diseases such as ulcer, eye infections, haemorrhoids, hypertension, infections, inflammation, leprosy, malaria, nephritis, pain, rheumatism, skin infection, tumours, varicose, venereal diseases, wound and peptic ulcers (Table 5.1). The seed oil of C. apetalum is used by traditional practitioners for the treatment of leprosy (Watt 2014). The latex of the seed of C. inophyllum has also been used for the management of leprosy. The seed oil of C. apetalum and C. soulattri was used in the treatment of skin infections (Stevens 1980; Watt 2014). The infusion of C. apetalum mixed with the honey is used for treating scabies (Watt 2014). C. apetalum, C. tacamahaca and C. inophyllum are reported to be used in the treatment of rheumatism (Dorla et al. 2019; Lavergne 2001; Watt 2014). Trunk bark decoction of C. brasiliense along with the root bark of Coutarea hexandra is used for the treatment of diabetes (Yasunaka et al. 2005). Root decoction of C. inophyllum is used locally in the treatment of ulcers and the leaf decoction is used in the treatment of eye infections (10). Furthermore, infusion of the roots of C. soulattri is rubbed on the skin to alleviate rheumatic pain. The oil extracted from the seeds of C. tomentosum is used in the treatment of skin disease (Stevens 1980).
4 Phytochemistry
The genus Calophyllum is a rich source of bioactive compounds such as xanthones and coumarins. The first phytochemical analysis of the genus was carried out in 1950 by Polonsky and Ormancey-Potier (Ormancey-Potier et al. 1951; Polonsky 1957). Since then several species of the genus have been explored for their phytochemical content. The phytochemical investigation has revealed the presence of various classes of secondary metabolites among which coumarins, xanthones, chromanones, triterpenes, steroid and glycosides are the predominant classes of phytoconstituents present in the genus (Subramanian and Nair 1971; Kashman et al. 1992; McKee et al. 1996; Dharmaratne and Wijesinghe 1997).
4.1 Coumarins
Coumarins are commonly found in the genus Calophyllum. Most of these coumarins are biosynthesized in the leaves. The coumarins have heterocyclic structure and their biosynthesis is related to the biosynthetic scheme for neo-flavonoids. Coumarins isolated from Calophyllum exhibit various pharmacological activities and can be used as a biomarker. The coumarins of the genus are further subclassified as simple coumarins, furanocoumarins, pyranocoumarins and furo-pyranocoumarins. Calanolide A (1), costatolide (2) (also known as calanolide B), calanolide C (3) and calanolide D (4) were isolated from the fruits and twigs of C. lanigerum. Calanolide E1 (5) and calanolide E2 (6) (diastereoisomer of calanolide E1) were isolated from the stem bark of C. lanigerum (Kashman et al. 1992; McKee et al. 1996). Patil et al. (1993) isolated two tetracyclic dipyranocoumarins, i.e. calophyllic acid (7) and isocalophyllic acid (8), from the leaves of C. inophyllum. Similarly, recedesolide (9), a tricyclic pyranocoumarin, was isolated from C. blancoi (Shen et al. 2004). Shen et al. (2003) isolated inocalophyllin A (10) and B (12) along with their methyl esters (11and 13, respectively) from the seeds of C. inophyllum. Furthermore, Yasunaka et al. (2005), Gomez-Verjan et al. (2014) and Pires et al. (2014) isolated mammea-type coumarins [A/BA (14), A/BB (15) and B/BB (16)] from the leaves of C. brasiliense. Tomentolide A (17) and B (18) were isolated from the nut kernels of C. tomentosum (Nigam and Mitra 1968). Recently, a new coumarin Wallimarin T (19) was isolated from the stem bark of C. wallichianum (Fig. 5.1; Table 5.2).
4.2 Xanthones
Xanthones have been isolated from the bark and wood of several species of the genus. These xanthones differ in structure by oxygenation pattern and position of isoprenyl group in the xanthone nucleus. Xanthones are also substituted with various other functionalities like OH, OMe, OCOMe, 3-carboxybutyl, 1,1-dimethylprop-2-enyloxy, 2,3-dihydroxy-3-methylbutyl, 4-hydroxy-3-methylbutyl and 4-hydroxy-3-methylbut-2-enyl and 4-hydroxy-3-methylbut-2-enyl. Guanidine (39), a 1,5-dihydroxy-6-(3,3-dimethylbut-2-enyl)-1,5-dihydroxy xanthone, was isolated from the timber of C. walker (Dahanayake et al. 1974). Apetalinone A (40) was isolated from C. apetalum. Apetalinone A bears 1,1-dimethylprop-2-enyloxy ether substitution which suggested that its biosynthesis involves Claisen rearrangement and Diels–Alder reaction (Iinuma et al. 1997). Acetylblancoxanthone (41), caloxanthone A (42), caloxanthone C (43), jacareubin (44), 6-deoxyjacareubin (45), dombakinaxanthone (46) and osajaxanthone (47) are pyranoxanthones possessing a pyran ring at C5-C6, C6-C7 or C7-C8 isolated from the Calophyllum spp. (Dharmaratne and Wijesinghe 1997; Yimdjo et al. 2004; Shen et al. 2005; Taher et al. 2005; Mah et al. 2015). Dombakinaxanthone (46), a trioxygenated diprenylated chromen-xanthone, and calozeyloxanthone (48) were also isolated from C. moonii. (Dharmaratne and Wijesinghe 1997). Furthermore, caloxanthone (49) and pyranojacaeubin (50) possess two pyran rings. Caloxanthone G (51) possesses 2,2-dimethyl-3,4-dihydropyrane ring while caloxanthone B (52) possesses a furan ring. Gunasekera et al. (1977) isolated three xanthones calabaxanthone (53), trapezifolixanthone (54) and 1,3,5-trihydroxy-2-(3-methylbut-2-enyl)xanthone (55) from the bark of C. cuneifolium. Similarly, brasixanthone B (56) was isolated from the stem bark of C. inophyllum (Mah et al. 2015) (Fig. 5.1; Table 5.2).
