Summary
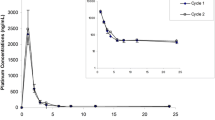
A total of 13 patients with ovarian cancer were studied during the initial two courses of i. v. thio-TEPA treatment they underwent after primary surgery. Following an increase in the dose from 60 to 80 mg for the second course, no sign of saturation of thio-TEPA elimination processes or of formation of the metabolite TEPA occurred, indicating dose-independent pharmacokinetics. Myelosuppression after courses was registered by serial measurements of platelets and leukocytes. The time to platelet nadir was quite uniformly 3 weeks and tended to be longer than that of leukocytes, which averaged 2 weeks but showed greater interindividual variation. Linear regression analyses of pharmacokinetic parameters versus myelosuppression revealed statistically significant correlations between thio-TEPA pharmacokinetics and the percentage of reductions in leukocytes and platelets at their mean nadirs. In contrast, no such correlation could be demonstrated for TEPA despite its greater exposure to the body in terms of AUC. We advocate further investigation of this pharmacokinetic-pharmacodynamic relationship so as to establish individualized dosing of thio-TEPA.
Similar content being viewed by others
References
Ackland SP, Choi KE, Ratain MJ, Egorin MJ, Williams SF, Sinkule JA, Bitran JD (1988) Human plasma pharmacokinetics of thiotepa following administration of high-dose thiotepa and cyclophosphamide. J Clin Oncol 6: 1192
Angelli G, De Cunto M, Gresele P, Del Favero A (1982) Early onset life-threatening myelosuppression after low-dose intravesical thiotepa. Postgrad Med J 58: 380
Bateman JC (1955) Chemotherapy of solid tumors with triethylene thiophosphoramide. N Engl J Med 252: 879
Boone IU, Rogers BS, Williams DL (1962) Toxicity, metabolism, and tissue distribution of carbon 14-labeledNN′N″-triethylenethiophosphoramide (thio-TEPA) in rats. Toxicol Appl Pharmacol 4: 344
Calvert AH, Newell DR, Gumbrell LA, O'Reilly S, Burnell M, Boxall FE, Siddik ZH, Judson IR, Gore ME, Wiltshaw E (1989) Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol 7: 1748
Cohen BE, Egorin MJ, Kohlhepp EA, Aisner J, Gutierrez PL (1986) Human plasma pharmacokinetics and urinary excretion of thiotepa and its metabolites. Cancer Treat Rep 70: 859
Craig AW, Fox BW, Jackson H (1959) Metabolic studies of32P-labeled triethylenethiophosphoramide. Biochem Pharmacol 3: 42
Eder JP, Antman K, Elias A, Shea TC, Teicher B, Henner WD, Schryber SM, Holden S, Finberg R, Critchlow J, Flaherty M, Mick R, Schnipper LE, Frei E III (1988) Cyclophosphamide and thiotepa with autologous bone marrow transplantation in patients with solid tumors. J Natl Cancer Inst 80: 1221
Egorin MJ, Van Echo DA, Tipping SJ, Olman EA, Whitacre MY, Thompson BW, Aisner J (1984) Pharmacokinetics and dosage reduction ofcis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Cancer Res 44: 5432
Friedman OM, Boger E (1961) Colorimetric estimation of nitrogen mustards in aqueous media. Anal Chem 33: 906
Hagen B, Walseth F, Walstad RA, Iversen T (1985) Gas chromatographic assay of triethylenethiophosphoramide in serum and urine. J Chromatogr Biomed Appl 345: 173
Hagen B, Walseth F, Walstad RA, Iversen T, Nilsen OG (1987) Single and repeated dose pharmacokinetics of thio-TEPA in patients treated for ovarian carcinoma. Cancer Chemother Pharmacol 19: 143
Hagen B, Walstad RA, Nilsen OG (1988) Pharmacokinetics of thio-TEPA at two different doses. Cancer Chemother Pharmacol 22: 356
Hagen B, Neverdal G, Walstad RA, Nilsen OG (1990) Long-term pharmacokinetics of thio-TEPA, TEPA and total alkylating activity following i. v. bolus adminsitration of thio-TEPA in ovarian cancer patients. Cancer Chemother Pharmacol 25: 257
Heideman RL, Cole DE, Balis F, Sato J, Reaman GH, Packer RJ, Singher LJ, Ettinger LJ, Gillespie A, Sam J, Poplack DG (1989) Phase I and pharmacokinetic evaluation of thiotepa in cerebrospinal fluid and plasma of pediatric patients: evidence for dose-dependent plasma clearance of thiotepa. Cancer Res 49: 736
Henner WD, Shea TC, Furlong EA, Flaherty MD, Eder JP, Elias A, Begg C, Antman K (1987) Pharmacokinetics of continuous-infusion high-dose thiotepa. Cancer Treat Rep 71: 1043
Hollister D, Coleman M (1980) Hematologic effects of intravesical thiotepa therapy for bladder carcinoma. JAMA 244: 2065
Kapadia SB, Krause JR (1978) Ovarian carcinoma terminating in acute nonlymphocytic leukemia following alkylating agent therapy. Cancer 41: 1676
Lazarus HM, Reed MD, Spitzer TR, Salah Rabaa M, Blumer JL (1987) High-dose i. v. thiotepa and cryopreserved autologous bone marrow transplantation for therapy of refractory cancer. Cancer Treat Rep 71: 689
Lokich JJ, Egorin MJ, Cohen BE, Bern MM, Zipoli TE, Moore C (1989) A phase I study of thiotepa administered by short-term and protracted continuous intravenous infusion. Cancer 63: 46
McDermott BJ, Double JA, Bibby MC, Wilman DEV, Loadman PM, Turner RL (1985) Gas chromatographic analysis of triethylenethiophosphoramide and triethylenephosphoramide in biological specimens. J Chromatogr Biomed Appl 338: 335
Mellet LB, Woods LA (1960) The comparative physiological disposition of thio-TEPA and TEPA in the dog. Cancer Res 20: 524
Tanum G, Sønstevold A, Engeset A (1987) The effect of pretreatment with thio-TEPA and cytosine arabinoside on megakaryocytopoiesis in rats given a sublethal dose of thio-TEPA. Blut 54: 33
Waxman DJ, Clarke L, Ng S (1989) Oxidative metabolism of thio-TEPA. Role of hepatic cytochrome P-450. Proc Am Assoc Cancer Res 30: 463
Author information
Authors and Affiliations
Additional information
The work described in this paper was supported by grants from the Cancer Fund at the Regional Hospital (Trondheim) and the Norwegian Cancer Society (Oslo). During this work the author was a research fellow for the Norwegian Cancer Society
Rights and permissions
About this article
Cite this article
Hagen, B. Pharmacokinetics of thio-TEPA and TEPA in the conventional dose-range and its correlation to myelosuppressive effects. Cancer Chemother. Pharmacol. 27, 373–378 (1991). https://doi.org/10.1007/BF00688860
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00688860