Abstract
Status asthmaticus is a life-threatening episode of asthma that is refractory to usual therapy. Recent studies report an increase in the severity and mortality associated with asthma. In the airways, inflammatory cell infiltration and activation and cytokine generation produce airway injury and edema, bronchoconstriction and mucus plugging. The key pathophysiological consequence of severe airflow obstruction is dynamic hyperinflation. The resulting hypoxemia, tachypnea together with increased metabolic demands on the muscles of respiration may lead to respiratory muscle failure.
The management of status asthmaticus involves intensive pharmacological therapy particularly with β-adrenoceptor agonists (β-agonists) and corticosteroids. Albuterol (salbutamol) is the most commonly used β2-selective inhaled bronchodilator in the US. Epinephrine (adrenaline) or terbutaline, administered subcutaneously, have not been shown to provide greater bronchodilatation compared with inhaled β-agonists. Corticosteroids such as methylprednisolone should be administered early. Aerosolized corticosteroids are not recommended for patients with status asthmaticus. Inhaled anticholinergic agents may be useful in patients refractory to inhaled β-agonists and corticosteroids. In patients requiring mechanical ventilation, the strategy aims to avoid dynamic hyperinflation by enhancing expiratory time to allow complete exhalation. Complications of dynamic inflation are hypotension and barotrauma. Sedation with opioids, benzodiazepines or propofol is required to facilitate ventilator synchrony but neuromuscular blockade should be avoided as myopathy has been a reported complication.
Overall, in the management of patients with status asthmaticus, the challenge to the pulmonary/critical care clinician is to provide optimal pharmacological and ventilatory support and avoid the adverse consequences of dynamic hyperinflation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Status asthmaticus is a severe, life-threatening episode of asthma which is unresponsive to the usual initial therapy. Status asthmaticus necessitates aggressive pharmacological therapy and mechanical ventilation.[1]
Recent studies emphasize the increasing severity and mortality associated with asthma.[2] An increased risk of death from asthma has been found among inner-city minority populations of children and adults.[2] The 1991 mortality rates for African American individuals was reported as 3.5 per 100000 compared with 1.2 per 100000 for Caucasians.[2] Severe asthma and increased mortality from asthma have been identified in particular geographic areas of the United States.[3] Even within cities, there are particular geographic areas of high risk; one study found that there were certain zip code areas within New York City with especially high hospital admission rates related to asthma.[4]
The incidence of near-fatal asthma is difficult to assess, particularly because of lack of a standard disease definition. Patients requiring assisted ventilation, patients with respiratory arrest, as well as patients who experience hypercapnia may be considered to have near-fatal asthma.
The literature reports of patients with status asthmaticus admitted to the medical intensive care unit (ICU)[5–9] are mainly descriptive case series; thus, management and outcome data are dependent on reports mainly from urban, academic medical centers. The series also reflect on individual clinical decisions for intubation, ICU admission, and certain medication and ancillary treatments.
This article focuses on the medical and ventilatory management of adults with status asthmaticus in the ICU. In order to understand the rationale for treatment, an understanding of the pathophysiology of airflow obstruction is important.
1. Pathophysiology of Status Asthmaticus
Structural changes and cellular infiltration of the airways are major features of asthma. Eosinophils[10] and T helper lymphocytes[11] infiltrate the airways. Stimulated airway T helper lymphocytes release cytokines such as interleukin (IL)-3, IL-4, IL-5 and granulocyte-macrophage colony-stimulating factors.[12] Cytokines such as IL-4 stimulate immunoglobulin (Ig) E production.[11] Activated mast cells produce cytokines such as IL-4 and IL-5. Activation of eosinophils leads to the release of toxic proteins, leukotrienes, and platelet activating factor. The consequences are bronchoconstriction, airway infiltration, and mucus gland secretion.