4.3 Chromanones
Secondary metabolites having chromanone nucleus, viz. flavonoids, biflavonoids and pyranochromanones, have also been isolated from the genus Calophyllum. Epicatechin (80), carpachromene (81) and myricetin (82) flavonoids have been isolated from the stem bark, leaves and androecium of flowers, from C. enervosum, C. symingtonianum and C. inophyllum, respectively (Table 5.2) (Subramanian and Nair 1971; Taher et al. 2005; Aminudin et al. 2015). Quercetin (83) was also isolated from the androecium of flowers of C. inophyllum (Subramanian and Nair 1971).
The biflavonoids of type flavanone-flavonol [GB-1 (84) and GB-2 (85)], flavanone-flavanone [GB-1a (86) and GB-2a (87)], flavanone-flavonol [garcinianin (88) and pancibiflavonol (89)], flavanone-flavone [GD-IV (90)] and flavone-flavone [amentoflavone (91)] have also been isolated from the species of genus Calophyllum (Table 5.2) (Ito et al. 1999; Reyes-Chilpa et al. 2004). Pyranoamentoflavone 4′-methyl ether (92) and pyranoamentoflavone 7-methyl ether (93) were also isolated from the leaves of C. venulosum (Cao et al. 2001).
Isocalolongic acid (94), isoinophynone (95) and inophynone (96) are 1-benzopyran-4-one class of compounds possessing an additional pyran ring which is fused at C7-C8 bond. Inophynone (96) and isoinophynone (95) which are a pair of epimers were isolated from the ethanolic extract of the fresh leaves of C. inophyllum (Ali et al. 1999). Compounds 97–103 are pyranochromanone derivatives, isolated from various species of the genus Calophyllum (Table 5.2) (Shen et al. 2004; Aminudin et al. 2015).
Brasiliensophyllic acid A (104), isobrasiliensophyllic acid A (105), brasiliensophyllic acid B (106), isobrasiliensophyllic acid B (107), brasiliensophyllic acid C (108) and isobrasiliensophyllic acid A (109) are novel chromanone acids which were isolated from the bark of C. brasiliense (Cottiglia et al. 2004) (Fig. 5.1; Table 5.2).
4.4 Triterpenes and Steroid
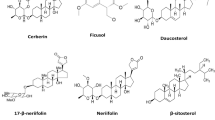
Betulinic acid (111), canophyllol (112) and friedelin (113) are the most common triterpenes found in the genus Calophyllum (Table 5.2) (Karunanayake et al. 1981; Reyes-Chilpa et al. 2004; Yasunaka et al. 2005). Genus Calophyllum is a rich source of various triterpenes, which belong to various groups like adianane [3β-simiarenol (114)], fridelane [frideline (115) and canophyllol (116)], lupane [betulinic acid(111)], oleanane [canophyllicacid (117) and apetalactone (118)] and taraxerane [taraxerol (119) and taraxerone (120)]. Furthermore, stigmasterol (121) was also isolated from the stem bark of C. wallichianum (Tee et al. 2018) (Fig. 5.1; Table 5.2).
4.5 Glycosides
Flavonoid glycoside, myricetin-7-glucoside (128), was isolated from the androecium of flowers of C. inophyllum (Subramanian and Nair 1971). Similarly, Ming et al. (2016) isolated a new C-glycoside calophymembranside C (124) from the stem of C. membranaceum. Further, Zhu et al. (2018) isolated three C-glycosides calophymembranside D(125), calophymembranside E (126) and calophymembranside F (127), from the stem of C. membranaceum (Fig. 5.1).
