Abstract
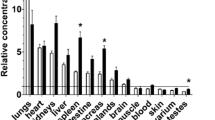
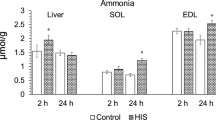
To investigate the rate of metabolism of nitrogen-13 labelled ammonia (13NH3) in different conditions, we have determined the relative amount of unchanged13NH3 in the blood of dogs, volunteers and transplant patients at different times following injection. In dogs, the determinations were made under basal conditions, during adenosine administration and after coronary occlusion. The results show that adenosine administration increases the metabolic rate whereas coronary occlusion does not affect13NH3 metabolism. For both human volunteers and transplant patients the metabolic rate of13NH3 was assessed under basal conditions and during adenosine administration.13NH3 metabolism proceeds faster in transplant patients than in volunteers under both conditions. Adenosine administration causes a faster13NH3 turnover in volunteers but not in transplant patients. Application of individual metabolite correction resulted in a 16% decrease in the calculated blood flow compared to uncorrected values. A smaller difference (5%) was observed between correction with mean metabolite values and individually acquired metabolite values.
Similar content being viewed by others
References
Hutchins G, Schwaiger M, Rosenspire K, Krivokapich J, Schelbert H, Kuhl D. Noninvasive quantification of regional blood flow in the human heart using N-13 ammonia and dynamic positron emission tomographic imaging.J Am Coll Cardiol 1990; 15: 1032–1042.
Muzik O, Beanlands R, Hutchins G, Mangner T, Nguyen N, Schwaiger M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET.J Nucl Med 1993; 34: 83–91.
Kuhle WG, Porenta G, Huang SC, Buxton D, Gambhir SS, Hansen H, Phelps ME, Schelbert HR. Quantification of regional myocardial blood flow using13N-ammonia and reoriented dynamic positron emission tomographic imaging.Circulation 1992; 86: 1004–1017.
Bol A, Melin J, Vanoverschelde JL, Baudhuin T, Vogelaers D, De Pauw M, Michel C, Luxen A, Labar D, Cogneau M. Direct comparison of [13N]ammonia and [15O]water estimates of perfusion with quantification of regional myocardial blood flow by microspheres.Circulation 1993; 87: 512–525.
Duda GD, Handler P. Kinetics of ammonia metabolism in vivo.J Biol Chem 1958; 232: 303–314.
Lockwood AH, McDonald JM, Reiman RE, Gelbard AS, Laughlin J, Duffy TE, Plum F. The dynamics of ammonia metabolism in man. Effects of liver disease and hyperammonemia.J Clin Invest 1979; 63: 449–460.
Rosenspire KC, Schwaiger M, Mangner TJ, Hutchins GD, Sutorik A, Kuhl DE. Metabolic fate of [13N]ammonia in human and canine blood.J Nucl Med 1990; 31: 163–167.
Weinberg IN, Huang SC, Hofmann EJ, Araujo L, Nienaber C, Grover-McKay M, Dahlbom M, Schelbert H. Validation of PET-acquired input functions for cardiac studies.J Nucl Med 1988; 29: 241–247.
Wieland B, Bida G, Padgett H, Hendry G, Zippi E, Kabalka G, Morelle JL, Verbruggen R, Ghyoot M. In-target production of [13N]ammonia via proton irradiation of dilute aqueous ethanol and acetic acid mixtures.Int J Appl Radiat Isot 1991; 42: 1095–1098.
Bormans G, Langendries W, Mortelmans L, Verbruggen A. On-line anion exchange purification of [13N]NH3 produced by 10-MeV proton irradiation of dilute aqueous ethanol.Int J Appl Radiat Isot 1994; 35: 183–185.
Gupta NC, Esterbrooks D, Mohiuddin S, Hilleman D, Sunderland J, Shine CY, Frick MP. Adenosine in myocardial perfusion imaging using positron emission tomography.Am Heart J 1991; 122: 293–301.
Nuyts J, Suetens P, Oosterlinck A, De Roo M, Mortelmans L. Delineation of ECT images using global constraints and dynamic programming.IEEE Trans Med Imaging 1991; 10: 489–498.
Muzik O, Beanlands RSB, Hutchins G, Mangner TJ, Nguyen N, Schwaiger M. Validation of nitrogen-13-ammonia tracer kinetic model for quantification of myocardial blood flow using PET.J Nucl Med 1993; 34: 83–91.
Krivokapich J, Stevenson LW, Kobashigawa J, Huang SC, Schelbert HR. Quantification of absolute myocardial perfusion at rest and during exercise with positron emission tomography after human cardiac transplantation.J Am Coll Cardiol 1991; 18: 512–517.
Senneff MJ, Hartman J, Sobel BE, Geltman EM, Bergmann SR. Persistence of coronary vasodilator responsivity after cardiac transplantation.Am J Cardiol 1993; 71: 333–338.
Olsson RA, Pearson JD. Cardiovascular purinereceptors.Physiol Rev 1990; 70:761–845.
Botvinick E, Frias M, O'Connell WJ, et al. Phase image evaluation of patients with ventricular pre-excitation syndromes.J Am Coll Cardiol 1984; 3: 799–814.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bormans, G., Maes, A., Langendries, W. et al. Metabolism of nitrogen-13 labelled ammonia in different conditions in dogs, human volunteers and transplant patients. Eur J Nucl Med 22, 116–121 (1995). https://doi.org/10.1007/BF00838940
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00838940