An autopsy study in patients with fatal asthma demonstrated an increase in airway wall area in large and small airways, and increases in smooth muscle, mucus gland and cartilage.[13] Rapidly fatal asthma has been characterized by infiltration of the airways with neutrophils, increased numbers of mucous glands[14] and increased infiltration of the larger airways with eosinophils.[15]
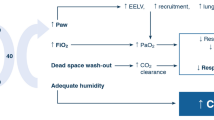
Acute increases in airflow obstruction result from progressive inflammation and airway edema, smooth muscle mediated bronchoconstriction or intraluminal mucus plugging. The pathophysiological consequence is expiratory airflow obstruction leading to hypoxemia and air-trapping.[1,16] The initial tachypnea achieves an increase in minute ventilation and hypocapnia. However, the increased minute ventilation in the setting of airflow obstruction leads to dynamic hyperinflation, i.e. incomplete exhalation and air-trapping. Because exhalation is incomplete, alveolar pressure remains positive at the end of expiration; this is termed auto-positive end-expiratory pressure (PEEP). Lung hyperinflation places the diaphragm at a mechanical disadvantage. Areas of ventilation-perfusion (V/Q) mismatch result in hypoxemia. In addition, sustained functioning of the respiratory muscles increase their metabolic demands. Increased metabolic demands, hypoxemia, and hypoperfusion may cause respiratory muscle failure and worsening of hypercapnia. Alterations in consciousness may ensue and respiratory arrest can follow if respiration is not assisted. Although it is expiratory airflow that is obstructed, it is the inspiratory muscle, the diaphragm, that fails thereby necessitating mechanical ventilation.
The approach to treatment of patients with status asthmaticus therefore involves treatment of inflammation, reduction of bronchoconstriction and provision of oxygen and ventilatory support, if necessary. Aggressive attempts to treat airflow obstruction will often prevent intubation. Although intubation may be life-saving in the case of respiratory failure due to status asthmaticus, the complications due to dynamic hyperinflation are of major concern.
2. Pharmacological Therapy
Initial pharmacological therapy for the patient with severe asthma includes oxygen and inhaled bronchodilators.[1] Low-flow oxygen is generally sufficient to achieve adequate arterial oxygenation. Severe hypoxemia may be due to additional complications such as atelectasis, pneumonia, and barotrauma. Bronchodilator therapy and corticosteroids are the most important treatments in status asthmaticus (table I).[1,17,18]
2.1 β-Adrenoceptor Agonist (β-Agonist) Therapy
2.1.1 Inhaled β-Agonists
β-Adrenoceptor agonist (β-agonist) therapy is the cornerstone of the initial management of status asthmaticus.[1] β-agonists relax airway smooth muscle and decrease mucosal edema. The onset of action is rapid. Albuterol (salbutamol) is the most commonly used β2 selective inhaled bronchodilator in the United States. Inhaled therapy may be administered via a nebulizer or metered-dose inhaler (MDI) with a spacer (table II). Nebulization of 2.5mg (in 2ml of saline) or four puffs (0.36mg) of albuterol have been shown to be equivalent.[1] The onset of bronchodilator action is rapid, occurring within 5 minutes, with peak effect within 30 minutes. The medication may be administered every 20 minutes for three doses and then hourly. For some adult patients with severe acute asthma, higher doses of albuterol and continuous inhaled therapy are required.[19] Administration of β2-agonist via an MDI may be effective; however, in patients with severe airflow obstruction administration via a nebulizer may provide greater subjective relief.
The administration of inhaled therapy to patients who are intubated is challenging.[17,20] Higher doses are required for an adequate amount of drug delivery to the lower airways. Many factors affect aerosol deposition in the airways of intubated patients including ventilator settings, ventilator circuit, humidification, location of device, amount of drug, endotracheal tube dimensions, severity of airways disease, and patient effort and synchrony.[17] Nebulized albuterol 2.5mg is administered with the nebulizer placed 30cm distal to the Y connector with an airflow rate of 6–8 L/min.[17] The use of an MDI with a spacer placed 10cm from the Y connector for the administration of 6–8 puffs of albuterol has also been advocated. MDI actuation directly into the endotracheal tube has been found to supply the least effective bronchodilation, as more drug impacts on the endotracheal tube and large airways.[20]
The best approach is to assess the effect of a drug dose or delivery system for the individual patient.[1,17] Corbridge and Hall[1] recommend assessing changes in airflow resistance. The ventilatory parameter of peak-to-plateau airway pressure gradient may be examined for the effect of the administered bronchodilator. A fall in the gradient of >15% indicates a significant effect on airflow resistance. The drug can then be administered every 30 or 60 minutes. With improvement, the time interval between doses can be lengthened.