4.6 Miscellaneous
Secondary metabolites belonging to other chemical classes apart from the above-mentioned ones have also been isolated from the genus Calophyllum. A C24 terpenoid, soulattrone A, was isolated from the bark of C. soulattri (Nigam et al. 1988). Enervosanone (129) and cambogin (130), two phloroglucinols, were isolated from the stem bark of C. enervosum (Taher et al. 2005). Similarly, two 3-propylpropanoic acid moiety-bearing phloroglucinols, i.e. sundaicumone A (131) and sundaicumone B (132), were isolated in bioassay-guided fractionation using glucocorticoid receptor assay from the leaves of C. sundaicum (Cao et al. 2006a, b) (Fig. 5.1; Table 5.2).
5 Bioactivities of Genus Calophyllum
The genus Calophyllum exhibited several biological activities such as antiviral, antimalarial, chemopreventive, antisecretory, antibacterial, cytoprotective, analgesic, antitumour-promoting and cytotoxic activity.
5.1 Antiviral Activity
The dipyrano-tetracyclic coumarins such as calanolides, inophyllums and cordatolides isolated from the genus have exhibited potential anti-HIV activity (Kashman et al. 1992; Patil et al. 1993; Dharmaratne et al. 1998a, b). Studies on its mechanism of action showed that these compounds inhibit reverse transcriptase enzyme and are classified as non-nucleoside reverse transcriptase inhibitors (Creagh et al. 2001). Researchers from the National Cancer Institute studied anticancer potential of Malaysian trees in Sarawak’s forest. Unfortunately, they did not get any anticancer compound; instead they found an anti-HIV compound calanolide A from leaves and twigs of C. lanigerum. The isolated compound calanolide A showed complete protection against HIV-1 replication, with an IC50 of 5.9 ± 1.9 μM. The relocation efforts of the tree were failed and percentage of calanolide A was very less in other species of the genus Calophyllum. Further research showed that calanolide B, an isomer of calanolide A, is slightly less active. Calanolide B has the advantage of being readily available from the latex without causing any harm to the trees (Kashman et al. 1992). The development of calanolides was licensed by NCI/NIH to MediChem Research, Inc. (now Advanced Life Sciences) which negotiated an agreement with the Sarawak State Government for the development of calanolides as an anti-HIV drug. A joint venture company named Sarawak Medichem Pharmaceuticals was incorporated for the development of the lead molecule. After the joint venture arrangement proved unworkable, the lead role in the development was transferred to a Sarawakian company named Craun Research Sendirian Berhad. MediChem Research successfully synthesized (+)-calanolide A which is in early clinical trials, while (−)-calanolide B is in preclinical development. 11-Demethyl-12-oxo, an analogue of calanolide A, possesses comparable in vitro anti-HIV-1 activity and is used as a template to study structure–activity relationship of other congeners (Hanna 1999).
Inophyllums such as inophyllum B and P, isolated from C. inophyllum, displayed activity against HIV with an IC50 value of 38 nM and 130 nM, respectively (Patil et al. 1993). Similarly, cordatolides A and B isolated from C. cordato-oblongum showed anti-HIV activity with an IC50 value of 12.3 nM and 19 μM, respectively. Five pyranoxanthones isolated from C. blancoi also showed activity against the coronavirus with EC50 of 3–15 μg/ml (Shen et al. 2005) (Table 5.3).
5.2 Antimicrobial Activity
Undi oil (C. inophyllum) showed antibacterial activity against several Gram-positive bacteria. The activity of ethanol extract was 14 times of the original oil (Bhat et al. 1954). Novel chromanone acids, brasiliensophyllic acid A–C and isobrasiliensophyllic acid A–C, isolated from the bark of C. brasiliense exhibited antibacterial activity against Bacillus cereus and Staphylococcus epidermidis (Cottiglia et al. 2004). In addition, different parts of C. soulattri plant (methyl alcohol extracts of root, stem bark and leaf barks) exhibited a wide range of antibacterial activities (Khan et al. 2002) (Table 5.3).
5.3 Inhibition of the Multidrug Transporter P-glycoprotein
The coumarins of genus Calophyllum as well as their synthetic analogues inhibited the multidrug transporter P-glycoprotein. Structure–activity relationship study of these compounds showed a favourable region of electrostatic and steric volume. The study also revealed the importance of hydrophobic and neutral-charge group for the activity (Raad et al. 2006).
5.4 Anticancer Activity
Mammea-type coumarins (A/BA, A/BB, B/BB and B/BA) showed anticancer activity against various cancer cell lines including BV173, HL60, HTC116, K562, MALM6, PC3, SEM, U251 and a P-glycoprotein overexpressing cell line. Study of their mechanism of action suggested that these anticancer activities are due to the induction of caspase-mediated cell death (Kimura et al. 2005). Brasixanthones A–D displayed significant anti-proliferative activity against TPA-induced Epstein-Barr virus early antigen (EBV-EA) activation in Raji cells lines (Ito et al. 2002). Similarly, 8-desoxygartanin, calanolide A, brasimarin A, brasimarin B, brasimarin C, calanolide C, calanone and mammea B/BB also showed inhibition of TPA-induced EBV-EA activation in Raji cell lines (Ito et al. 2003) (Table 5.3).
