Summary
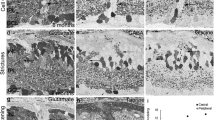
Measures of rabbit eyes and retinal whole-mounts were used to evaluate the development of retinal area and shape. The retina is shown to have a horizontal axis about a third longer than the vertical axis just before birth, and to adopt an almost symmetrical shape during postnatal development to adulthood. In general, retinal thickness is shown to decrease after birth, but differently in particular retinal regions: the reduction is marked in the periphery, and less pronounced in the visual streak. As an exception, the myelinated region — after it becomes really myelinated, from 9 days p.p. — even increases in thickness. In all regions of the retina, the absolute and relative thickness of the nuclear layers decreases, whereas the relative thickness of plexiform and fibrous layers increases. Proliferation of cells within the rabbit retina was studied during the first three postnatal weeks. 3H-thymidine incorporation was used to demonstrate DNA synthesis autoradiographically in histological sections as well as in enzymatically isolated retinal cells. A first proliferation phase occurs in the neuroblastic cell layer and ceases shortly after birth in the retinal center, but lasts for about one week in the retinal periphery. We found, however, a few 3H-thymidine-labeled cells as late as in the third postnatal week.
These late-labeled cells were found within the nerve fiber layer and in the inner plexiform layer. The latter cells were shown to express antigens detected by antibodies directed to the intermediate-sized filament protein vimentin, which are known to label Müller cells and neuroepithelial stem cells. This was confirmed in our preparation of enzymatically isolated cells; all cells with autoradiographically labeled nuclei revealed a characteristic elongated morphology typical for Müller radial glia (and also for early neuroepithelial stem cells). 3H-thymidine-labeled cells in the nerve fiber layer were most probably astrocytic. In analogy to the brain, we conclude that the mammalian retina undergoes a series of proliferation phases: first an early phase producing both neurons and glial cells, and then a late phase producing glial cells, e.g., in the nerve fiber layer. Most probably, the late phase within the inner nuclear layer is glial as well, i.e., consists of dividing Müller cells; it cannot be excluded, however, that there may remain some mitotically active stem cells.
Similar content being viewed by others
References
Anderson DH, Stern WH, Fisher SK, Erickson PA, Borgula GA (1983) Retinal detachment in the cat: the pigment epithelial- photoreceptor interface. Invest Ophthalmol Vis Sci 24:906–926
Anderson DH, Guérin CJ, Erickson PA, Stern WH, Fisher SK (1986) Morphological recovery in the reattached retina. Invest Ophthalmol Vis Sci 27:168–183
Beach DA, Jacobson M (1979) Patterns of cell proliferation in the retina of the clawed frog during development. J Comp Neurol 183:603–614
Bennett GS (1987) Changes in intermediate filament composition during neurogenesis. In: Current Topics in Developmental Biology, Vol 21, Academic Press, New York, pp 151–183
Blanks JC, Bok D (1977) An autoradiographic analysis of postnatal cell proliferation in the normal and degenerative mouse retina. J Comp Neurol 174:317–328
Bulliman BT, Kuchel PW (1988) A series expression for the surface area of an ellipsoid and its application to the computation of the surface area of avian erythrocytes. J Theor Biol 134:113–123
Erickson PA, Fisher SK, Anderson DH, Stern WH, Borgula GA (1983) Retinal detachment in the cat: the outer nuclear and outer plexiform layers. Invest Ophthalmol Vis Sci 24:927–942
Fernald RD (1989) Retinal rod neurogenesis. In: Finlay BL, Sengelaub DR (eds) Development of the vertebrate retina, Plenum Press, New York London, pp 31–42
Fernald RD, Johns P (1980) Retinal specialization and growth in the cichlid fish, H. burtoni. Am Zool 20:943
Foresman GE, Cohen RJ, Das ND (1985) Ornithine decarboxylase in developing neonatal rabbit ocular tissue. Ophthalmic Res 17:262–265
Greiner JV, Weidman TA (1982) Embryogenesis of the rabbit retina. Exp Eye Res 34:739–765
Hollyfield JG (1972) Histogenesis of the retina in the killifish Fundulus heteroclitus. J Comp Neurol 144:373–380
Holt CE, Bertsch TW, Ellis HM, Harris WA (1988) Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron 1:15–26
Hughes A (1975) A quantitative analysis of the cat retinal ganglion cell topography. J Comp Neurol 163:107–128
Johns PR (1977) Growth of the adult goldfish eye. III. Source of the new retinal cells. J Comp Neurol 176:343–358
Kelling ST, Sengelaub DR, Wikler KC, Finlay BL (1989) Differential elasticity of the immature retina: a contribution to the development of the area centralis? Vis Neurosci 2:117–120
Korr H (1982) Proliferation of different cell types in the brain of senile mice. Autoradiographic studies with 3H- and 14C-thymidine. Exp Brain Res [Suppl] 5:51–57
Lemmon V, Rieser G (1983) The developmental distribution of vimentin in the chick retina. Dev Brain Res 11:191–197
Mares V (1980) The time and space pattern of gliogensis, its morphogenetic significance and some regulatory aspects. In: Di-Bonadetta C et al. (eds) Multidisciplinary Approach to Brain Development, Elsevier, Amsterdam, pp 79–90
Mastronarde DN, Thibeault MA, Dublin MW (1984) Non-uniform postnatal growth of the cat retina. J Comp Neurol 228:598–608