Potential adverse effects of inhaled β-agonist therapy include arrhythmias, tremor, and hypokalemia.[17]
2.1.2 Parenteral β-Agonist Therapy
Although patients with status asthmaticus have severe airflow obstruction, inhaled medications are still generally effective in reaching the lower airways. Epinephrine (adrenaline) or terbutaline given subcutaneously have not been shown to provide greater bronchodilatory effect compared with an inhaled β-agonist.[18] Such therapy may be considered in younger patients (<40 years) who demonstrate refractory status asthmaticus.[1] Epinephrine 0.3ml of 1 : 1 000 solution can be administered subcutaneously every 20 minutes for 3 doses.[18] Terbutaline 0.25mg subcutaneously every 20 minutes for three doses is preferred in the pregnant patient.[21] Complications include metabolic disturbances such as hypokalemia and lactic acidosis, cardiac arrhythmias and myocardial ischemia. In adults with status asthmaticus, intravenous β-agonist administration has shown no improvement in bronchodilation compared with the inhaled route,[1] and is not recommended.
There is rarely a need for parenteral β-agonist therapy in status asthmaticus. Oral β-agonists are not useful in status asthmaticus. Status asthmaticus will usually respond to appropriate inhaled therapy, corticosteroids and supportive measures.
2.2 Corticosteroids
Corticosteroid therapy is a mainstay of treatment of status asthmaticus. Corticosteroids decrease synthesis of inflammatory mediators such as leukotrienes and prostaglandins, inhibiting macrophage and T cell mediator release, and decreasing endothelial adhesion molecules.[16] Corticosteroids thus decrease airway wall inflammation and mucus production and also improve responsiveness to β-agonist therapy. Their mechanism of action involves binding to cell cytosol receptors and regulating gene transcription; the latter effects are not seen for 6–12 hours. Corticosteroids should be administered early and may be administered as methylprednisolone 60–125mg intravenously or prednisone 30–40mg orally every 6 hours.[1,18] Aerosolized corticosteroids are not generally administered for status asthmaticus.
Complications related to corticosteroid administration include hyperglycemia, hypertension, and psychosis. Myopathy is a specific complication of corticosteroid use in patients intubated for status asthmaticus who are receiving neuromuscular blocking agents.[22]
2.3 Anticholinergic Agents
Inhaled anticholinergic agents may be useful in status asthmaticus, particularly in those patients refractory to inhaled β-agonist and corticosteroid therapy.[1,18] Ipratropium blocks acetylcholine action at muscarinic receptors on airway smooth muscle. Peak effect occurs within 90 minutes and lasts up to 6 hours. Anticholinergic agents alone are weak bronchodilators but may enhance bronchodilation provided by β-agonists. A recent study found significant benefit from the addition of ipratropium to albuterol, via a MDI, as initial therapy in the emergency department for adult patients with acute asthma.[23]
There is little systemic toxicity related to inhaled ipratropium. Its use may be of particular importance in patients with β-blockade. Ipratropium bromide may be administered as a 0.5mg solution via nebulization alone or in combination with albuterol 2.5mg. Ipratropium may be administered as 4–10 puffs via a MDI with spacer.
2.4 Theophylline
Theophylline has a long history of use in the treatment of asthma with the potential beneficial effects of potentiation of β-agonist-induced bronchodilation, improved diaphragmatic contractility, inotropic and diuretic effects. Recent work also suggests an immunomodulatory role of theophylline in inhibiting activation of airway T lymphocytes.[24] However, theophylline has not been demonstrated to benefit patients with status asthmaticus when added to standard bronchodilator therapy.[18] Adverse effects include tachycardia, nausea and tremor, and there are many drug interactions to consider.