5.5 Antimalarial Activity
Hay et al. (2004) isolated and tested seven xanthone compounds against chloroquino-resistant strain of Plasmodium falciparum. The IC50 values of tested compounds range from 0.8 to 4.4 μg/ml. SAR study showed that the OH group position is critical for the activity and the presence of 1,1-dimethylallyl, an additional pyran ring, two isopentenyl chains or one isopentenyl chain with a pyranic ring is favourable for the activity. It was also concluded that the hydroxylation of the prenyl side chain is not required for higher activity. The resin of C. antillanum showed potent activity against P. falciparum with an IC50 value of 0.3 μg/ml (Cuesta-Rubio et al. 2015).
5.6 Anti-parasite Activity
Mammea-type coumarins (A/BA, A/BB and B/BB) showed anti-parasitic activity against Trypanosoma cruzi and Leishmania amazonensis. The observed anti-parasitic activity was due to disruption of mitochondrial swelling, which in turn loses normal ultrastructure (Reyes-Chilpa et al. 2004). Similarly, three xanthones (jacareubin, 6-deoxyjacareubin, and 1,3,5,6-tetrahydroxy-2-(3-methyl-2-butenyl)xanthone) isolated from the heartwood of C. brasiliense showed in vitro trypanocidal activity against epimastigotes and trypomastigotes of T. cruzi. Further, xanthones isolated from C. brasiliense showed potential against Chagas disease with IC100 value of 153–213 μM against trypomastigotes (Abe et al. 2004).
5.7 Sulphotransferase Inhibitor
Xanthones isolated from the heartwood of C. brasiliense showed reversible inhibition of sulphotransferases 1A1 (SULT1A1) with IC50 value ranging from 1.6 to 7.4 μM. Similarly, coumarins isolated from C. brasiliense showed inhibition of SULT1A1 with IC50 value ranging from 47 to 185 μM and SULT2A1 with IC50 value ranging from 16 to 31 μM (Mesía-Vela et al. 2001).
5.8 Anti-dyslipidaemic Activity
The canophyllic acid, amentoflavone and a mixture of calophyllic acid and isocalophyllic acid isolated from C. inophyllum showed dose-dependent lipid-lowering activity under in vivo condition in triton-induced hyperlipidaemia model (Prasad et al. 2012).
5.9 Antioxidant Activity and Anti-inflammatory Activity
Oil obtained from the nuts of C. inophyllum showed antioxidant activity by inhibiting lipid peroxidation. The antioxidant activity of the oil helps to protect skin cells from damage by reactive oxygen species (Mahmud et al. 1998). Xanthones isolated form C. inophyllum exhibited in vivo anti-inflammatory activity when administered through intraperitoneal or oral routes in rats (Gopalakrishnan et al. 1980).
5.10 Hypotensive Activity
Oku et al. (2005) studied inhibitory effects of 22 xanthones on exogenous platelet-activating factor (PAF)-induced hypotension in in vivo assay. The result of the study showed that caloxanthone E, 1,3,5,6-tetrahydroxy-2-isoprenylxanthone, 6-deoxyjacareubin and guanidine showed 60% inhibition in PAF-induced hypotension (Oku et al. 2005).
5.11 α-Glucosidase Activity
Two flavonoids amentoflavone and carpachromene along with two coumarins, inophyllum D and inophyllum H, isolated from the crude extracts of the bark and leaves of C. symingtonianum showed promising α-glucosidase activity with IC50 ranging from 6.4 to 62.3 μM, which was better than the synthetic drug acarbose (IC50 456.4 μM) (Aminudin et al. 2015).
5.12 Other Activities
Coumarin named inophyllolide isolated by bioassay-guided fractionation from the nuts of C. inophyllum showed anti-piscicidal activity (Kawazu et al. 1968). Calofloride isolated from the seeds of C. verticillatum showed significant molluscicidal activity (Ravelonjato et al. 1992). Calophyllolide, a coumarin isolated from the C. inophyllum, showed anticoagulant action in in vivo experiments. The coagulation activity was in between the dicoumarol (slow and long acting) and ethyl biscoumacetate (very fast and short acting) (Arora et al. 1962). Amentoflavone isolated from the bark and leaves of C. symingtonianum showed potential 15-LOX inhibitory activity with an IC50 value of 0.04 μM (Aminudin et al. 2015).
6 Conclusions
Genus Calophyllum is a rich source of bioactive secondary metabolites of class xanthones, coumarins and chromanone. Other classes of secondary metabolites like triterpenoid and glycoside found in the genus Calophyllum have shown a wide range of biological activities like antiviral, anticancer, antimalarial, antibacterial and anti-proliferative and inhibition of P-glycoprotein (involved in the multidrug transport process) and inhibition of sulphotransferases. Calanolide A, isolated from the genus Calophyllum, has shown potential anti-HIV activity and continues to be in the clinical developmental stage. The dependence of calanolide A availability on isolation from the natural source has posed a problem in its further development. Various synthetic routes and alternative sources of the compounds with better yields are being explored to overcome this problem. Further studies to explore the molecular mechanism of the phytochemicals need to be done to exploit it for health benefits.