McArdle CB, Dowling JE, Masland RH (1977) Development of outer segments and synapses in the rabbit retina. J Comp Neurol 175:253–278
Mott JC (1965) Haemorrhage as a test of the function of the cardiovascular system in rabbits of different ages. J Physiol (Lond) 181:728–752
Narang HK, Wisniewski HM (1977) The sequence of myelination in the epiretinal portion of the optic nerve in the rabbit. Neuropathol Appl Neurobiol 3:15–27
Noell WK (1958) Differentiation, metabolic organization, and viability of the visual cells. Arch Opthalmol 60:702–733
Rapaport DH, Robinson SR, Stone J (1984) Cell movement and birth in the developing cat retina. In: Development of Visual Pathways in Mammals, Alan R Liss, New York, pp 23–38
Rapaport DH, Robinson SR, Stone J (1985) Cytogenesis in the developing retina of the cat. Austr New Zealand J Ophthalmol 13:113–124
Reichenbach A (1987) Quantitative and qualitative morphology of rabbit retinal glia. A light microscopical study on cells both in situ and isolated by papaine. J Hirnforsch 28:213–220
Reichenbach A (1989) Glia: neuron index: review and hypothesis to account for different values in various mammals. Glia 2:71–77
Reichenbach A, Birkenmeyer G (1984) Preparation of isolated Müller cells of the mammalian (rabbit) retina. Z Mikrosk Anat Forsch 98:789–792
Reichenbach A, Wohlrab F (1986) Morphometric parameters of Müller (glial) cells dependent on their topographic localization in the nonmyelinated part of the rabbit retina. A consideration of functional aspects of radial glia. J Neurocytol 15:451–459
Reichenbach A, Reichelt W, Schumann R (1987) Use of Pappenheim's panoptic staining method on enzymatically isolated cells for demonstration of postnatal development of the rabbit retina. Z Mikrosk Anat Forsch 101:597–608
Reichenbach A, Hagen E, Schippel K, Eberhardt W (1988) Quantitative electron microscopy of rabbit Müller (glial) cells in dependence of retinal topography. Z Mikrosk Anat Forsch 102:721–755
Reichenbach A, Schnitzer J, Friedrich A, Knothe A-K, Henke A (1991a) Development of the rabbit retina. II. Müller cells. Submitted
Reichenbach A, Schnitzer J, Reichelt E, Fritzsche B, Friedrich A, Knothe A-K, Schober W, Timmermann A (1991b) Development of the rabbit retina. III. Differential growth and ganglion cell density. In preparation
Reichenbach A, Eberhardt W, Scheibe R, Deich C, Seidel B, Reichelt W, Dähnert K, Rödenbeck M (1991c) Development of the rabbit retina. IV. Tissue tensility and elasticity in dependence on topographic specializations. Exp Eye Res (in press)
Rentsch F (1973) Preretinal proliferation of glial cells after mechanical injury of the rabbit retina. Albr v Graefe's Arch Clin Exp Ophthalmol 188:79–90
Robinson SR, Dreher B, McCall M (1989) Non-uniform retinal expansion during the formation of the rabbit's visual streak: implications for the ontogeny of mammalian retinal topography. Vis Neurosci 2:201–219
Schimke RT (1959) Effects of prolonged light deprivation on the development of retinal enzymes in the rabbit. J Biol Chem 234:700–703
Schnitzer J (1985) Distribution and immunoreactivity of glia in the retina of the rabbit. J Comp Neurol 240:128–142
Schnitzer J (1988a) The development of astrocytes and blood vessels in the postnatal rabbit retina. J Neurocytol 17:433–449
Schnitzer J (1988b) Astrocytes in the mammalian retina. In: Osborne N, Chader J (eds) Progress in retinal research, Pergamon Press, Oxford, pp 210–231
Schnitzer J (1988c) Immunocytochemical studies on the development of astrocytes, Müller (glial) cells, and oligodendrocytes in the rabbit retina. Dev Brain Res 44:59–72
Schnitzer J (1990) Postnatal gliogenesis in the nerve fiber layer of the rabbit retina: an autoradiographic study. J Comp Neurol 292:551–562
Schnitzer J, Karschin A (1986) The shape and distribution of astrocytes in the retina of the adult rabbit. Cell Tissue Res 246:91–102
Sidman RL (1961) Histogenesis of mouse retina studied with thymidine-H3. In: Smelser (ed) The Structure of the Eye, Academic Press, New York, pp 487–506
Sternberger LA (1979) Immunocytochemistry, 2nd edn, John Wiley and Sons, New York
Stone J (1981) The Whole Mount Handbook, Maitland, Sydney
Stone J, Egan M, Rapaport DH (1985) The site of commencement of retinal maturation in the rabbit. Vision Res 25:309–317
Straznicky K, Gaze RM (1971) The growth of the retina in Xenopus laevis: an autoradiographic study. J Embryol Exp Morphol 26:67–79
Tout S, Ashwell K, Stone J (1988) The development of astrocytes in the albino rabbit retina and their relationship to retinal vasculature. Neurosci Lett 90:241–247
Turner DL, Cepko CL (1987) A common progenitor for neurons and glia persists in rat retina late in development. Nature (Lond) 328:131–136
Uga S, Smelser GK (1973) Electron microscopic study of the development of retinal Müllerian cells. Invest Opthhalmol 12:295–307
Wässle H, Levick WR, Cleland BG (1975) The distribution of the alpha type ganglion cells in the cat's retina. J Comp Neurol 159:419–437
Wetts R, Fraser SE (1988) Multipotent precursors can give rise to all major cell types of the frog retina. Science 239:1142–1145
Young RW (1983) The life history of retinal cells. Trans Am Ophthalmol Soc 81:193–228
Young RW (1985) Cell differentiation in the retina of the mouse. Anat Rec 212:199–205
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reichenbach, A., Schnitzer, J., Friedrich, A. et al. Development of the rabbit retina. Anat Embryol 183, 287–297 (1991). https://doi.org/10.1007/BF00192216
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00192216