2.5 Magnesium Sulfate
Magnesium has been administered as a bronchodilator in patients with severe status asthmaticus.[1] Magnesium may work by inhibiting calcium-mediated as well as parasympathetic nerve-mediated constriction of airway smooth muscle. A meta-analysis of seven trials using intravenous magnesium sulfate in the emergency department for the treatment of asthma indicated that although routine use was not beneficial, use in patients with severe asthma led to improvement in peak expiratory flow rates.[25] Therefore, individual patients with status asthmaticus may benefit from intravenous magnesium. Magnesium sulfate is administered as a 2g intravenous infusion over 20 minutes.
2.6 Heliox
Heliox is a mixture of helium and oxygen which is less dense than air and thus may produce less resistance in airways with turbulent air flow.[26] Heliox does not affect airway inflammation or bronchoconstriction. The objective of administering heliox is to reduce the work of breathing while giving time for pharmacological therapy to become effective.[1] Several small studies have shown improvement in peak flow and reduction in pulsus paradox,[1] as well as an improvement in respiratory acidosis.[26] At this time there is insufficient evidence to recommend the use of heliox in status asthmaticus.
3. Ancillary Treatments
Because viral respiratory tract infections are more common than bacterial infections, antibacterials are usually unnecessary in the treatment of status asthmaticus. Antibacterials should, however, be administered for suspected pneumonia or sinusitis.[1,18]
There is no benefit in the administration of intravenous fluid to adult patients with status asthmaticus.[18] Chest physical therapy is not beneficial and may be stressful.[18] The use of mucolytic therapy may worsen cough and bronchospasm and should not be administered unless there is evidence of lobar atelectasis.[1] Leukotriene receptor antagonists have not been used in the treatment of patients with status asthmaticus.
4. Response to Therapy
The majority of patients show a clinical improvement following initial therapy. Patients who remain in respiratory distress (use of accessory muscles of respiration, clinical fatigue), those with a peak expiratory flow rate below 150 L/min or arterial carbon dioxide partial pressure (PaCO2) that is normal or elevated should be admitted to a monitored unit.
5. Mechanical Ventilation
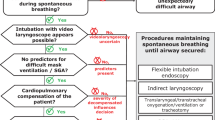
Mechanical ventilation is clearly indicated in patients with cardiopulmonary arrest or near arrest. Intubation is performed in patients with status asthmaticus showing clinical deterioration and exhaustion [1,27] signaled by alterations in the level of consciousness, posture, ability to speak, and air movement. The decision for intubation is a clinical decision by the physician who must weigh the chance of improvement with further therapy, the potential for respiratory arrest, and the potential complications of intubation. Arterial blood gas analysis is often performed and an elevated PaCO2 is certainly worrisome. If the patient is clinically responding to therapy, the hypercarbia itself is not a reason for intubation and, in fact, most patients with hypercarbia do not require intubation.[28]
The goals of mechanical ventilation are to provide adequate oxygenation and ventilation and allow the respiratory muscles to rest while the airway inflammation subsides. Fears of mechanical ventilation involve the reported risk of mortality and potential complications of barotrauma and hypotension. Noninvasive positive pressure ventilation has been applied to patients with respiratory failure due to status asthmaticus. In one series, noninvasive ventilation without intubation was successful in correcting the respiratory acidosis in the majority of patients with status asthmaticus.[29] A trial of noninvasive ventilation may be considered in selected patients who are alert, cooperative, and who do not have secretions.
Prevention of dynamic hyperinflation is the current guiding principle in mechanical ventilation in patients with status asthmaticus.[27,30,31] With expiratory airflow limitation, incomplete exhalation of the ventilator-delivered tidal volume leads to trapped air. At elevated levels of minute ventilation, the amount of trapped air can be significant.[30] Elevated end-expiratory pressure (auto-PEEP) raises intrathoracic pressure, decreases venous return and can result in hypotension. Barotrauma (pneumothorax and pneumomediastinum) is a potential complication of the alveolar overdistension.
