Abstract
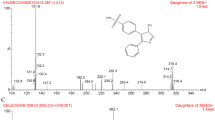
Granisetron is a selective 5-HT3 receptor antagonist that is used therapeutically for the prevention of vomiting and nausea associated with emetogenic cancer chemotherapy. Although forms of the drug are commercially available for intravenous and oral dosage, there is a need for intranasal delivery formulations in specific patient populations in which the use of these dosage forms may be unfeasible and/or inconvenient. A rapid and specific high-performance liquid chromatography method with mass spectrometric detection (LC-MS) was developed and validated for the analysis of granisetron in plasma after nasal administration in rats. Granisetron was separated in a reverse-phase C-18 column without interference from other components of plasma. This method involves a rapid assay for the determination of granisetron in a small volume of plasma with a run time of 12 min using ondansetron as an internal standard. Data were acquired in the electrospray ionization (ESI) mode with positive ion detection and application of single ion recording (SIR). Granisetron and ondansetron were detected at m/z values of 313.2 and 294.2, respectively. The method described was found to be suitable for the analysis of all samples collected during preclinical pharmacokinetic investigations of granisetron in rats after nasal administration. To date, the first pharmacokinetic study after intranasal administration of granisetron was performed and some pharmacokinetic parameters were presented in this paper. Granisetron was found to be well absorbed through nasal route and the bioavailability of this drug following nasal administration was comparable with that of intravenous administration.
Similar content being viewed by others
References
Bjerre, C., Björk, E., and Camber, O., Bioavailability of the sedative propiomazine after nasal administration in rats.Int. J. Pharm., 144, 217–224 (1996).
Blum, R. A., Majumdar, A., McCrea, J., Busillo, J., Orlowski, L. H., Panebianco, D., Hesney, M., Petty, K. J., Goldberg, M. R., Murphy, M. G., Gottesdiener, K. M., Hustad, C. M., Lates, C., Kraft, W. K., Van Buren, S., Waldman, S. A., and Greenberg, H. E., Effects of aprepitant on the pharmacokinetics of ondansetron and granisetron in healthy subjects.Clin. Ther., 25, 1407–1419 (2003).
Boppana, V. K., Simultaneous determination of granisetron and its 7-hydroxy metabolite in human plasma by reversed-phase high-performance liquid chromatography utilizing fluorescence and electrochemical detection.J. Chromatogr. A, 692, 195–202 (1995).
Boppana, V. K., Miller-Stein, C., and Schaefer, W. H., Direct plasma liquid chromatographic-tandem mass spectrometric analysis of granisetron and its 7-hydroxy metabolite utilizing internal surface reversed-phase guard columns and automated column switching devices.J. Chromatogr. B., 678, 227–236 (1996).
Bressolle, F., Bromet-Petit, M., and Audran, M., Validation of liquid Chromatographic and gas Chromatographic methods Applications to pharmacokinetics.J. Chromatogr. B., 686, 3–10 (1996).
Carmichael, J., Cantwell, B. M., Edwards, C. M., Zussman, B. D., Thompson, S., Rapeport, W. G., and Harris, A. L., A pharmacokinetic study of granisetron (BRL 43694A), a selective 5-HT3 receptor antagonist: correlation with antiemetic response.Cancer Chemother. Pharmacol., 24, 45–49 (1989).
Huang, C.-T., Chen, K.-C., Chen, C.-F., and Tsai, T.-H., Simultaneous measurement of blood and brain microdialysates of granisetron in rat by high-performance liquid chromatography with fluorescence detection.J. Chromatogr. B., 716, 251–255 (1998).
Hussain, A. A., Dakkuri, A., and Itoh, S., Nasal absorption of ondansetron in rats: an alternative route of drug delivery.Cancer Chemother. Pharmacol., 45, 432–434 (2000).
Hussain, A. A., Hirai, S., and Bawarshi, R., Nasal absorption of propranolol from different dosage forms by rats and dogs.J. Pharm. Sci., 69, 1411–1413 (1980).
International Conference on Harmonization, Note for Guidance on Validation of Analytical Procedures: Methodology, Committee for Proprietary Medical Products, CPMP/ICH/281/95, Approval 18 December 1996.
Kudoh, S., Sato T., Okada, H., Kumakura, H., and Nakamura, H., Simultaneous determination of granisetron and 7- hydroxygranisetron in human plasma by high-performance liquid chromatography with fluorescence detection. J.Chromatogr. B., 660, 205–210 (1994).
Physicians’ Desk Reference, 59th ed (2005) Medical Economics, Montvale, New Jersey, pp. 2901–2903
Sanger, G. J. and Nelson, D. R., Selective and functional 5- hydroxytryptamine3 receptor antagonism by BRL 43694 (granisetron).Eur. J. Pharmacol., 159, 113–124 (1989).
United States Pharmacopoeia 23, Section 1225, Validation of Compendial Methods, United States Pharmacopoeia Convention, Inc., Rockville, MD, 1995.
Upward, J. W., Arnold, B. D., Link, C., Pierce, D. M., Allen, A., and Tasker, T. C., The clinical pharmacology of granisetron (BRL 43694), a novel specific 5-HT3 antagonist.Eur. J. Cancer, 26, S12-S15 (1990).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Woo, J.S. Nasal absorption studies of granisetron in rats using a validated high-performance liquid chromatographic method with mass spectrometric detection. Arch Pharm Res 30, 778–784 (2007). https://doi.org/10.1007/BF02977642
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02977642