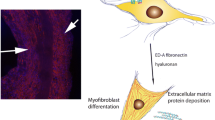
Summary
Cells contracting connective tissue matrices generate tractional forces in tissues. Studies of fibroblast contraction, using collagen gels in an in vitro model, demonstrate that it involves the actin cytoskeleton, specific extracellular matrix receptors and requires stimulation by exogenous promoters. Fibroblast contraction is stimulated by factors released by platelets and potentially secreted within the contracting tissue. Endothelial cells secrete a potent promoter of fibroblast contraction which has been identified as endothelin 1. The pathway through which fibroblast contraction is stimulated appears to require activation of protein kinase C. Tumor cells can also secrete endothelin. These mechanisms may be relevant to tumor progression.
Similar content being viewed by others
References
Tomasek JJ, Hay ED, Fujiwara K: Collagen modulates cell shape and cytoskeleton of embryonic corneal and fibroma fibroblasts: distribution of actin, alpha actinin, and myosin. Dev Biol 92: 107, 1982
Stopak D, Harris AK: Connective tissue morphogenesis by fibroblast traction. Dev Biol 90: 383, 1982
Stopak D, Wessells NK, Harris AK: Morphogenetic rearrangement of injected collagen in developing chicken limb buds. Proc Natl Acad Sci USA 82: 2804–2808, 1985
Machemer R: Proliferative Vitreoretinopathy (PVR): A personal account of its pathogenesis and treatment. Invest Ophthal and Vis Sci 29: 1771–1783, 1989
Glaser BM, Cardin A, Biscoe B: Proliferative vitreoretinopathy: The mechanism of development of vitreoretinal traction. Ophthalmol 94: 327–332, 1987
Robinson BW, Gross AN: The intrauterine healing of fetal rat cheek wounds. Cleft Palate J 18: 251–255, 1981
Roswell AR: The intrauterine healing of foetal muscle wounds: Experimental study in the rat. Br J Plast Surg 37: 635–642, 1984
Rowlatt U: Intrauterine wound healing in a 20 week human fetus. Virchow Arch 381: 353–361, 1979
Longaker MT, Harrison MR, Crombleholme TM, Langer JC, Decker M, Verrier ED, Spendlove R, Stern R: Studies in fetal wound healing: I. A factor in fetal serum that stimulates deposition of hyaluronic acid. J Ped Surg 24: 789–792, 1989
Longaker MT, Whitby DJ, Adzick NS, Crombleholme TM, Langer JC, Duncan BW, Bradley SM, Stern R, Ferguson MJW, Harrison MR: Studies in fetal wound healing, VI. Second and early third trimester fetal wounds demonstrate rapid collagen deposition without scar formation. J Ped Surg 25: 63–69, 1990
DePalma RL, Krummel TM, Durham LA, Michna BA, Thomas BL, Nelson JM, Diegelmann RF: Characterization and quantitation of wound matrix in the fetal rabbit. Matrix 9: 224–231, 1989
Mast BA, Flood LC, Haynes JH, DePalma RL, Cohen IK, Diegelmann RF, Krummel TM: Hyaluronic acid is a major component of the matrix of retal rabbit skin and wounds: Implications for healing by regeneration. Matrix 11: 63–68, 1991
Harris AK, Stopak D, Wild P: Fibroblast traction as a mechanism for collagen morphogenesis. Nature 290: 249–251, 1981
Harris AK: Traction, and its relations to contraction in tissue cell locomotion. In: Bellairs R, Curtis A, Dunn G (eds) Cell Behaviour. Cambridge University Press, Great Britain, 1982
Bell E, Ivarsson B, Merrill C: Production of a tissue-like structure by contraction of collagen lattices by human skin fibroblasts in vitro. Proc Natl Acad Sci USA 76: 1274, 1979
Grinnell F, Lamke C: Reorganization of hydrated collagen lattices by human skin fibroblasts. J Cell Sci 66: 51–63, 1984
Guidry C, Grinnell F: Studies on the mechanism of hydrated collagen gel reorganization by human skin fibroblasts. J Cell Sci 79: 67, 1985
Guidry C, Grinnell F: Contraction of hydrated collagen gels by fibroblasts: evidence for two mechanisms by which collagen fibrils are stabilized. Coll Rel Res 6: 515, 1986
Guidry C, Grinnell F: Heparin modulates the organization of hydrated collagen gels and inhibits gel contraction by fibroblasts. J Cell Bio 104: 1097, 1987
Guidry C, Hohn S, Hook M: Endothelial cells secrete a factor that promotes fibroblast contraction of hydrated collagen gels. J Cell Bio 110: 519, 1990
Guidry C, Hook M: Endothelins produced by endothelial cells promote collagen gel contraction by fibroblasts. J Cell Bio 115: 873, 1991
Steinberg BM, Smith K, Colozzo M, Pollack R: Establishment and transformation diminish the ability of fibroblasts to contract a native collagen gel. J Cell Biol 87: 304, 1980
De Juan E, Dickson J, Hatchell DL: Interaction of retinal glial cells with collagen matrices: implication for pathogenesis of cell-mediated traction. Graefe's Arch Clin Exp Ophthalmol 227: 494, 1989
Kelley C, D'Amore, Hechtman HB, Shepro D: Microvascular pericyte contractility in vitro: comparison with other cells of the vascular wall. J Cell Bio 104: 483, 1987