The crucial factor in dynamic hyperinflation is the minute ventilation. Optimal ventilator management involves allowing sufficient expiratory time to prevent dynamic hyperinflation. The patient is given a slow respiratory rate in order to allow greater time for expiration. With a reduced minute ventilation in the setting of severe airflow obstruction, it is expected that the PaCO2 may be elevated.
Controlled hypoventilation and permissive hypercapnia are favored approaches in the management of patients with status asthmaticus.[27,32] An elevated PaCO2 is acceptable providing that oxygenation is adequate, and PaCO2 levels up to 90mm Hg are generally tolerated. Complications of hypercapnia are cerebral vasodilatation, depression of cardiac contractility, systemic vasodilatation, and pulmonary arterial vasoconstriction.[32] Contraindications are elevated intracranial pressure, myocardial ischemia or severe myocardial dysfunction. The approach of permissive hypercapnia, with sodium bicarbonate administration to correct the acidosis, has been applied in the treatment of status asthmaticus in several patients.[33] Controversy surrounds whether a buffer should be administered to maintain a specific level of blood pH.
Clinical signs of dynamic hyperinflation include auscultation revealing continued expiration at the time of a subsequent ventilator-delivered breath, hyperinflation of the chest on examination, hemodynamic effects of tachycardia or hypotension,[1] and respiratory efforts that do not trigger a ventilator-assisted breath (auto-PEEP). Respiratory and hemodynamic compromise may also be a sign of tension pneumothorax in intubated patients with status asthmaticus.[1]A brief disconnection from the ventilator to allow exhalation of trapped gas may improve the hemodynamic disturbance resulting from dynamic hyperinflation.
The recommended ventilator management is designed to prevent dynamic hyperinflation and its consequences.[30] There are actually no controlled studies comparing different ventilatory strategies in patients with status asthmaticus; the literature is composed of case series. Initial ventilation is targeted to allow sufficient expiratory time (table III).[1] Tachypnea due to intubation, airways disease, and hypercapnia is the usual response by the patient and may lead to further dynamic hyperinflation. Sedation is necessary to achieve a slow respiratory rate and sufficient expiratory time. Adequate oxygenation with saturation above 90% is generally accomplished without difficulty.
6. Sedation
Opioids, benzodiazepines and propofol are agents used for sedation in mechanically ventilated patients with status asthmaticus (table IV).[1] Despite the theoretical potential for histamine release and worsening of airflow obstruction, morphine is commonly used successfully in these patients. Fentanyl is a synthetic opioid with less hypotensive effects. Benzodiazepines such as lorazepam and midazolam are often used alone or in combination with an opiate.
Sedation with propofol offers the benefits of rapid onset and short half-life, as well as a potential bronchodilating effect.[34] In adult patients, hypertriglyceridemia, hypotension and bradycardia may be seen.[35] Propofol is no longer approved for sedation in pediatric patients.
Choosing the desired sedative for an individual patient with status asthmaticus may be based on issues such as anticipated duration of ventilation, hemodynamic stability and cost. Using a short-acting sedative such as propofol may reduce the chance of prolonged sedation when the patient is ready for extubation; for general ICU patients, the reduced time on mechanical ventilation has not, however, translated into a decrease in length of stay in the ICU.[35]
7. Neuromuscular Blocking Agents
Neuromuscular blocking agents have been administered to ICU patients requiring mechanical ventilation.[36] Neuromuscular blockade causes paralysis of the skeletal muscle and does not affect airway smooth muscle contraction. Neuromuscular blockade is indicated to facilitate ventilatory control, achieve synchrony and eliminate dangerous respiratory efforts only when complete sedation is not effective. There are no analgesic or sedative effects, so the patient must receive an agent such as an opioid or benzodiazepine.