References
Abe F, Nagafuji S, Okabe H, Akahane H, Estrada-Muñiz E, Huerta-Reyes M, Reyes-Chilpa R (2004) Trypanocidal constituents in plants 3. Leaves of Garcinia intermedia and heartwood of Calophyllum brasiliense. Biol Pharm Bull 27(1):141–143
Abraham E. Materia Medica Guian. Britt. Timehri, 3rd ser. 1912;2
Ali MS, Mahmud S, Perveen S, Ahmad VU, Rizwani GH (1999) Epimers from the leaves of Calophyllum inophyllum. Phytochemistry 50(8):1385–1389
Aminudin NI, Ahmad F, Taher M, Zulkifli RM (2015) α-Glucosidase and 15-lipoxygenase inhibitory activities of phytochemicals from Calophyllum symingtonianum. Nat Prod Commun 10(9):1585–1587
Arora R, Mathur C, Seth S (1962) Calophyllolide, a complex coumarin anticoagulant from Calophyllum inophyllum Linn. J Pharm Pharmacol 14(1):534–535
Babu V, Arya R, Ilyas M, Nasim K (1994) 9-Hydroxy-2, 2, 6, 7-tetramethyl-2H-[1]-benzopyran-(1-phenylethylene-10-yl)-[3, 2-b]-dihydropyran-4-one from Calophyllum tomentosum. Phytochemistry 35(2):507–510
Bhat SG, Kane JG, Sreenivasan A (1954) The in vitro evaluation of the antibacterial activity of Undi oil (Calophyllum inophyllum Linn.). J Am Pharm Assoc 43(9):543–546
Cao S, Low K-N, Glover RP, Crasta SC, Ng S, Buss AD, Butler MS (2006a) Sundaicumones a and B, Polyprenylated Acylphloroglucinol derivatives from Calophyllum sundaicum with weak activity against the glucocorticoid receptor. J Nat Prod 69(4):707–709
Cao S-G, Sim KY, Goh S (2001) Minor methylated pyranoamentoflavones from Calophyllum venulosum. Nat Prod Lett 15(5):291–297
Cao S-G, Sim KY, Goh SH (2006b) Minor methylated Pyranoamentoflavones from Calophyllum venulosum. Nat Prod Lett 15(5):291–297
Cottiglia F, Dhanapal B, Sticher O, Heilmann J (2004) New chromanone acids with antibacterial activity from Calophyllum brasiliense. J Nat Prod 67(4):537–541
Creagh T, Ruckle JL, Tolbert DT, Giltner J, Eiznhamer DA, Dutta B, Flavin MT, Xu Z-Q (2001) Safety and pharmacokinetics of single doses of (+)-Calanolide a, a novel, naturally occurring nonnucleoside reverse transcriptase inhibitor, in healthy, human immunodeficiency virus-negative human subjects. Antimicrob Agents Chemother 45(5):1379–1386
Cuesta-Rubio O, Oubada A, Bello A, Maes L, Cos P, Monzote L (2015) Antimicrobial assessment of resins from Calophyllum antillanum and Calophyllum inophyllum. Phytother Res 29(12):1991–1994
Dahanayake M, Kitagawa I, Somanathan R, Sultanbawa MUS (1974) Chemical investigation of ceylonese plants. Part VII. Extractives of Calophyllum thwaitesii planch and Triana and Calophyllum walkeri Wight (Guttiferae). J Chem Soc, Perkin Trans 1:2510–2514
Dharmaratne HRW, Sajeevani JRDM, Marasinghe GPK, Ekanayake EMHGS (1998a) Distribution of pyranocoumarins in Calophyllum cordato-oblongum. Phytochemistry 49(4):995–998
Dharmaratne HRW, Wanigasekera WMAP, Mata-Greenwood E, Pezzuto JM (1998b) Inhibition of human immunodeficiency virus type 1 reverse transcriptase activity by Cordatolides isolated from Calophyllum cordato-oblongum. Planta Med 64(05):460–461
Dharmaratne HRW, Wijesinghe WMNM, Thevanasem V (1999) Antimicrobial activity of xanthones from Calophyllum species, against methicillin-resistant Staphylococcus aureus (MRSA). J Ethnopharmacol 66(3):339–342
Dharmaratne HRW, Wijesinghe WNM (1997) A trioxygenated diprenylated chromenxanthone from Calophyllum moonii. Phytochemistry 46(7):1293–1295
Díaz DMV (2013) Multivariate analysis of morphological and anatomical characters of Calophyllum (Calophyllaceae) in South America. Bot J Linn Soc 171(3):587–626
Dorla E, Grondin I, Hue T, Clerc P, Dumas S, Gauvin-Bialecki A, Laurent P (2019) Traditional uses, antimicrobial and acaricidal activities of 20 plants selected among Reunion Island’s flora. S Afr J Bot 122:447–456
Eckenwalder JE (1980) Taxonomy of the West Indian cycads. J Arnold Arbor 61(4):701–722