Wang SY, Merrill C, Bell E: Effects of ageing and long-term subcultivation on collagen lattice contraction and intra-lattice proliferation in three rat cell types. Mech Age Dev 44: 127–141, 1988
Schafer IA, Shapiro A, Kovach M, Lang C, Fratianne RB: The interaction in human papillary and reticular fibroblasts and human keratinocytes in the contraction of three-dimensional floating collagen lattices. Exp Cell Res 183: 112–125, 1989
Raymond MC, Thompson JT: RPE-mediated collagen gel contraction: Inhibition by colchicine and stimulation by TGF-beta. Invest Ophthalmol Vis Sci 31: 1079, 1990
Jelenska M, Kopec M: Blood platelets cause retraction of collagen gels. Thromb Haemo 44: 161–164, 1980
Buttle DJ, Ehrlich HP: Comparative studies of collagen lattice contraction utilizing a normal and transformed cell line. J Cell Physiol 116: 159, 1983
Montesano R, Orci L: Transforming growth factor beta stimulates collagen-matrix contraction by fibroblasts: implications for wound healing. Proc Natl Acad Sci USA 85: 4894, 1988
Anderson SN, Ruben Z, Fuller GC: Cell-mediated contraction of collagen lattices in serum-free medium: effect of serum and nonserum factors. In Vitro Cell Dev Biol 26: 61–66, 1990
Clark RAF, Folkvord JM, Hart CE, Murray MJ, McPherson JM: Platelet isoforms of platelet-derived growth factor stimulate fibroblasts to contract collagen matrices. J Clin Invest 84: 1036, 1989
Gullberg D, Tingstrom A, Thuresson AC, Olsson L, Terracio T, Rubin K: Beta1 integrin-mediated collagen gel contraction is stimulated by PDGF. Exp Cell Res 186: 264, 1990
Pierce GF, Mustoe TA, Lingelbach J, Masakowski VR, Griffin GL, Senior RM, Deuel TF: Platelet-derived growth factor and transforming growth factor-beta enhance tissue repair activities by unique mechanisms. J Cell Bio 109: 429–440, 1989
Loef EB, Proper JA, Coustin S, Shipley GD, DeCorleto PE, Moses HL: Induction of c-cis mRNA and activity similar to platelet-derived growth factor by transforming growth factor beta. Proc Natl Acad Sci USA 83: 2453–2457, 1986
Yanagisawa M, Kurihara H, Kimura S, Tombe Y, Kobayashi M, Mitsui Y, Goto K, Masaki T: A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415, 1988
Simonson MS, Dunn MJ: review. Cellular signalling by peptides of the endothelin gene family. FASEB 4: 2989, 1990
Rubanyi GM, Botelho HP: Endothelins. FASEB: 5: 2713–2720, 1991
Takuwa N, Takuwa Y, Yanagisawa M, Yamashita K, Masaki T: A novel vasoactive peptide endothelin stimulates mitogenesis through inositol lipid turnover in Swiss 3T3 fibroblasts. J Biol Chem 264: 7856–7861, 1989
Devesly P, Phillips PE, Johns A, Rubanyi G, Parker-Botelho LH: Receptor kinetics differ for endothelin-1 and endothelin-2 binding to Swiss 3T3 fibroblasts. Biochem Biophys Res Comm 172: 126–134, 1990
Ohnishi-Suzaki A, Yamaguchi K, Kusuhara M, Adachi I, Abe K, Kimura S: Comparison of biological activities of endothelin-1, -2 and -3 in murine and human fibroblast cell lines. Biochem Biophys Res Comm 166: 608–614, 1990
Muldoon L, Rodland KD, Forsythe ML, Magun BE: Stimulation of phosphatidylinositol hydrolysis, diacylglycerol release, and gene expression in response to endothelin, a potent new agonist for fibroblasts and smooth muscle cells. J Biol Chem 264: 8529–8536, 1989
MacCumber MW, Ross CA, Glaser BM, Snyder SH: Endothelin: Visualization of mRNAs by in situ hybridization provides evidence of local action. Proc Natl Acad Sci USA 86: 7285–7289, 1989
Brown KD, Littlewood CJ: Endothelin stimulated DNA synthesis in Swiss 3T3 cells; Synergy with polypeptide growth factors. Biochem J 263: 977, 1989
Koseki C, Imai M, Hirata Y, Yanagisawa M, Masaki Y: Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide? Amer J Physiol 256: R858-R866, 1989
Griendling KK, Tsuda T, Alexander RW: Endothelin stimulates diacylglycerol accumulation and activates protein kinase C in cultured vascular smooth muscle cells. J Biol Chem 264: 8237–8240, 1989
Nishizuka Y: Studies and perspectives of protein kinase C. Science 233: 305–312, 1986
Collins MKL, Rozengurt E: Binding of phorbol esters to high-affinity sites on murine fibroblast cells elicits a mitogenic response. J Cell Physiol 112: 42–50, 1982
Danowski BA, Harris AK: Changes in fibroblast contractility, morphology, and adhesion in response to a phorbol ester tumor promoter. Exp Cell Res 177: 47–59, 1988
Albelda SM, Buck CA: Integrins and other cell adhesion molecules. FASEB J 4: 2868, 1990
Staatz WD, Fok KF, Zutter MM, Adams SP, Rodriguez BA, Santoro SA: Identification of a tetrapeptide recognition sequence for the alpha2-beta1 integrin in collagen. J Biol Chem 266: 7363, 1991
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guidry, C. Extracellular matrix contraction by fibroblasts: Peptide promoters and second messengers. Cancer Metast Rev 11, 45–54 (1992). https://doi.org/10.1007/BF00047602
Issue Date:
DOI: https://doi.org/10.1007/BF00047602