Paralytic agents such as pancuronium, vecuronium and atracurium have been used in status asthmaticus.[1] Paralytic agents may be administered as an intermittent bolus or a continuous infusion. Despite monitoring by train-of-four peripheral nerve stimulation, prolonged neuromuscular blockade can occur. The paralytic agent vecuronium may have a prolonged effect in patients with renal failure. Cisatracurium and atracurium are degraded in plasma and are recommended for patients with hepatic or renal dysfunction.[36]
The use of neuromuscular blocking agents to facilitate ventilator synchrony in status asthmaticus has been associated with the severe complication of myopathy.[22,37,38] This myopathy has been linked to the concomitant administration of corticosteroids. The mechanism of action is unclear; however, the denervation injury may predispose to a corticosteroid-induced myopathic insult. The incidence of this complication has been difficult to establish because of dependency on the clinicians’ pursuit of diagnostic investigations. In one series, muscle weakness was found in 20 of 69 patients with status asthmaticus treated with corticosteroids and neuromuscular blocking agents.[37] Myopathy was diagnosed in 9 of 30 patients with status asthmaticus who received neuromuscular blockade.[38] The association of myopathy with the dose and duration of administration of corticosteroids and neuromuscular blocking agents have been variable. Creatine kinase may be normal or elevated. Diagnosis is made by electromyography and muscle biopsy. The duration of muscle weakness can range from days to months and severe weakness can contribute to prolonged mechanical ventilation.[22,37] The general consensus is that neuromuscular blocking agents should be avoided unless absolutely necessary.
8. Anesthetic Agents
Ketamine is an intravenous general anesthetic with bronchodilating effects and has been used in children with status asthmaticus.™ Complications include sympathomimetic effects leading to tachycardia and hypertension, decrease in seizure threshold and delirium, and increase in salivation.
General inhalation anesthetics, particularly isoflurane, has been used in the management of exceptional cases of status asthmaticus with refractory airflow obstruction.[1,40] With the approach of permissive hypercapnia and complete sedation in the management of status asthmaticus, there is a rare need to consider anesthesia. A recent report described the use of isoflurane anesthesia and permissive hypercapnia in patients with severe status asthmaticus.[41] These authors reported the difficulty in ventilating patients with severe asthma using an anesthesia ventilator. General anesthesia is also complicated by significant hemodynamic changes.
9. Buffer Therapy
Management of patients with severe status asthmaticus using the ventilatory approach may lead to hypercapneic acidosis. The administration of sodium bicarbonate to maintain a pH of 7.2 has been investigated in the management of patients with status asthmaticus using combined ventilator and bicarbonate strategy.[33] No studies have demonstrated a benefit when pH is maintained at a specific level with bicarbonate infusion.
10. Bronchoscopy
In certain patients with status asthmaticus, mucus impaction may be a significant factor in airflow obstruction and hypoxemia due to atelectasis. In this case, bronchoscopic examination of the airways and removal of secretions may be beneficial.[42] However, there is a risk of worsening lung hyperinflation during examination due to the presence of the bronchoscope. In an early series of patients with status asthmaticus, pneumothorax occurred only among patients subjected to bronchial lavage.[43]
11. Special Circumstance: Pregnancy
The same principles of therapy apply to the pregnant patient with status asthmaticus.[44] Inhaled bronchodilator and corticosteroids are standard treatments. If a parenteral β-agonist is considered, subcutaneous terbutaline is recommended.[21] The management of the pregnant patient with status asthmaticus allowing permissive hypercapnia during mechanical ventilation has not been addressed.[44] Hypercapnia has been reported in pregnancy without adverse outcome.[45] Opioids, benzodiazepines, and propofol all cross the placenta, as do neuromuscular blocking agents.[44] Morphine and fentanyl have not been associated with congenital malformations and have been used in the pregnant patient intubated for status asthmaticus. Propofol has also been used in pregnant patients with status asthmaticus.[34]
12. Outcome
Recent studies of patients with status asthmaticus have revealed a mortality rate of 0–22%.[5–9] Authors have stressed the importance of ventilator management geared to preventing dynamic hyperinflation for improved outcome. It is clear that standard therapy with bronchodilators and corticosteroids are the cornerstones of treatment for airways disease related to status asthmaticus. Ventilator management plays a purely supportive role until medical therapy is effective; in several series, the average duration of mechanical ventilation has been 3 days.[7,9] Careful ICU management is essential for optimal outcome. Despite many complications associated with the management of patients with status asthmaticus, as in critical care in general, these patients survive. In our series of 87 patients requiring intubation for status asthmaticus, the mortality was 2% and these deaths occurred in individuals who experienced a pre-ICU cardiopulmonary arrest.[9,46]
13. Conclusion
The management of patients with status asthmaticus requires intensive standard pharmacological therapy, particularly with corticosteroids and β-agonists. Mechanical ventilation may be necessary in cases of respiratory failure or respiratory arrest. The challenge to the pulmonary/critical care clinician is to provide optimal pharmacological and ventilatory support and avoid the sequelae of dynamic hyperinflation.