Ee GCL, Ng KN, Taufiq-Yap YH, Rahmani M, Ali AM, Muse R (2004) Mucigerin, a new coumarin from Calophyllum mucigerum (Guttiferae). Nat Prod Res 18(2):123–128
Gomez-Verjan JC, Estrella-Parra EA, González-Sánchez I, Vázquez-Martínez ER, Vergara-Castañeda E, Cerbón MA, Reyes-Chilpa R (2014) Molecular mechanisms involved in the cytotoxicity induced by coumarins from C alophyllum brasiliense in K 562 leukaemia cells. J Pharm Pharmacol 66(8):1189–1195
Gopalakrishnan C, Shankaranarayanan D, Nazimudeen S, Viswanathan S, Kameswaran L (1980) Anti-inflammatory and CNS depressant activities of xanthones from Calophyllum inophyllum and Mesua ferrea. Indian J Pharmacol 12(3):181
Govindachari T, Prakash D, Viswanathan N (1968) Apetalactone, a new triterpene lactone from Calophyllum species. J Chem Soc C: Organic:1323–1324
Govindachari TR, Viswanathan N, Pai BR, Rao UR, Srinivasan M (1967) Triterpenes of Calophyllum inophyllum Linn. Tetrahedron 23(4):1901–1910
Grenand P, Moretti C, Jacquemin H. Pharmacopées Traditionnelles en Guyane, Créoles, Palikur. Wayapi. Edit. ORSTOM, Paris. 1987
Group AP (2009) An update of the angiosperm phylogeny group classification for the orders and families of flowering plants: APG III. Bot J Linn Soc 161(2):105–121
Guerreiro E, Kunesch G, Polonsky J (1973) Chromanones de l'écorce de Calophyllum recedens. Phytochemistry 12(1):185–189
Guilet D, Seraphin D, Rondeau D, Richomme P, Bruneton J (Oct 2001) Cytotoxic coumarins from Calophyllum dispar. Phytochemistry 58(4):571–575
Gunasekera SP, Jayatilake GS, Selliah SS, Sultanbawa MUS (1977) Chemical investigation of ceylonese plants. Part 27. Extractives of Calophyllum cuneifolium Thw. And Calophyllum soulattri Burm. F.(Guttiferae). J Chem Soc, Perkin Trans 1 13:1505–1511
Hanna L (1999) Calanolide A: a natural non-nucleoside reverse transcriptase inhibitor. BETA 12(2):8
Hay AE, Guilet D, Morel C, Larcher G, Macherel D, Le Ray AM, Litaudon M, Richomme P (2003) Antifungal Chromans inhibiting the mitochondrial respiratory chain of pea seeds and new Xanthones from Calophyllum caledonicum. Planta Med 69(12):1130–1135
Hay A-E, Hélesbeux J-J, Duval O, Labaïed M, Grellier P, Richomme P (2004) Antimalarial xanthones from Calophyllum caledonicum and Garcinia vieillardii. Life Sci 75(25):3077–3085
Iinuma M, Ito T, Tosa H, Tanaka T, Miyake R, Chelladurai V (1997) Prenylated xanthonoids from Calophyllum apetalum. Phytochemistry 46(8):1423–1429
Iinuma M, Tosa H, Tanaka T, Yonemori S (1995) Two xanthones from roots of Calophyllum inophyllum. Phytochemistry 38(3):725–728
Iinuma M, Tosa H, Toriyama N, Tanaka T, Ito T, Chelladurai V (1996) Six xanthones from Calophyllum austroindicum. Phytochemistry 43(3):681–685
Ito C, Itoigawa M, Mishina Y, Filho VC, Enjo F, Tokuda H, Nishino H, Furukawa H (2003) Chemical constituents of Calophyllum brasiliense. 2. Structure of three new coumarins and cancer chemopreventive activity of 4-substituted coumarins. J Nat Prod 66(3):368–371
Ito C, Itoigawa M, Mishina Y, Filho VC, Mukainaka T, Tokuda H, Nishino H, Furukawa H (2002) Chemical constituents of Calophyllum brasiliensis: structure elucidation of seven new Xanthones and their cancer Chemopreventive activity. J Nat Prod 65(3):267–272
Ito C, Itoigawa M, Miyamoto Y, Rao KS, Takayasu J, Okuda Y, Mukainaka T et al (1999) A new Biflavonoid from Calophyllum panciflorum with antitumor-promoting activity. J Nat Prod 62(12):1668–1671
Itoigawa M, Ito C, Tan HT-W, Kuchide M, Tokuda H, Nishino H, Furukawa H (2001) Cancer chemopreventive agents, 4-phenylcoumarins from Calophyllum inophyllum. Cancer Lett 169(1):15–19
Joshi SP, Kulkarni SR, Phalgune UD, Puranik VG (2013) New dipyranocoumarin from the leaves of Calophyllum apetalum Willd. Nat Prod Res 27(20):1896–1901