References
Corbridge TC, Hall JB. The assessment and management of adults with status asthmaticus. Am J Respir Crit Care Med 1995; 151: 1296–316
Sly RM. Changing asthma mortality. Ann Allergy 1994; 73: 259–68
Weiss KB, Wagener DK. Changing patterns of asthma mortality. JAMA 1990; 264: 1683–7
De Palo VA, Mayo PH, Friedman P, et al. Demographic influences on asthma hospital admission rates in New York City. Chest 1994; 106: 447–51
Braman SS, Kaemmerlen JT. Intensive care of status asthmaticus. JAMA 1990; 264: 366–8
Mansel JK, Stogner SW, Petrini MF, et al. Mechanical ventilation in patients with acute severe asthma. Am J Med 1990; 89: 42–8
Zimmerman JL, Dellinger P, Shah AN, et al. Endotracheal intubation and mechanical ventilation in severe asthma. Crit Care Med 1993; 21: 1727–30
Afessa B, Morales I, Cury JD. Clinical course and outcome of patients admitted to an ICU for status asthmaticus. Chest 2001; 120: 1616–21
Shapiro JM, McLeroth P. Status asthmaticus: a large MICU experience. Clin Intensive Care. In press
Bousquet J, Chanez P, Lacoste JY, et al. Eosinophilic inflammation in asthma. N Engl J Med 1990; 323: 1033–9
Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol 2001; 54: 577–89
Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med 1992; 326: 298–304
Carroll N, Elliot J, Morton A, et al. The structure of large and small airways in nonfatal and fatal asthma. Am Rev Respir Dis 1993; 147: 405–10
Carroll N, Carello S, Cooke C, et al. Airway structure and inflammatory cells in fatal attacks of asthma. Eur Respir J 1996; 9: 709–15
Synek M, Beasley R, Frew AJ, et al. Cellular infiltration of the airways in asthma of varying severity. Am J Respir Crit Care Med 1996; 154: 224–30
Madison JM, Irwin RS. Status asthmaticus. In: Rippe JM, Irwin RS, Fink MP, et al., editors. Intensive care medicine. 3rd ed. Boston (MA): Little, Brown and Company, 1996: 605–18
Dhand R, Tobin MJ. Inhaled bronchodilator therapy in mechanically ventilated patients. Am J Respir Crit Care Med 1997; 156: 3–10
National Asthma Education and Prevention Program. Expert panel report 2. Guidelines for the diagnosis and management of asthma. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute, 1997 Apr. NIH publication no. 97-4051
Shrestha M, Bidadi K, Gourlay S, et al. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest 1996; 110: 42–7
Marik P, Hogan J, Krikorian J. A comparison of bronchodilator therapy delivered by nebulization and metered-dose inhaler in mechanically ventilated patients. Chest 1999; 115: 1653–7
National Asthma Education and Prevention Program. Report of the Working Group on Asthma and Pregnancy. Management of asthma during pregnancy. Bethesda (MD): National Institutes of Health, National Heart, Lung, and Blood Institute, 1993 Sep. NIH publication no. 93-3279
Shapiro JM, Condos R, Cole RP. Myopathy in status asthmaticus: relation to neuromuscular blockade and corticosteroid administration. J Intensive Care Med 1993; 8: 144–52
Rodrigo GJ, Rodrigo C. First-line therapy for adult patients with acute asthma receiving a multiple-dose protocol of ipratropium bromide plus albuterol in the emergency department. Am J Respir Crit Care Med 2000; 161: 1862–8
Kidney J, Dominguez M, Taylor PM, et al. Immunomodulation by theophylline in asthma. Am J Respir Crit Care Med 1995; 151: 1907–14
Rowe BH, Bretzlaff JA, Bourdon C, et al. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med 2000; 36: 181–90
Kass JE, Castriotta RJ. Heliox therapy in acute severe asthma. Chest 1995; 107: 757–60
Koh Y. Ventilatory management of patients with severe asthma. Int Anesthesiol Clin 2001; 39: 63–73
Mountain RD, Sahn SA. Clinical features and outcome in patients with acute asthma presenting with hypercapnia. Am Rev Respir Dis 1988; 138: 535–9
Meduri GU, Cook TR, Turner RE, et al. Noninvasive positive pressure ventilation in status asthmaticus. Chest 1996; 110: 767–74
Tuxen DV, Lane S. The effects of ventilatory pattern on hyperinflation, airway pressures, and circulation in mechanical ventilation of patients with severe air-flow obstruction. Am Rev Respir Dis 1987; 136: 872–9
Williams TJ, Tuxen DV, Scheinkestel CD, et al. Risk factors for morbidity in mechanically ventilated patients with acute severe asthma. Am Rev Respir Dis 1992; 146: 607–15
Tuxen DV. Permissive hypercapnic ventilation. Am J Respir Crit Care Med 1994; 150: 870–4
Menitove SM, Goldring RM. Combined ventilator and bicarbonate strategy in the management of status asthmaticus. Am J Med 1983; 74: 898–901
Papadakos PJ. Using sedative agents in special ICU circumstances. Crit Care Med 2002; 30: S113–7
Jacobi J, Fraser GL, Coursin DB, et al. Clinical practice guidelines for the sustained use of sedatives and analgesics in the critically ill adult. Crit Care Med 2002; 30: 119–41
Murray MJ, Cowen J, DeBlock H. Clinical practice guidelines for sustained neuromuscular blockade in the adult critically ill patient. Crit Care Med 2002; 30: 142–56
Leatherman JW, Fluegel WL, David WS, et al. Muscle weakness in mechanically ventilated patients with severe asthma. Am J Respir Crit Care Med 1996; 153: 1686–90
Behbehani NA, Al-Mane F, D’yachkova Y, et al. Myopathy following mechanical ventilation for acute severe asthma: the role of muscle relaxants and corticosteroids. Chest 1999; 115: 1627–31
Werner HA. Status asthmaticus in children. Chest 2001; 119: 1913–29
Maltais F, Sovilj M, Goldberg P, et al. Respiratory mechanics in status asthmaticus: effects of inhalational anesthesia. Chest 1994; 106: 1401–6
Mutlu GM, Factor P, Schwartz DE, et al. Severe status asthmaticus: management with permissive hypercapnia and inhalation anesthesia. Crit Care Med 2002; 30: 477–80
Henke CA, Hertz M, Gustafson P. Combined bronchoscopy and mucolytic therapy for patients with severe refractory status asthmaticus on mechanical ventilation: a case report and review of the literature. Crit Care Med 1994; 22: 1880–3
Luksza AR, Smith P, Coakley J, et al. Acute severe asthma treated by mechanical ventilation: 10 years’ experience from a district general hospital. Thorax 1986; 41:459–63
Lapinsky SE, Kruczynski K, Slutsky AS. Critical care in the pregnant patient. Am J Respir Crit Care Med 1995; 152: 427–55
Ivankovic AD, Elam JO, Huffman J. Effect of maternal hypercarbia on the newborn infant. Am J Obstet Gynecol 1970; 107: 939–46
Shapiro JM. Intensive care management of status asthmaticus. Chest 2001; 120: 1439–41
Acknowledgements
No sources of funding were used to assist in the preparation of this manuscript. The author has no conflicts of interest that are directly relevant to the content of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shapiro, J.M. Management of Respiratory Failure in Status Asthmaticus. Am J Respir Med 1, 409–416 (2002). https://doi.org/10.1007/BF03257168
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03257168