Karunanayake S, Sotheeswaran S, Uvais M, Sultanbawa S, Balasubramaniam S (1981) Xanthones and triterpenes of Calophyllum tomentosum. Phytochemistry 20(6):1303–1304
Kashman Y, Gustafson KR, Fuller RW, Cardellina JH, McMahon JB, Currens MJ, Buckheit RW et al (1992) The calanolides, a novel HIV-inhibitory class of coumarin derivatives from the tropical rainforest tree, Calophyllum lanigerum. J Med Chem 35(15):2735–2743
Kawazu K, Ohigashi H, Mitsui T (1968) The piscicidal constituents of Calophyllum inophyllum Linn. Tetrahedron Lett 9(19):2383–2385
Khan M, Kihara M, Omoloso A (2002) Antimicrobial activity of Calophyllum soulattri. Fitoterapia 73(7–8):741–743
Khan NU-D, Parveen N, Singh MP, Singh R, Achari B, Dastidar PP, Dutta PK (1996) Two isomeric benzodipyranone derivatives from Calophyllum inophyllum. Phytochemistry 42(4):1181–1183
Kimura S, Ito C, Jyoko N, Segawa H, Kuroda J, Okada M, Adachi S et al (2005) Inhibition of leukemic cell growth by a novel anti-cancer drug (GUT-70) from Calophyllum brasiliense that acts by induction of apoptosis. Int J Cancer 113(1):158–165
Lavergne R. Tisaneurs et plantes médicinales indigènes: l'île de La Réunion: Éd. Orphie; 2001
Mah SH, Cheng Lian Ee G, Teh SS, Sukari MA (2015) Antiproliferative xanthone derivatives from Calophyllum inophyllum and Calophyllum soulattri. Pak J Pharm Sci 28(2):425–429
Mahmud S, Rizwani G, Ahmad M, Ali S, Perveen S, Ahmad V (1998) Antimicrobial studies on fractions and pure compounds of Calophyllum inophyllum Linn. Pak J Pharmacol 15(2):13–25
McKee TC, Fuller RW, Covington CD, Cardellina JH, Gulakowski RJ, Krepps BL, McMahon JB, Boyd MR (1996) New pyranocoumarins isolated from Calophyllum lanigerum and Calophyllum teysmannii. J Nat Prod 59(8):754–758
Mesía-Vela S, Sańchez R, Estrada-Muniz E, Alavez-Solano D, Torres-Sosa C, Jiménez-Estrada M, Reyes-Chilpa R, Kauffman F (2001) Natural products isolated from Mexican medicinal plants: novel inhibitors of sulfotransferases, SULT1A1 and SULT2A1. Phytomedicine 8(6):481–488
Ming M, Zhang X, Chen H-F, Zhu L-J, Zeng D-Q, Yang J, Wu G-X, Wu Y-Z, Yao X-S (2016) RXRα transcriptional inhibitors from the stems of Calophyllum membranaceum. Fitoterapia 108:66–72
Morel C, Séraphin D, Teyrouz A, Larcher G, Bouchara J-P, Litaudon M, Richomme P, Bruneton J (2002) New and antifungal xanthones from Calophyllum caledonicum. Planta Med 68(01):41–44
Nigam S, Mitra C (1968) Constituents of Calophyllum tomentosum and C. apetalum nuts. Planta Med 16(04):450–457
Nigam S, Mitra C (1969) Constituents of Calophyllum apetalum and Calophyllum tomentosum trunk bark. Phytochemistry 8(01):223–224
Nigam SK, Banerji R, Rebuffat S, Pascard C, Bodo B (1988) Soulattrone a, a C24 terpenoid from Calophyllum soulattri. Phytochemistry 27(2):527–530
Oger J-M, Morel C, Helesbeux J-J, Litaudon M, Séraphin D, Dartiguelongue C, Larcher G, Richomme P, Duval O (2003) First 2-hydroxy-3-methylbut-3-enyl substituted xanthones isolated from plants: structure elucidation, synthesis and antifungal activity. Nat Prod Res 17(3):195–199
Oku H, Ueda Y, Iinuma M, Ishiguro K (2005) Inhibitory effects of xanthones from Guttiferae plants on PAF-induced hypotension in mice. Planta Med 71(01):90–92
Ormancey-Potier A, Buzas A, Lederer E (1951) Sur le calophyllolide et 1′ acide calophyllique isolés des graines de Calophyllum inophyllum. Bulletin de la Société Chimique de France. 577
Patil AD, Freyer AJ, Eggleston DS, Haltiwanger RC, Bean MF, Taylor PB, Caranfa MJ, Breen AL, Bartus HR (1993) The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J Med Chem 36(26):4131–4138
Pengsuparp T, Serit M, Hughes SH, Soejarto DD, Pezzuto JM (1996) Specific inhibition of human immunodeficiency virus type 1 reverse transcriptase mediated by soulattrolide, a coumarin isolated from the latex of Calophyllum teysmannii. J Nat Prod 59(9):839–842
Pires CTA, Brenzan MA, Scodro RB, DAG C, LDG L, VLD S, Cardoso RF (2014) Anti-mycobacterium tuberculosis activity and cytotoxicity of Calophyllum brasiliense Cambess (Clusiaceae). Mem Inst Oswaldo Cruz 109(3):324–329
Polonsky J (1957) Structure Chimique Du Calophyllolide, De Linophyllolide Et De Lacide Calophyllique, Constituants Des Noix De Calophyllum inophyllum. Bull Soc Chim Fr 7:1079–1087
Prasad J, Shrivastava A, Khanna A, Bhatia G, Awasthi S, Narender T (2012) Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum. Phytomedicine 19(14):1245–1249
Raad I, Terreux R, Richomme P, Matera E-L, Dumontet C, Raynaud J, Guilet D (2006) Structure–activity relationship of natural and synthetic coumarins inhibiting the multidrug transporter P-glycoprotein. Bioorg Med Chem 14(20):6979–6987
Ravelonjato B, Libot F, Ramiandrasoa F, Kunesch N, Gayral P, Poisson J (1992) Molluscicidal constituents of Calophyllum from Madagascar: activity of some natural and synthetic neoflavonoids and khellactones. Planta Med 58(01):51–55
Reyes-Chilpa R, Estrada-Muñiz E, Apan TR, Amekraz B, Aumelas A, Jankowski CK, Vázquez-Torres M (2004) Cytotoxic effects of mammea type coumarins from Calophyllum brasiliense. Life Sci 75(13):1635–1647
Sakagami Y, Kajimura K, Wijesinghe W, Dharmaratne H (2002) Antibacterial activity of calozeyloxanthone isolated from Calophyllum species against vancomycin-resistant enterococci (VRE) and synergism with antibiotics. Planta Med 68(06):541–543
Shen Y-C, Hung M-C, Wang L-T, Chen C-Y (2003) Inocalophyllins A, B and their methyl esters from the seeds of Calophyllum inophyllum. Chem Pharm Bull 51(7):802–806
Shen Y-C, Wang L-T, Khalil AT, Chiang LC, Cheng P-W (2005) Bioactive pyranoxanthones from the roots of Calophyllum blancoi. Chem Pharm Bull 53(2):244–247
Shen Y-C, Wang L-T, Khalil AT, Kuo Y-H (2004) Chromanones and dihydrocoumarins from Calophyllum blancoi. Chem Pharm Bull 52(4):402–405
Stevens PF (1980) A revision of the Old World species of Calophyllum (Guttiferae). J Arnold Arbor 61(2):117–424
Subramanian SS, Nair A (1971) Myricetin-7-Glucoside from the androecium of the flowers of Calophyllum inophyllum. Phytochemistry 10(7):1679–1680
Taher M, Idris MS, Ahmad F, Arbain D (2005) A polyisoprenylated ketone from Calophyllum enervosum. Phytochemistry 66(6):723–726
Tee KH, Ee GCL, Ismail IS, Karunakaran T, Teh SS, Jong VYM, Mohd Nor SM (2018) A new coumarin from stem bark of Calophyllum wallichianum. Nat Prod Res 32(21):2565–2570
Watt G (2014) Dictionary of the economic products of India volume II (Cabbage to Cyperus). Cambridge University Press, Cambridge, pp 29–32
Yasunaka K, Abe F, Nagayama A, Okabe H, Lozada-Pérez L, López-Villafranco E, Muñiz EE, Aguilar A, Reyes-Chilpa R (2005) Antibacterial activity of crude extracts from Mexican medicinal plants and purified coumarins and xanthones. J Ethnopharmacol 97(2):293–299
Yimdjo MC, Azebaze AG, Nkengfack AE, Meyer AM, Bodo B, Fomum ZT (2004) Antimicrobial and cytotoxic agents from Calophyllum inophyllum. Phytochemistry 65(20):2789–2795
Zhu L-J, Yi S, Li X, Chen H-F, Ming M, Zhang X, Yao X-S (2018) C-glycosides from the stems of Calophyllum membranaceum. J Asian Nat Prod Res 20(1):49–54
Conflict of Interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Gupta, S., Gupta, P. (2020). The Genus Calophyllum: Review of Ethnomedicinal Uses, Phytochemistry and Pharmacology. In: Singh, J., Meshram, V., Gupta, M. (eds) Bioactive Natural products in Drug Discovery. Springer, Singapore. https://doi.org/10.1007/978-981-15-1394-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-15-1394-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-15-1393-0
Online ISBN: 978-981-15-1394-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)